Abstract
The high demand for renewable energy and increased biodiesel production lead to the surplus availability of crude glycerol. Due to the above reason, the bio-based value addition of crude glycerol into various bioproducts is investigated; among them, microbial lipids are attractive. The present study was dedicated to find the optimal glycerol concentration and carbon/nitrogen (C/N) ratio to produce maximum lipid using Yarrowia lipolytica SKY7. The glycerol concentration (34.4 to168.2 g/L) and C/N ratio (25 to 150) were selected to investigate to maximize the lipid production. Initial glycerol concentration 112.5 g/L, C/N molar ratio of 100, and with 5 % v/v inoculum supplementation were found to be optimum for biomass and lipid production. Based on the above optimal parameters, lipid concentration of 43.8 % w/w with a biomass concentration of 14.8 g/L was achieved. In the case of glycerol concentration, the maximum Yp/s (0.192 g/g); Yx/s (0.43 g/g) was noted when the initial glycerol concentration was 112.5 g/L with C/N molar ratio 100 and inoculum volume 5 % v/v. The glycerol uptake was also noted to increase with the increase in glycerol concentration. At low C/N ratio, the glycerol consumption was found to be high (79.43 g/L on C/N 25) whereas the glycerol consumption was observed to decrease when the C/N ratio was raised to 150 (40.8 g/L).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biofuels can be described as the high energy molecules produced by microbes through carbon fixation. These are biodegradable, nontoxic, and carbon neutral in characteristics [1]. The recent advancement in microbial-based triacylglycerol (TAG) production made a revolutionary change in biodiesel production. At present, biodiesel available in the market has been produced based on first generation biofuel technology. This includes the sugar, carbohydrates, edible oils, used cooking oils, animal fat, tallow, etc. [2–4]. The current US biodiesel market analysis (monthly) shows 122 million gallons of biodiesel has been produced in June 2015 and which is expected to grow further [5]. In the meantime, the global biodiesel market has been expected to expand to 140 billion liters at the end of 2016, which promises a substantial surplus availability of crude glycerol in the world market [6]. In the case of Canadian biodiesel market, about 400 million liters of biodiesel has been reported to be produced at the end of 2014 [7]. This shows 40 million letters of crude glycerol production on every year. Furthermore, the rapid growth in biodiesel market resulted in the low price of crude glycerol (8.50 to 10 US CTS/LB) in the beginning of 2014 [8]. Due to the low cost and surplus availability of crude glycerol, the research on third-generation biofuels and value-added product search was boosted in recent decades.
Biodiesel from crude glycerol using microbes as a biocatalyst was well established. Crude glycerol has been used to produce various value-added products such as single cell oil (SCO), polyhydroxyalkanoates (PHA), sugar alcohols, β-carotene, organic acids, etc. [9–13]. Among them, SCO from crude glycerol became one of the popular due to the high demand of energy in transportation and strict environmental protection policies. SCO can be produced by microbes in heterotrophic or autotrophic mode. In autotrophic, mainly algae were employed to produce lipid using sunlight/CO2/glycerol as a carbon source [14]. In heterotopic mode, mainly yeast, filamentous fungi, or bacteria were used. Among them, yeast was preferred over filamentous fungi due to its unicellular nature and capable of growing faster than fungi. The main yeast cultures used for SCO production were Rhodotorula, Yarrowia, Candida, Cryptococcus, Lypomyces, Rhodosporidium, Mortirella, and Mucor [15–17]. Yarrowia lipolytica became one of the important SCO producers due to its heterotrophic nature and multiple product formations and it regarded as the model organism to understand the lipid synthesis [18].
In the case of lipid production by heterotrophic microbes, the cultivation condition plays a crucial role. The culture parameters such as carbon concentration, carbon to nitrogen ratio (C/N molar ratio), and other macro- and microelements were investigated in the past decade. In a batch cultivation, a 30.1 % lipid accumulation (1.27 g/L) was attained in a media supplemented with 10 % w/w crude glycerol by Poli et al. 2014. This study compared the pure glycerol with crude glycerol and found no significant difference when crude glycerol was used for lipid production [2]. However, when Y. lipolytica was cultivated in a continuous culture with nutrient limited media, the biomass increased to 60 g/L with a volumetric lipid productivity of 0.43 g/L/h [19]. Based on the literature survey, it has been found that the carbon concentration is a critical factor that controls the biomass and lipid production. A high initial carbon concentration inhibits the biomass production. At the same time, the low glycerol concentration at lipid production phase significantly affects the volumetric lipid production. Due to the above reasons, the present study engaged to illustrate the impact of glycerol concentration and carbon/nitrogen (C/N) ratio on biomass and lipid production using Y. lipolytica SKY7.
