Abstract
Doxorubicin is an anticancer drug whose toxic effects on non-cancer cells are associated with increased oxidative stress. This study investigated the chemical composition, antioxidant activity of the methanolic extract of Schinus terebinthifolius Raddi leaves (MESL) as well as effects against doxorubicin-induced toxicity in human erythrocytes, K562 human erythroleukemia cells, and mouse hearts. The chemical composition indicated the presence of phenolic compounds, flavonoids, tannins, and ascorbic acid. MESL showed antioxidant activity by scavenging free radicals and inhibiting hemolysis and lipid peroxidation in human erythrocytes incubated with an oxidizing agent, and was able to increase the enzymatic activity of superoxide dismutase and glutathione peroxidase in human erythrocytes, without influencing the activity of enzyme catalase. The increase of oxidative hemolysis and malondialdehyde levels in erythrocytes incubated with doxorubicin was reduced by treatment with MESL. The cytotoxic activity of doxorubicin in erythroleukemia cells treated with MESL was unmodified. Additionally, the extract protected mice against the doxorubicin-induced cardiotoxicity. In conclusion, the MESL exhibits antioxidant activity, reducing doxorubicin-induced oxidative stress without changing the anticancer action of the drug, and protects against doxorubicin-induced cardiotoxicity. Hence, these findings suggest that these effects are via anti-oxidative by inhibiting free radicals, decreased oxidative stress, and increased antioxidant enzyme activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Doxorubicin is widely used for treating various cancers including acute leukemias, malignant lymphomas, and various solid tumors. However, prolonged use can cause toxic side effects such as cardiotoxicity [1, 2]. The antitumor action mechanisms of doxorubicin include inhibition of topoisomerase II and intercalation of the drug between pairs of DNA, and increased oxidative stress, which is considered a limiting factor for its long-term use due to the accumulation of reactive oxygen species [3, 4]. In view of the oxidative stress induced by the clinical use of doxorubicin, compounds with antioxidant properties that do not interfere with the therapeutic potential of the drug have been investigated [5, 6]. Among the antioxidant compounds most widely used against the toxicity of doxorubicin, terpenoids [7, 8] and phenolic compounds [9,10,11] stand out. In recent years, there has been a growing interest in the pharmacological properties of medicinal plants in order to produce new herbal, semi-synthetic drugs and derivatives of natural products with fewer side effects that can assist in the treatment of various diseases [12, 13].
Schinus terebinthifolius Raddi (Anacardiaceae) is a medicinal plant, popularly known as Brazilian pepper tree, aroeira, rose pepper, or broadleaved pepper tree, and is a tree species native to Brazil. This plant is included in the Brazilian Pharmacopeia and is popularly used in the treatment of wounds and skin ulcers, tumors, diarrhea, arthritis, and urinary and respiratory tract infections [14, 15]. The anti-inflammatory [16], antimicrobial [17], and antitumor [18, 19] activities of the leaves and fruits of this plant have been investigated and described in various pharmacological models and have been attributed to its chemical constituents such as terpenoids and phenolic compounds.
In this context, the aim of this study was to evaluate the chemical composition, antioxidant activity of the methanolic extract of S. terebinthifolius Raddi leaves (MESL) as well as effects against doxorubicin-induced toxicity in human erythrocytes, K562 human erythroleukemia cells, and mouse hearts.
Materials and Methods
Plant Material and Preparation of MESL
S. terebinthifolius Raddi leaves were collected under coordinates 22° 11′ 43.7568″ S and 54° 56′ 8.0916″ W. A voucher specimen was deposited in the Herbarium of the Federal University of Grande Dourados, Brazil DDMS (No. 4889). Dried leaves (2.128 g) were kept in thorough maceration in absolute methanol. The filtrate was concentrated under vacuum at 45 °C and lyophilized to obtain the methanolic extract of S. terebinthifolius leaves (MESL). The yield was approximately 13%.
Chemical Composition
Total Phenolic Compound Content
The content of phenolic compounds in the MESL was determined using the Folin-Ciocalteu colorimetric method, as detailed by Meda et al. [20], with some modifications. The MESL (200 μg/mL) was diluted in absolute ethanol, and a 0.5-mL aliquot was added to 2.5 mL of Folin-Ciocalteu reagent (diluted 1:10 with distilled water). This solution was allowed to stand for 5 min at room temperature. After this period, 2 mL of a 14% sodium carbonate solution was added to the samples, the mixture was incubated for 2 h at room temperature, and the absorbance was read at 760 nm in a T70 UV/VIS spectrophotometer (PG Instruments Limited, UK). A standard curve was prepared using gallic acid in the concentration range of 0.4–11.0 μg/mL. The total amount of phenolic compounds was expressed in milligrams of gallic acid equivalents per gram of extract (mg GAE/g extract). Assays were performed in triplicate.
Flavonoid Content
The total flavonoids present in the MESL were determined according to the method described by Liberio et al. [21], with some modifications. Briefly, 4.5 mL of a hexahydrate aluminum chloride solution (AlCl3·6H2O) at 2% in absolute methanol was mixed with 0.5 mL of the MESL (200 μg/mL). The mixture was incubated for 30 min at room temperature, and the absorbance was read at 415 nm in a T70 UV/VIS spectrophotometer (PG Instruments Limited, UK). A standard curve was prepared using quercetin in the concentration range of 0.4–11.0 μg/mL. The total flavonoids were expressed as milligrams of quercetin equivalents per gram of extract (mg QE/g extract). Assays were performed in triplicate.
Tannin Content
One milliliter of MESL (2 mg/mL) was mixed with 2 mL of 4% vanillin solution in methanol and 2 mL of 8% HCl in methanol. The mixture was allowed to 30 °C stand for 20 min, and absorption was measured at 500 nm against methanol as a blank. Quantitation was performed using a calibration curve with increasing concentrations of catechin. The amount of total condensed tannins is expressed as milligrams of catechin equivalents per gram of extract (mg CE/g extract). Assays were performed in triplicate.
Ascorbic Acid Content
The ascorbic acid content was investigated using the titration method described by Benassi and Antunes [22], being expressed as milligrams of ascorbic acid equivalent per gram of extract (mg AAE/g extract). Assays were performed in triplicate.
Presence of Saponins
The presence of saponins was determined using method described by Tirloni et al. [23]. For this assay, 5 mg of MESL was solubilized in 1 mL of 80% ethanol and then, 2.5 mL of boiling water was added. The mixture was vigorously stirred and allowed to stand for 20 min. In this assay, the presence of foam indicates the presence of saponins. Assays were performed in triplicate.
Antioxidant Activity Determination
DPPH· Free Radical Scavenging Assay
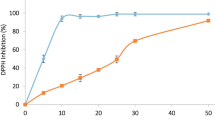
The 2,2-diphenyl-1-picrylhydrazyl (DPPH·) free radical scavenging assay was performed according to method described by Campos et al. [24]. Different MESL concentrations (0.1–1000 μg/mL) were mixed with DPPH· solution (0.11 mM) in 80% ethanol. The mixture was homogenized and incubated at room temperature in the dark for 30 min and read at 517 nm. Ascorbic acid was used as standard. Three independent experiments were performed in triplicate. The percentage of DPPH· inhibition in samples was calculated by the equation described: % inhibition of DPPH· = (1 − Abssample / AbsDPPH·) × 100.
Assessment of the Activity of Antioxidant Enzymes
In all assays, dilution of erythrocytes at 1:200 was used (25 mmol/L potassium phosphate buffer, pH 7.5, supplemented with 0.1% bovine serum albumin and 1 mmol/L EDTA). All assays were performed in microplates (Multiskan GO Microplate Spectrophotometer; Thermo Scientifc®, Vantaa, Finland). Erythrocytes were incubated with different MESL concentrations (50–500 μg/mL) for 20 min at 37 °C. Two independent experiments were performed in triplicate.
SOD Activity
SOD was determined using the Fluka® commercial kit (Sigma-Aldrich®, Seelze, Germany) according to manufacturer’s instructions. Data are expressed as the total international unit (IU) normalized to the hemoglobin concentration in milligrams/milliliter (IU/Hb, mmol/L).
CAT Activity
CAT activity was spectrophotometrically determined by the hydrogen peroxide (H2O2) decomposition rate, according to method described by Xu et al. [25], with modifications for microplates. Data were normalized for hemoglobin levels, and activity was expressed as micromoles per minute per milliliter per millimolar hemoglobin.
GPx Activity
GPx activity was determined using colorimetric method described by Paglia and Valentine [26], adapted for microplates. Data were normalized by hemoglobin levels and the enzyme activity was expressed as nanomoles per minute per milliliter per hemoglobin, millimolar per liter.