Optimizing the glycerol concentration (carbon) is one of the important parameters to achieve high biomass and lipid content. Due to the variation in glycerol composition derived from biodiesel industries, the concentration of glycerol in crude glycerol varies from 15 to 80 % w/w. The other major components were soap, free fatty acids, methanol, catalyst, and water. Among them, FFA and soap are known for its inhibitory effects on biomass and lipid accumulation. In spite of carbon concentration, C/N ratio regarded as the controller of biochemical events of lipid production since the limitation of external nitrogen activates the AMP-deaminase and the intracellular AMP degraded to release NH4 + ions. The low intracellular AMP reverses the citric acid cycle and accumulates citrate in mitochondria, and then it transported to the cytoplasm [18, 20]. Thus, studying the impact of C/N ratio could improve the biomass and lipid production.
Materials and Methods
Culture and Inoculum
A pure culture of Y. lipolytica SKY 7 was stored in YPD (yeast extract, peptone, and dextrose) plates at 4 °C. The culture was activated by inoculating a loop full of culture in YPD broth and incubated for 24 h at 28 °C in a shaking incubator (180 rpm). The activated culture was further used for the inoculum preparation. The inoculum for the current study was prepared in YPG broth (yeast extract, peptone, and crude glycerol). The prepared YPG broth was sterilized by autoclaving at 121 °C for 20 min, and after the sterilization, 10 % v/v of the activated culture of Y lipolytica SKY7 was inoculated aseptically and the culture was incubated for 24 h at 28 °C with an agitation of 180 rpm.
Crude Glycerol Characterization
The composition of the crude glycerol was analyzed before the fermentation. The glycerol content in the present crude glycerol was measured according to the method described by Bondioli and Della Bella (2005) [21]. Appropriately diluted crude glycerol samples were mixed with 1:1 ethanol-water solution, and then 1.2 mL of 20 mM sodium periodate solution was added. After well mixing, 1.2 mL of 200-mM acetylacetone solution was added. The resulting mixture was incubated at 70 °C for 1 min. The yellow-colored compound developed by this reaction was measured at 410 nm using a Varian Cry 500 spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). A calibration standard was made with pure glycerol procured from Sigma-Aldrich Canada.
The density of the glycerol was measured by weighing 5 mL of crude glycerol by a conventional gravimetric method. The methanol content was measured by distilling the crude glycerol at 60 °C by an automated rotary evaporator. Thus, obtained methanol was measured gravimetrically and recorded. The pH was measured by dissolving 1 g of glycerol in 10 mL of distilled water and the pH was measured with a galvanometric pH meter. The FFA and catalyst content were measured by adjusting the pH to1 by adding 85 % orthophosphoric acid and thus resulted mixture was centrifuged at 5000 rpm for 10 min. Due to the density difference, three distinct layers were formed. The top dark red layer is composed of FFA and soap, and the middle turbid layer represents the catalyst, other insoluble matters and the bottom layer with glycerol and water.
Effect of Glycerol Concentration on Biomass and Lipid Production
The impact of glycerol concentration on biomass and lipid accumulation was investigated from the range of 34.43 to 168.25 g/L. Experiments were carried out in duplicates in 2 L Erlenmeyer flask with a working volume of 500 mL. The C/N ratio was adjusted to 100 with required amount of yeast extract powder. The fermentation media was formulated with crude glycerol, Na2HPO4 1 g/L, KH2PO4 2.7 g/L, MgSO4.7H2O 0.5 g/L, and yeast extract (varied according to the C/N ratio). The prepared media was sterilized by autoclaving at 121 °C for 15 min, and the sterile media was inoculated with 15 mL (10 % v/v) of the well-grown pure culture of Y. lipolytica SKY7. The flasks were incubated at 28 °C with an agitation rate of 180 rpm for a period of 120 h. Samples were collected at 0, 72, and 120 h. The collected samples were analyzed for biomass lipid and residual glycerol.