Antioxidant Assay Using Model of Human Erythrocytes
Preparation of Erythrocyte Suspensions
About 15 mL of peripheral blood was collected from healthy donors (procedure approved by the Ethics Research Committee of the Federal University of Grande Dourados - UNIGRAN - No. 123/12). Blood was centrifuged at 700×g; plasma and blood buffy coat were discarded. Erythrocytes were washed three times in 0.9% sodium chloride (NaCl) and after washing, two erythrocyte suspensions were prepared at 10 to 20% in 0.9% NaCl.
Hemolysis Assay and Inhibition of Oxidative Hemolysis
The hemolytic capacity and inhibition of oxidative hemolysis of MESL was determined according to method described by Campos et al. [24], with modifications. The assays were performed with 10% erythrocyte suspensions. Erythrocytes were preincubated at 37 °C for 30 min in test tubes in the presence of different concentrations of ascorbic acid or MESL (50–500 μg/mL). Subsequently, 0.9% NaCl or 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH) 50 mM was added to evaluate the hemolytic capacity and oxidative hemolysis inhibition, respectively. Samples remained at 37 °C for 240 min with periodic shaking. Ethanol was used as solvent control at final concentration of 0.8%. Total hemolysis was induced by incubating erythrocytes with distilled water. Three independent experiments were performed in duplicate. The hemolysis percentage was determined by measuring absorbance at 540 nm, by the equation described: % hemolysis = Abssample / Abstotal hemolysis × 100.
Effect of Lipid Peroxidation Inhibition in Erythrocytes
Assays were carried out with 20% erythrocyte suspensions. Erythrocytes were preincubated at 37 °C for 30 min in the presence of different concentrations of ascorbic acid or MESL (50–500 μg/mL). Then, 50 mM AAPH was added to the erythrocyte solution and incubated at 37 °C for 4 h with periodic shaking. After this period, samples were centrifuged at 700×g and aliquots of 500 μL of the supernatant were transferred to tubes with 1 mL of 10 nmol thiobarbituric acid (TBA). As a standard, 500 μL of 20-mM malondialdehyde (MDA) solution was added to 1 mL of TBA. Samples were incubated at 96 °C for 45 min. Then, 4 mL of n-butyl alcohol was added and centrifuged at 1600×g. The absorbance of supernatants was measured at 532 nm. Two independent experiments were performed in triplicate. The MDA concentration in samples was expressed in nanomoles per milliliter, obtained from the equation described: MDA = Abssample × (20 × 220.32 / Absstandard).
Oxidative Hemolysis and Lipid Peroxidation Doxorubicin-Induced in vitro and in vivo
Oxidative Hemolysis
Erythrocytes 10% were preincubated at 37 °C for 30 min with different concentrations of MESL (50–500 μg/mL). Later, doxorubicin was added (160 μg/mL), being maintained at 37 °C for 240 min with periodic shaking. After this period, the hemolysis percentage was determined by measuring the absorbance at 540 nm, by the equation described: % hemolysis = Abssample / Abstotal hemolysis × 100. Total hemolysis was induced by incubating erythrocytes with distilled water. Three independent experiments were performed in duplicate.
Lipid Peroxidation
Erythrocytes were preincubated at 37 °C for 30 min in the presence of different MESL concentrations of (50–500 μg/mL). Then, doxorubicin (160 μg/mL) was added to the erythrocyte solution and incubated at 37 °C for 4 h with periodic shaking. After this period, samples were centrifuged at 700×g and aliquots of 500 μL of the supernatant were transferred to tubes with 1 mL of 10 nmol TBA. As a standard, 500 μL of MDA solution (20 mM) was added to 1 mL of TBA. Samples were incubated at 96 °C for 45 min. Then, 4 mL of n-butyl alcohol was added and centrifuged at 1600×g. The supernatants of samples were removed and the absorbance was measured at 532 nm. Two independent experiments were performed in triplicate. The MDA concentration in samples was expressed in nanomoles per milliliter, obtained from the equation described: MDA = Abssample × (20 × 220.32 / Absstandard).
Cell Viability Assay by MTT
K562 cells 2 × 104 in RPMI medium supplemented with 10% inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin were placed in a 96-well plate, followed by addition of doxorubicin 0.5 μg/mL and different concentrations of MESL (50–500 μg/mL). Cells were incubated in a final volume of 200 μL for 48 h at 37 °C in CO2 atmosphere at 5% of humidity. After a 48-h incubation, the plates were centrifuged to pellet the cells, the supernatant was removed, and 100 μL of MTT (1 mg/mL) dissolved in RPMI medium was added followed by incubation for 4 h at 37 °C in a humid, 5% CO2 atmosphere. After this period, the plates were centrifuged again, the supernatant was removed, and the insoluble formazan crystals were dissolved in 100 μL of dimethyl sulfoxide. The absorbance was read in ELISA plate reader at 630 nm. The results were expressed as percentage inhibition relative to control cells (considered as 100%).