Evaluation of C/N Ratio
In all the experiments, 100 g/L of crude glycerol was used as the carbon source. The C/N ratio was adjusted from 25 to 150 using yeast extract as the source of nitrogen. The C/N ratio was calculated based on the carbon present in the glycerol (39.1 % w/w) and the nitrogen present in the commercially available yeast extract approximately (12 % w/w). The other media components remain the same as mentioned in the previous section. The flask containing 500 mL of media was inoculated with 15 mL of inoculum (10 % v/v) and incubated at 28 °C with an agitation rate of 180 rpm for a period of 120 h. The samples were collected at 0, 72, and 120 h and were analyzed for biomass, lipid, and residual glycerol.
Influence of Inoculum Concentration on Biomass and Lipids
An actively dividing inoculum has a huge impact on biomass and lipid accumulation; hence, it is one of the factors to determine the final biomass and lipid accumulation. In the present study, 2.5 to 10 % v/v inoculum was studied. The pre-inoculum and the fermentation media was prepared as explained in previous sections. The flasks were inoculated with well-grown pre-culture (36 h) of a Y. lipolytica SKY7. The culture was incubated for 120 h at 28 °C with an agitation of 180 rpm. The samples were withdrawn at 0, 72, and 120 h, and the biomass and lipid and residual glycerol were analyzed.
The Importance of Trace Elements on Growth and Lipid Accumulation
The trace elements have a high influence on biomass and lipid accumulation. In order to assess the influence of trace elements in biomass and lipid accumulation, a trace element solution was prepared with the following composition and was studied in a range from 1 to 10 mL/L. Composition of trace element solution (g/L) FeSO4.7H2O 4 g, CaCl3 3.6 g, ZnSO4.7H2O 0.75 g, CuSO4.5H2O 0.13 g, MnSO4.H2O 0.5 g, CoCl2.6H2O 0.13 g, Na2MoO4.2H2O 0.17 g. Samples were withdrawn at 0, 72, and 120 h and analyzed for biomass, lipid accumulation, and residual glycerol.
Quantification of Biomass and Lipid
Twenty milliliters of fermented broth was used for biomass and lipid quantification. The collected samples were centrifuged at 4000 rpm for 10 min, and the supernatant was collected in a clean tube. The biomass was washed with distilled water for two times, and the cell pellets were collected by centrifugation. For biomass quantification, the samples were transferred into a clean pre-weighed aluminum pan and it was kept at 105 °C until it reaches a constant weight. For lipid extraction, a modified method of Folch (1957) [22] was employed. Wet sample was mixed with 2:1 chloroform-methanol mixture, and to that, zirconium beads (0.7 mm) was added, the cells were disrupted by bead beater for 3 min (Biospecs, USA), and the samples were filtered using Whatman filter paper with a pore size of 0.45 μm. The solid sample was resuspended in 1:1 chloroform-methanol mixture, and the procedure was repeated. The resulting filtrate was pooled into a pre-weighed glass tube and concentrated under reduced nitrogen pressure, and the samples were further dried at 60 °C in a hot air oven until the sample reaches a constant weight.
Characterization of Lipid
Twenty-five milligrams of lipid was trans-esterified using acidified methanol as the catalyst. Docosahexaenoic acid was used as the internal standard. The trans-esterified lipid fraction was extracted using hexane, and the samples were further characterized by GC equipped with flame ionization detector (FID).
Results and Discussion
Characterization of Crude Glycerol
The composition of the crude glycerol used for the present study is presented in Table 1. The glycerol concentration was 78.3 % w/w, with other components methanol, soap, free fatty acids, and catalyst. The pH of the crude glycerol was 6.8. The pH may vary according to the catalyst used for the trans-esterification process. Based on the pH of the current glycerol, it can be assumed that the catalyst used was a base. The near acidic pH reduces the addition or adjustment of pH during the fermentation since the final fermentation pH is 6.5. The impurities present in the biodiesel industry-driven crude glycerol were mainly methanol, soap, minerals, and catalyst. These impurities can influence the growth and metabolite production in either way. In a study conducted by Chatzifragkou et al., 2012 reported positive effects on certain metabolite production [23].
Impact of Glycerol Concentration on Biomass and Lipid Production
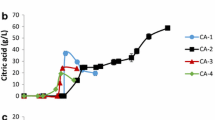
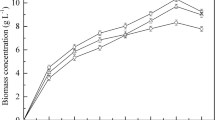
Four different concentrations (34.43, 52.37, 112.58, and 168.25 g/L) of glycerol were studied, and the C/N ratio was maintained 100 in all conditions. The comparison of biomass production and glycerol consumption on each glycerol concentrations was given in Fig. 1a, b. Biomass concentration of 16.08 g/L was obtained on 52.3 g/L glycerol supplements experiment and followed by 14.2 g/L in 112.5 g/L glycerol supplemented experiments. Whereas in the case of 34.4 g/L glycerol supplemented experiment, the biomass concentration was reached to 11.76 g/L, and in the case of 168.2 g/L glycerol supplemented experiments, it was 11.04 g/L. In the case of glycerol consumption, 30.08, 34.1, 48.66, and 32.58 g/L of glycerol were assimilated by Y. lipolytica SKY7 on 34.4, 52.3, 112.5, and 168.2 g/L glycerol supplemented experiments, respectively. From the experimental data, it has been concluded that there could be two reasons for low biomass concentrations in 34.4 and 168.2 g/L glycerol supplemented experiments. Primarily, the available nitrogen content in the fermentation media can influence the biomass concentration. When 34.4 g/L glycerol was studied, the amount of nitrogen added to the media was 0.135 g/L in order to achieve a C/N ratio of 100 (13.5 g of carbon in 34.4 g of glycerol). Hence, the low biomass production in 34.4 g/L glycerol supplemented studies could be due to the low nitrogen concentration in the media. The second reason could be the inhibition of high substrate concentration in the case of 168.2 g/L glycerol supplemented experiments. From Fig. 1a, it can be observed the when the glycerol concentration increased, the biomass concentration also increases up to the glycerol concentration reaches 52.3 g/L. However, it declined when the glycerol concentration was increased to 112.5 and 168.2 g/L. In the case of 168.2 g/L, 0.6 g/L available nitrogen was supplemented in the media. However, due to the high glycerol concentration, the growth was comparatively low than other conditions. To confirm the above results, the specific growth rates were plotted (Fig. 2) and it showed a declining trend from the early stage of fermentation in all conditions, and this can be due to minimal nutrient supplementation and high substrate concentration.
Figure 3a represents the Yx/s (yield of biomass/gram of glycerol consumed) showing a higher substrate to biomass conversion at the beginning of fermentation (0 to 24 h) in the case of 34.4 and 112.5 g/L glycerol supplemented experiments. Whereas, it was slightly different in the case of 52.3 and 168.2 g/L glycerol supplemented experiments. In the case of 52.3 g/L glycerol supplemented experiments, the maximum Yx/s (0.789 g/g) was achieved at 48 h, and in 168.2 g/L (0.448 g/g), it was on 60 h. The specific growth rate shows (Fig. 2) that the higher the glycerol concentration, the low specific growth was obtained. The experiment with 52.3 g/L glycerol showed a slightly higher specific growth than 34.4 and 112.5 g/L glycerol-contained experiments. In the case of 34.4 g/L glycerol supplemented experiments, the original concentration of nitrogen supplied is low compared to 52.3 and 168.2 g/L glycerol supplemented experiments. Due to the low concentration of available nitrogen, cell growth is also affected. Whereas in the case of 112.5 and 168.2 g/L glycerol supplemented experiments, even though the nitrogen concentration is higher than 52.3 g/L glycerol supplemented experiments, the high glycerol concentration inhibits the growth. From this observation, it can be concluded that the glycerol concentration from 52.3 to 112.5 g/L could be optimal for biomass production. Moreover, the recent reports on Y. lipolytica cultivated on the crude glycerol show either low or approximately similar biomass production ( 6.7 g/L, (2), 19.14 g/L [24], and 12 g/L [25]) on batch fermentations. From the current reports and the experimental data on Y. lipolytica SKY7, it has been clear that this stain has great potential to produce biomass and TAG in minimal media.