Doxorubicin-Induced Cardiac Oxidative Stress: Levels of Malondialdehyde
Experimental procedures followed the standards of the National Council for the Control of Animal Experimentation (CONCEA) and were approved by the Ethics Committee for Animal Use of the Federal University of Grande Dourados (protocol no. 37/2015 CEUA/UFGD). Female C57Bl/6 mice were maintained in 12-h light/dark cycle under controlled temperature (22 ± 2 °C) and given food and water ad libitum. The doxorubicin-induced cardiac stress was performed according to method described by Momin et al. [27], with modifications. The animals weighing approximately 30 g treated for 18 days, divided into three groups of five animals each: Group 1: control—mice treated with 0.9% NaCl intraperitoneal injection (i.p.), Group 2: DOX—mice treated with doxorubicin with total cumulative dose of 24 mg/kg i.p. in six divided dosages, and Group 3: DOX + MESL—mice pretreated with methanolic extract of Schinus terebinthifolius Raddi leaves 200 mg/kg oral gavage (p.o.) along with doxorubicin treatment. Groups 2 and 3 received doxorubicin at alternate days from the 7th day (the days selected for doxorubicin injection was on the 7th, 9th, 11th, 13th, 15th, and 17th. On the 18th day, parameters studied were general appearance and heart weight.
To verify the levels of MDA in mouse hearts with doxorubicin-induced cardiac oxidative stress, 1:4 (m/v) of cardiac tissue was homogenized with 1.15% KCl. Thereafter, the samples were centrifuged at 1600×g for 10 min and 0.5-mL aliquots of the supernatant were mixed with 1 mL of trichloroacetic acid (10% TCA) and 1 mL of 10 nM TBA and incubated at 96 °C for 45 min. After cooling, they were added with 3 mL of n-butyl alcohol and centrifuged at 1600×g for 10 min; the supernatants of samples were removed and the absorbance was measured at 532 nm. The MDA concentration in samples was expressed in nanomoles per milliliter, obtained from the equation described: MDA = Abssample × (60 × 220.32 / Absstandard). As a standard, 0.5 mL of MDA solution (60 mM) was added to 1 mL of TCA and 1 mL TBA.
Statistical Analysis
Data are expressed as mean ± standard error of the mean (SEM). For analysis and comparison of results, the one-way analysis of variance (ANOVA) test and Student-Newman-Keuls post-test were used. Data were considered significant when P < 0.05.
Results
Total Phenolic Compound, Flavonoid, Tannin, Ascorbic Acid, and Saponin Contents
MESL showed the presence of phenolic compounds 145.45 ± 4.5 mg GAE/g of extract, flavonoid 12.46 ± 1.6 QE/g of extract, tannin 129.36 ± 0.9 CE/g mg of extract, and ascorbic acid 7.78 ± 0.5 mg AAE/g of extract. The presence of saponins was not verified.
Antioxidant Activity
DPPH· Free Radical Scavenging Activity
Considering the presence of substances with antioxidant potential, in vitro assay was performed to evaluate the DPPH· free radical scavenging activity by MESL. The concentration that inhibited 50% of DPPH· free radicals (IC50) and the maximum activity shown by MESL was similar to that presented by ascorbic acid as shown in Table 1.
Activity of SOD, CAT, and GPx Antioxidant Enzymes
The activities of SOD, CAT, and GPx antioxidant enzymes were evaluated in human erythrocyte lysates. MESL increased the enzymatic activity of SOD at all concentrations evaluated by approximately 25 ± 2% (Fig. 1a), but did not influence the activity of CAT (Fig. 1b) and GPx enzymes (Fig. 1c), except for the GPx in most evaluated concentration (500 μg/mL).
Oxidative Hemolysis Inhibition Assay
MESL was also evaluated for its hemolytic property and capacity to protect erythrocytes against oxidative hemolysis. MESL showed no hemolytic activity at concentrations up to 250 μg/mL during the experimental period of 240 min (Fig. 2a), indicating that these are not toxic for this cell model at the evaluated concentrations. However, at the highest concentrations tested, 500 μg/mL, MESL induced hemolysis. Additionally, MESL exhibited anti-hemolytic activity at all concentrations evaluated during the entire experimental period of 240 min (Fig. 2b).