Figure 1c shows the variation in lipid concentration observed in different glycerol concentrations. The maximum lipid concentration (6.6 g/L) was obtained in 112.5 g/L glycerol supplemented experiment. The low concentration of lipid (3.95 g/L) was observed in 34.4 g/L glycerol congaing experiment. The experimental data shows that when the glycerol concentration in the media was increased, the lipid concentration also increased. However, when the glycerol concentration is increased above 112.5 g/L, the lipid concentration tends to drop due to low biomass concentration even though the lipid content was higher in the cells (Fig. 1d).
Comparisons of lipid yield factors were presented in Fig. 3b. A low yield of 0.161 g/g (30 h) was observed when the glycerol concentration was 34.4 g/L. The yield of lipid (Yp/s) was noted to be increased with increase in glycerol concentration. In the case of 52.3 g/L glycerol, the Yp/s was 0.22 g/g at 30 h and which was noted to decline with the fermentation time. However, the maximum yield of lipid (0.243 g/g) was attained at 18 h in 112.5 g/L glycerol supplemented experiments. From 18 h onwards, the Yp/s declines. In the case of 168.2 g/L glycerol supplemented study, the Yp/s reached a maximum at 48 h (0.315 g/g). When considering the above four conditions, the yield of lipid was higher in the case of 112.5 g/L glycerol supplemented experiment within 18 h of fermentation. This indicated that the lipid production occurred at the early stage of fermentation. This could be due to the low nutrient concentration of the media. When the media is poor, the growth decelerates at early and the cells start to produce lipids. In the first three cases, the lipid production was noted to decline after 18 to 30 h of fermentation. Whereas in the case of 168.2 g/L glycerol supplemented experiment, the decline in yield was observed after 48 h. This could be due to the inhibition of growth by high glycerol concentration. Due to the slow growth of the cells, the available nitrogen could be utilized slowly; thus, the lipid yield was higher at 48 h. Based on the Yp/s and total concentration of lipid, 112.5 g/L of glycerol supplemented could be better for lipid and biomass production.
Impact of C/N Ratio on Biomass and Lipid Production
Based on the studies conducted on glycerol concentration, 112.5 g/L glycerol concentration was chosen for evaluating the C/N ratio. Figure 4a compares the difference in biomass obtained in four experimental conditions. The biomass concentration was noted to decrease with respect to the increase in C/N ratio. Comparatively higher biomass (22.62 g/L) was obtained in C/N ratio of 25, and the low biomass (13.57 g/L) was in C/N ratio of 150. This explains when the C/N ratio increases, the available nitrogen content of the fermentation medium decreases and it directly affects the biomass production. To support the above fact, the specific growth rate was calculated and presented in Fig. 5. In the case of C/N ratio 25 and 50, the specific growth rate was identical throughout the fermentation. However, in the case of C/N ratio of 100 and 150, the specific growth rates were significantly lower compared to the other two conditions. This confirms that the C/N ratio affects the overall growth, and thus, it reduces the biomass generation in high C/N ratios. Moreover, the most recent studies on the influence of C/N ratio on biomass and lipid production by Y. lipolytica also confirmed the C/N ratio of 75 to 100 could be the optimum. A study conducted by Dobrowolski et al. 2016 with Y. lipolytica A101 showed a biomass production of 8.46 ± 0.23 g/L in the case of C/N 75 and 7.97 ± 0.23 g/L on C/N 100 [26]. However, in the case of Y. lipolytica SKY7, the biomass concentrations were found to be 16.37 ± 0.1 g/L at 120 h of fermentation.
The yield of biomass is presented in Fig. 6a. It reveals a contradicting observation. The biomass yield was low in the case of C/N 25 and 150. However, the Yx/s was high in the case of C/N 50 and 100. The above observations reveal that more substrates were converted into biomass in the case of 50 and 100 C/N ratios. In the case of C/N 25, the assimilated substrate was converted into biomass and other metabolites. To ensure the above observation, the substrate consumption rate (dg/dt) was calculated and presented in Fig. 7. The substrate consumption was noted to be high in the case of C/N 25 followed by C/N 150. On the other hand, the substrate consumption rate shows a gradual increase in C/N 50 and 100. The monotonous increase in substrate consumption and an increase in biomass and lipid yield (Figs. 4a and 6b) in C/N 100 indicate that most of the assimilated substrates could be converted into biomass at initial fermentation time and lipid and less byproduct formation at a later stage of fermentation.
Figure 4b represents the lipid produced on each condition. The maximum lipid was produced when the C/N ratio was 100 (6.66 g/L) and the low lipid concentration was in C/N 25 (4.55 g/L). In the case of C/N 100, 50, and 25, the maximum lipid concentration was reached at 72 h, and in C/N 150, it was at 60 h of fermentation. Further, the lipid content (Fig. 4d) of the cells also showed a higher lipid content in the case of C/N ratio 100 and 150. This confirms further that the C/N ratio above 50 will enhance the lipid production by altering the cellular metabolism towards lipid synthesis.
The lipid yield (Yp/s Fig. 6b) shows higher substrate to lipid conversion in C/N 100, and this could be due to the low utilization of substrate (glycerol). In C/N 50 and 100, the Yp/s was approximately similar throughout the fermentation. Whereas, the low substrate to lipid conversion was found in C/N ratio 25. Based on the observations, C/N 100 yielded a comparatively high concentration of lipid even though the yield (Yp/s) was higher in C/N 150. This concludes that C/N 100 could be suitable for lipid production in Y. lipolytica SKY7.
Importance of Inoculum Density and Trace Elements and Its Effect on Biomass and Lipid Accumulation
In this experiment, four different inoculum sizes were evaluated and Table 2 represents the biomass, lipid accumulation at 120 h. From the experimental results, inoculum concentration 5 % v/v seems to be suitable for an initial glycerol concentration of 10 % v/v and C/N ratio 100. A lipid concentration of 6.28 g/L was achieved when the inoculum concentration was maintained at 5 % v/v. On another hand, the biomass concentrations of all these conditions were comparable and there were no significant increase or decrease. The yield of lipid from the amount of substrate utilized by different inoculum size was represented in Table 2, and it suggests that the Yp/s was decreasing when the inoculum concentration was increased. The higher Yp/s (0.192 g/g) is observed in the case of 5 % v/v inoculum. Whereas, the glycerol assimilation increases when the inoculum size was increased and the substrate to lipid conversion dropped significantly. Based on the Yp/s and the lipid concentration, it is concluded that the inoculum concentration 5 %v/v could be the optimum.
The effect of trace elements on lipid and biomass accumulation is presented in Table 3. As expected, the trace elements improve the biomass accumulation when the trace element concentration was increased from 1 to 10 mL/L. Decreasing the trace elements shows a positive effect on lipid accumulation. Whereas, the glycerol utilization shows that the assimilated glycerol was mostly channelized to biomass accumulation; this indicated that the enzymes related to the lipid accumulation were under limited activity when the microelements are provided in sufficient amount. But this could be inverted when the fermentation time progresses due to the microbial consumption of trace elements. The present study illustrates that when the fermentation was carried out without any addition of trace elements, the biomass and lipid accumulation was 14.68 and 6.65 g/L (Fig. 1a), respectively. In experiments supplemented with trace elements, lipid accumulation dropped to 4.8 g/L (trace elements 10 mL/L) from 5.56 g/L (trace elements 1 mL/L) and the biomass accumulation increased to 18 to 19 g/L from 14.68 g/L. From the above observations and from the literature, it is well clear that the composition of the crude glycerol mainly contains the metal ions in the acceptable amount due to the addition of chemical catalyst during trans-esterification. In the meantime, the literature survey showed that the addition of trace elements resulted in higher biomass conversion rather than lipid production; however, the lipid concentration was found higher in the case of non-trace element supplemented media [27].
Composition of TAG
The composition of lipid considerably depends on the carbon source, the concentration of carbon, and the C/N ratio. There were several studies showing that the lipid profile was changed upon the fermentation time increases. Table 4 represents the lipid composition of Y. lipolytica SKY7 at different glycerol concentration and C/N ratios at 120 h of fermentation. Oleic acid was found to be the major component in all the cases. Though Y. lipolytica is reported to produce high percentage of linoleic acid, this isolate seems to produce other unsaturated fatty acids such as Myristic (C14:0), Palmitic (C16:0), Stearic (C18:0), Oleic cis-9 (C18:1n9-cis), Linoleic (C18:2n6c), and Arachidonic (C20:4n6) acid in high quantity. Other than the listed components this organism produces C15:0, C15:1, Palmitoleate (C16:1n7), Elaidic (C18:1n9t), Linolenate (C18:3n3), Arachidic (C20:0), Eicosenoate (C20:1n9), and Lignoceric (C24:0) in minor quantities (data not shown).