Lipid Peroxidation Inhibition
The capacity of MESL to inhibit lipid peroxidation in human erythrocytes induced by AAPH was confirmed with the dosage of MDA generated. MESL inhibited lipid peroxidation induced by AAPH as indicated by the decrease in MDA levels by 76, 92, 90, 90, 84, and 72% at concentrations of 50, 75, 100, 125, 250, and 500 μg/mL, respectively, compared to the AAPH group, already to the effect of ascorbic acid at the same concentrations, decreased by 36, 43, 78, 80, 89, and 90% (Fig. 3).
Reduction of Oxidative Hemolysis and Doxorubicin-Induced Lipid Peroxidation in a Model of Human Erythrocytes
MESL was able to reduce doxorubicin-induced oxidative hemolysis by 34.83 ± 5.23 and 52.53 ± 5.6% at concentrations of 250 and 500 μg/mL, respectively (Fig. 4a), and MDA levels by 17.89 ± 5.24, 33.09 ± 5.04, and 46.23 ± 1.51% at concentrations of 125, 250, and 500 μg/mL, respectively (Fig. 4b).
Cell Viability in the Presence of Doxorubicin and MESL
The viability of K562 leukemic cells was measured using MTT assay. Doxorubicin was able to reduce approximately 73% cell viability, the MESL also reduce the cell viability (Fig. 5a); however, there was no change on the cytotoxic activity of doxorubicin in K562 cells at all the concentrations tested (Fig. 5b).
Cell viability of K562 incubated with DOX (0.5 μg/mL) and different MESL concentrations (50–500 μg/mL): only MESL (a) and MESL incubated with DOX (b). The control group represents untreated cells. Values are expressed as mean ± SEM. *P < 0.05 compared with control. #P < 0.05 compared with the DOX group
Malondialdehyde Levels in Mouse Hearts
Oxidative stress is a major cause of doxorubicin-induced cardiotoxicity. The MESL was able to protect mice against the doxorubicin-induced cardiotoxicity, as observed by reduced MDA levels, restoring the values to those observed in the control (Fig. 6).
Discussion
During cancer treatment, doxorubicin increases the production of free radicals and reduces the expression of antioxidant enzymes in the heart, indicating that this drug induces oxidative stress resulting in cardiotoxicity, which becomes the major limitation for its clinical use [28, 29].
Currently, combinations of anticancer drugs with antioxidant agents are being investigated to improve clinical response and reduce the toxic effects caused by anticancer drugs. It has been demonstrated that the administration of antioxidant agents like carotenoids and flavonoids may prevent damage caused by the use of doxorubicin [8, 30].
Our results show that the phytochemical constituents of MESL are phenolic compounds, and among these, tannins, and flavonoids, besides ascorbic acid, which are known as antioxidants, because of their ability of complexing metal ions and macromolecules, inactivating free radicals, and preventing the conversion of hydrogen peroxide [31,32,33,34]. The inhibition of free radicals can prevent hydrogen peroxide conversion into reactive oxyradicals resulting in cellular protection.
Phenolic compounds and some terpenoids such as carotenoids also inactivate radical reactions preventing hydroperoxide conversion into reactive oxyradicals [32]. In addition, the body produces endogenous enzymatic antioxidants including SOD, CAT, and GPx enzymes, which act in preventing as well as controlling the formation of reactive oxygen species [35]. Ascorbic acid may be associated with increased activity of SOD enzyme, which is capable of transforming superoxide anion radical (O2 ·−) into H2O2 [36]. Antioxidants maintain the balance between the formation and elimination of oxidative compounds, inhibiting the formation of lipid hydroperoxides and aldehydes, such as MDA, which has been associated with the development of various diseases and complications [37, 38].
In our study, the activity of CAT enzyme showed no significant changes, which may indicate that the capacity of reducing oxidative stress observed in erythrocytes in the presence of the oxidative agent AAPH and anticancer agent doxorubicin may be linked to the complexing of phenolic compounds present in MESL with iron metal, inhibiting Fenton reactions, resulting in the generation of hydroxyl radical (·OH) for which there is no specific defense enzymatic system [39]. Additionally, the aromatic ring with one or more hydroxyl groups of phenolic compounds can donate electron pairs to stabilize free radicals such as ·OH radicals, inhibiting lipid peroxidation [40].
There is cell damage during the oxidative stress process, some resulting from the formation of lipid hydroperoxides and aldehydes, such as MDA, substance known to be associated with the development of several diseases [41]. The reduction in the generation of MDA in erythrocytes incubated with AAPH or doxorubicin in the presence of MESL confirmed its antioxidant activity and ability to reduce oxidative stress in this cell model.