Summary and Conclusion
The culture parameters such as glycerol concentration C/N ratio, inoculum concentration, and trace elements are evaluated in flask level. The results show that the glycerol concentration C/N ratio and inoculum concentration has more influence in the case of lipid and biomass accumulation. According to the study, the preferable condition for Y. lipolytica SKY7 are 100 g/L (10 % w/v) glycerol, and C/N ratio of 100 with inoculum concentration of 5 % v/v shows better biomass and lipid production. Though the trace elements increase the biomass concentration, it fails to trigger the lipogenesis and it could be due to the nutrient availability that promotes the cell division and by mean it reduces the lipid production. Apart from nitrogen iron, phosphate and magnesium limitations are reported for lipid accumulation. Moreover, supply of iron enhances the citric acid production in Y. lipolytica. As reported in the present study and by several authors, the inoculum age plays a key role in lipid accumulation. From the observations, the exponential phase of Y. lipolytica SKY7 falls within 18 h and in the present study, the inoculum age was 36 h. By reducing the inoculum age, the fermentation can be efficiently altered by shifting the late lipid production and this will avoid the initial lag phase (in this case 0–6 h growth was insignificant).
References
Easterling, E. R., French, W. T., Hernandez, R., & Licha, M. (2009). The effect of glycerol as a sole and secondary substrate on the growth and fatty acid composition of Rhodotorula glutinis. Bioresource Technology, 100, 356–361.
Poli, J. S., da Silva, M. A., Siqueira, E. P., Pasa, V. M., Rosa, C. A., & Valente, P. (2014). Microbial lipid produced by Yarrowia lipolytica QU21 using industrial waste: a potential feedstock for biodiesel production. Bioresource Technology, 161, 320–326.
Papanikolaou, S., Chevalot, I., Komaitis, M., Marc, I., & Aggelis, G. (2002). Single cell oil production by Yarrowia lipolytica growing on an industrial derivative of animal fat in batch cultures. Applied Microbiology and Biotechnology, 58, 308–312.
Papanikolaou, S., Chevalot, I., Galiotou-Panayotou, M., Komaitis, M., Marc, I. and Aggelis, G. (2007) Industrial derivative of tallow: a promising renewable substrate for microbial lipid, single-cell protein and lipase production by Yarrowia lipolytica. Electronic Journal of Biotechnology, 10, 425–435.
EIA. (2015) Monthly Biodiesel Production Report. U.S Energy information administration.
Anand, P., & Saxena, R. K. (2012). A comparative study of solvent-assisted pretreatment of biodiesel derived crude glycerol on growth and 1,3-propanediol production from Citrobacter freundii. New Biotechnology, 29, 199–205.
CRFA. (2014) From biofuels to bioeconomy. Canadian renewable fuel Association.
Landress, L. (2014) Glycerine Prices, markets & analysis. ICIS. http://www.icis.com/globalassets/global/icis/pdfs/sample-reports/chemicals-glycerine.pdf.
Avila-Neto, P. M., da Silva, G. P., Lima, C. J. B., De Paula, F. C., & Contiero, J. (2014). Evaluation and optimization of growth and citric acid production by Yarrowia lipolytica nrrl y-1095 using glycerol as carbon source as an alternative to use biodiesel byproduct. Journal of Experimental Biology and Agricultural Sciences, 2, 25–31.
Morgunov, I., Kamzolova, S., Perevoznikova, O., Shishkanova, N., & Finogenova, T. (2004). Pyruvic acid production by a thiamine auxotroph of Yarrowia lipolytica. Process Biochemistry, 39, 1469–1474.
Mantzouridou, F., Naziri, E., & Tsimidou, M. Z. (2008). Industrial glycerol as a supplementary carbon source in the production of B-carotene by Blakeslea trispora. Journal of Agricultural and Food Chemistry, 56, 2668.