Antioxidants may help in the treatment of patients making use of anticancer drugs, reducing the impact of oxidative stress caused by drugs [42, 43], provided that they do not change their pharmacological properties. Although MESL presents an important cytotoxic effect, when combined administration with doxorubicin to leukemic cells, it did not change the cytotoxic activity of the drug. Extracts from plants that exhibit antioxidant activities and cytotoxic effects are well described in the literature [44, 45].
Although doxorubicin is an effective anticancer agent, its therapeutic use is limited by cardiotoxicity [29]. The treatment of mice with MESL protected against the doxorubicin-induced cardiotoxicity in mice, corroborating with results of reduction of oxidative hemolysis and doxorubicin-induced lipid peroxidation in human erythrocytes observed in this study. Data opened new study perspectives for the potential use of MESL and their compounds in the prevention and treatment of diseases related to oxidative stress or as a protective adjuvant against oxidative stress caused by anticancer drugs.
Other plant extracts have been evaluated against the toxicity induced by doxorubicin such as Vaccinium macrocarpon, Ficus racemosa, Olea europaea, and Uncaria tomentosa, the latter being used in clinical trials as an adjuvant treatment for breast cancer, with cardioprotective activity related to its high content of antioxidants [46,47,48,49].
Taken together, our results suggest that the methanolic extract of S. terebinthifolius leaves possesses beneficial properties reducing doxorubicin-induced oxidative stress in human erythrocytes without changing the cytotoxic action of the anticancer agent on K562 human erythroleukemia cells and protecting against doxorubicin-induced cardiotoxicity. Hence, these findings suggest that these effects are via anti-oxidative effects by inhibiting free radicals, decreased oxidative stress (MDA), and increased antioxidant enzyme activity (SOD and GPx).
References
Minotti, G., Menna, P., Salvatorelli, E., Cairo, G., & Gianni, L. (2004). Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews, 56, 185–229. https://doi.org/10.1124/pr.56.2.6.
Zhang, S., Liu, X., Bawa-Khalfe, T., Lu, L. S., Lyu, Y. L., Liu, L. F., & Yeh, E. T. (2012). Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nature Medicine, 18, 1639–1642. https://doi.org/10.1038/nm.2919.
Li, T., & Singal, P. K. (2000). Adriamycin-induced early changes in myocardial antioxidant enzymes and their modulation by probucol. Circulation, 102, 2105–2110. https://doi.org/10.1161/01.CIR.102.17.2105.
Octavia, Y., Tocchetti, C. G., Gabrielson, K. L., Janssens, S., Crijns, H. J., & Moens, A. L. (2012). Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. Journal of Molecular and Cellular Cardiology, 52, 1213–1225. https://doi.org/10.1016/j.yjmcc.2012.03.006.
Siveski-Iliskovic, N., Hill, M., Chow, D. A., & Singal, P. K. (1995). Probucol protects against adriamycin cardiomyopathy without interfering with its antitumor effect. Circulation, 91, 10–15. https://doi.org/10.1161/01.CIR.91.1.10.
Imbaby, S., Ewais, M., Essawy, S., & Farag, N. (2014). Cardioprotective effects of curcumin and nebivolol against doxorubicin-induced cardiac toxicity in rats. Human & Experimental Toxicology, 33, 800–813. https://doi.org/10.1177/0960327114527628.
Ghosh, J., Das, J., Manna, P., & Sil, P. C. (2011). The protective role of arjunolic acid against doxorubicin induced intracellular ROS dependent JNK-p38 and p53-mediated cardiac apoptosis. Biomaterials, 32, 4857–4866. https://doi.org/10.1016/j.biomaterials.2011.03.048.
Indu, R., Azhar, T. S., Nair, A., & Nair, C. K. (2014). Amelioration of doxorubicin induced cardio-and hepato-toxicity by carotenoids. Journal of Cancer Research and Therapeutics, 10, 62–67. https://doi.org/10.4103/0973-1482.131370.
Xiao, J., Sun, G. B., Sun, B., Wu, Y., He, L., Wang, X., Chen, R. C., Cao, L., Ren, X. Y., & Sun, X. B. (2012). Kaempferol protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. Toxicology, 292, 53–62. https://doi.org/10.1016/j.tox.2011.11.018.
Mantawy, E. M., El-Bakly, W. M., Esmat, A., Badr, A. M., & El-Demerdash, E. (2014). Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. European Journal of Pharmacology, 728, 107–118. https://doi.org/10.1016/j.ejphar.2014.01.065.