André, A., Diamantopoulou, P., Philippoussis, A., Sarris, D., Komaitis, M., & Papanikolaou, S. (2010). Biotechnological conversions of bio-diesel derived waste glycerol into added-value compounds by higher fungi: production of biomass, single cell oil and oxalic acid. Industrial Crops and Products, 31, 407–416.
Rymowicz, W., Rywinska, A., & Marcinkiewicz, M. (2009). High-yield production of erythritol from raw glycerol in fed-batch cultures of Yarrowia lipolytica. Biotechnology Letters, 31, 377–380.
Chen, Y. H., & Walker, T. H. (2011). Biomass and lipid production of heterotrophic microalgae Chlorella protothecoides by using biodiesel-derived crude glycerol. Biotechnology Letters, 33, 1973–1983.
Ageitos, J. M., Vallejo, J. A., Veiga-Crespo, P., & Villa, T. G. (2011). Oily yeasts as oleaginous cell factories. Applied Microbiology and Biotechnology, 90, 1219–1227.
Li, Q., Du, W., & Liu, D. (2008). Perspectives of microbial oils for biodiesel production. Applied Microbiology and Biotechnology, 80, 749–756.
Rossi, M., Amaretti, A., Raimondi, S., & Leonardi, A. (2011). Getting lipids for biodiesel production from oleaginous fungi. doi:10.5772/25864
Beopoulos, A., Chardot, T., & Nicaud, J. M. (2009). Yarrowia lipolytica: a model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie, 91, 692–696.
Mironczuk, A. M., Rakicka, M., Biegalska, A., Rymowicz, W., & Dobrowolski, A. (2015). A two-stage fermentation process of erythritol production by yeast Y. lipolytica from molasses and glycerol. Bioresource Technology, 198, 445–455.
Ratledge, C. and Wynn, J. P. (2002) The Biochemistry and Molecular Biology of Lipid Accumulation in Oleaginous Microorganisms. Advances In Applied Microbiology, 51, 1–51.
Bondioli, P., & Della Bella, L. (2005). An alternative spectrophotometric method for the determination of free glycerol in biodiesel. European Journal of Lipid Science and Technology, 107, 153–157.
Folch, J., Lees, M., & Slane-Stanley, G. H. (1957). Simple method for the isolation and purification of total lipids from animal tissues. The Journal of Biological Chemistry, 226, 497–509.
Chatzifragkou, A., & Papanikolaou, S. (2012). Effect of impurities in biodiesel-derived waste glycerol on the performance and feasibility of biotechnological processes. Applied Microbiology and Biotechnology, 95, 13–27.
Machado Jr., F. R. S., Michelon, M., Burkert, J. F. M., Dalcanton, F., Burkert, C. A. V., & Furlong, E. B. (2015). Biomass production by Yarrowia lipolytica as a source of lipids: bench scale cultivation on raw glycerol-based medium. International Food Research Journal, 22, 1253–1260.
Sestric, R., Munch, G., Cicek, N., Sparling, R., & Levin, D. B. (2014). Growth and neutral lipid synthesis by Yarrowia lipolytica on various carbon substrates under nutrient-sufficient and nutrient-limited conditions. Bioresource Technology, 164, 41–46.
Dobrowolski, A., Mitula, P., Rymowicz, W., & Mironczuk, A. M. (2016). Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Bioresource Technology, 207, 237–243.
Nambou, K., Zhao, C., Wei, L., Chen, J., Imanaka, T., & Hua, Q. (2014). Designing of a "cheap to run" fermentation platform for an enhanced production of single cell oil from Yarrowia lipolytica DSM3286 as a potential feedstock for biodiesel. Bioresource Technology, 173, 324–333.
Acknowledgments
The authors would like to acknowledge the Natural Sciences and Engineering Research Council of Canada (grant A4984, Strategic grant 412994 – 11, Canada Research Chair) for financial support. We are grateful to technical staffs of INRS-ETE for their timely help to analyze the samples on GC-FID.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuttiraja, M., Douha, A., Valéro, J.R. et al. Elucidating the Effect of Glycerol Concentration and C/N Ratio on Lipid Production Using Yarrowia lipolytica SKY7. Appl Biochem Biotechnol 180, 1586–1600 (2016). https://doi.org/10.1007/s12010-016-2189-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2189-2