Chen, R. C., Xu, X. D., Zhi Liu, X., Sun, G. B., Zhu, Y. D., Dong, X., Wang, J., Zhang, H. J., Zhang, Q., & Sun, X. B. (2015). Total flavonoids from Clinopodium chinense (Benth.) O. Ktze protect against doxorubicin-induced cardiotoxicity in vitro and in vivo. Evidence-based Complementary and Alternative Medicine, 1, 1–17. https://doi.org/10.1155/2015/472565.
Roopan, S. M. (2016). An overview of phytoconstituents, biotechnological applications, and nutritive aspects of coconut (Cocos nucifera). Applied Biochemistry and Biotechnology, 179, 1309–1324. https://doi.org/10.1007/s12010-016-2067-y.
Bauer, Lopes Galeno, D. M., Carvalho, R. P., Boleti, A. P., Lima, A. S., Oliveira de Almeida, P. D., Pacheco, C. C., Pereira de Souza, T., & Lima, E. S. (2014). Extract from Eugenia punicifolia is an antioxidant and inhibits enzymes related to metabolic syndrome. Applied Biochemistry and Biotechnology, 172, 311–324. https://doi.org/10.1007/s12010-013-0520-8.
Morton, J. F. (1978). Brazilian pepper: its impact on people, animals and the environment. Economic Botany, 32, 353–359.
Brandão, M. G. L., Consenza, G. P., Moreira, R. A., & Monte-Mor, R. L. M. (2006). Medicinal plants and other botanical products from the Brazilian official pharmacopeia. Revista Brasileira de Farmacognosia, 16, 408–420. https://doi.org/10.1590/S0102-695X2006000300020.
Jain, M. K., Yu, B. Z., Rogers, J. M., Smith, A. E., Boger, E. T. A., Ostrander, R. L., & Rheingold, A. L. (1995). Specific competitive inhibitor of secreted phospholipase A2 from berries of Schinus terebinthifolius. Phytochemistry, 39, 537–547. https://doi.org/10.1016/0031-9422(94)00960-2.
Alves, L. A., Freires, I. A., Pereira, T. M., Souza, A., Lima, E. O., & Castro, R. D. (2013). Effect of Schinus terebinthifolius on Candida albicans growth kinetics, cell wall formation and micromorphology. Acta Odontologica Scandinavica, 71, 965–971. https://doi.org/10.3109/00016357.2012.741694.
Bendaoud, H., Romdhane, M., Souchard, J. P., Cazaux, S., & Bouajila, J. (2010). Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. Journal of Food Science, 75, C466–C472. https://doi.org/10.1111/j.1750-3841.2010.01711.x.
Matsuo, A. L., Figueiredo, C. R., Arruda, D. C., Pereira, F. V., Scutti, J. A. B., Massaoka, M. H., Travassos, L. R., Sartorelli, P., & Lago, J. H. G. (2011). α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochemical and Biophysical Research Communications, 411, 449–454. https://doi.org/10.1016/j.bbrc.2011.06.176.
Meda, A., Lamien, C. E., Romito, M., Millogo, J., & Nacoulma, O. G. (2005). Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chemistry, 91, 571–577. https://doi.org/10.1016/j.foodchem.2004.10.006.
Liberio, S. A., Pereira, A. L. A., Dutra, R. P., Reis, A. S., Araújo, M. J. A. M., Mattar, N. S., Silva, L. A., Ribeiro, M. N., Nascimento, F. R., Guerra, R. N., & Monteiro-Neto, V. (2011). Antimicrobial activity against oral pathogens and immunomodulatory effects and toxicity of geopropolis produced by the stingless bee Melipona fasciculate Smith. BMC Complementary and Alternative Medicine, 11, 1–10. https://doi.org/10.1186/1472-6882-11-108.
Benassi, M. T., & Antunes, A. J. (1988). A comparison of meta-phosphoric and oxalic acids as extractants solutions for the determination of vitamin C in selected vegetables. Arquivos de Biologia e Tecnologia, 31, 507–513.
Tirloni, C. A. S., Macorini, L. F. B., Santos, U. P., Rocha, P. S., Barros, S. V., Mello, A. M. M. F., Vieira, M. C., de Picoli, S., & Santos, E. L. (2015). Evaluation of the antioxidant activity, antimicrobial effect and acute toxicity from leaves of Allophylus edulis (A. St.-Hil., A. Juss. Cambess &.) Hieron. ex Niederl. African Journal of Pharmacy and Pharmacology, 9, 353–362. https://doi.org/10.5897/AJPP2015.4270.
Campos, J. F., Santos, U. P., Macorini, L. F. B., Melo, A. M. M. F., Balestieri, J. B. P., Gamero, E. J. P., Cardoso, C. A. L., Souza, K. P., & Santos, E. L. (2014). Antimicrobial, antioxidant and cytotoxic activities of propolis from Melipona orbignyi (Hymenoptera, Apidae). Food and Chemical Toxicology, 65, 374–380. https://doi.org/10.1016/j.fct.2014.01.008.
Xu, P., Costa-Goncalves, A. C., Todiras, M., Rabelo, L. A., Sampaio, W. O., Moura, M. M., Santos, S. S., Luft, F. C., Bader, M., Gross, V., Alenina, N., & Santos, R. A. (2008). Endothelial dysfunction and elevated blood pressure in mas gene-deleted mice. Hypertension, 51, 574–580. https://doi.org/10.1161/HYPERTENSIONAHA.107.102764.
Paglia, D. E., & Valentine, W. N. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of Laboratory and Clinical Medicine, 70, 158–169.
Momin, F. N., Kalai, B. R., Shikalgar, T. S., & Naikwade, N. S. (2012). Cardioprotective effect of methanolic extract of Ixora coccinea Linn. leaves on doxorubicin-induced cardiac toxicity in rats. Indian J. Pharmacol., 44, 178–183. https://doi.org/10.4103/0253-7613.93844.
Volkova, M., & Russell, R. (2011). Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Current Cardiology Reviews, 7, 214–220. https://doi.org/10.2174/157340311799960645.
Thandavarayan, R. A., Giridharan, V. V., Arumugam, S., Suzuki, K., Ko, K. M., Krishnamurthy, P., Watanabe, K., & Konishi, T. (2015). Schisandrin B prevents doxorubicin induced cardiac dysfunction by modulation of DNA damage, oxidative stress and inflammation through inhibition of MAPK/p53 signaling. PloS One, 10, 1–18. https://doi.org/10.1371/journal.pone.0119214.
Dong, Q., Chen, L., Lu, Q., Sharma, S., Li, L., Morimoto, S., & Wang, G. (2014). Quercetin attenuates doxorubicin cardiotoxicity by modulating Bmi-1 expression. British Journal of Pharmacology, 171, 4440–4454. https://doi.org/10.1111/bph.12795.
Yen, G. C., Duh, P. D., & Tsai, H. L. (2002). Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chemistry, 79, 307–313. https://doi.org/10.1016/S0308-8146(02)00145-0.
Sundaram, S., Anjum, S., Dwivedi, P., & Rai, G. K. (2011). Antioxidant activity and protective effect of banana peel against oxidative hemolysis of human erythrocyte at different stages of ripening. Applied Biochemistry and Biotechnology, 164, 1192–1206. https://doi.org/10.1007/s12010-011-9205-3.
Okuda, T., & Ito, H. (2011). Tannins of constant structure in medicinal and food plants—hydrolyzable tannins and polyphenols related to tannins. Molecules, 16, 2191–2217. https://doi.org/10.3390/molecules16032191.
Kumar, S., & Pandey, A. K. (2013). Chemistry and biological activities of flavonoids: an overview. The Scientific World Journal., 29, 1–16. https://doi.org/10.1155/2013/162750.
Nickel, A., Kohlhaas, M., & Maack, C. (2014). Mitochondrial reactive oxygen species production and elimination. Journal of Molecular and Cellular Cardiology, 73, 26–33. https://doi.org/10.1016/j.yjmcc.2014.03.011.
Chen, X., Touyz, R. M., Park, J. B., & Schiffrin, E. L. (2001). Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension, 38, 606–611. https://doi.org/10.1161/hy09t1.094005.
Del Rio, D., Stewart, A. J., & Nicoletta Pellegrini, N. (2005). A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutrition, Metabolism, and Cardiovascular Diseases, 15, 316–328. https://doi.org/10.1016/j.numecd.2005.05.003.
Bonomini, F., Rodella, L. F., & Rezzani, R. (2015). Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis., 6, 109–120. 10.14336/AD.2014.0305.
Winterbourn, C. C. (1995). Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicology Letters, 82, 969–974. https://doi.org/10.1016/0378-4274(95)03532-X.
Amorati, R., & Valgimigli, L. (2012). Modulation of the antioxidant activity of phenols by non-covalent interactions. Organic & Biomolecular Chemistry, 10, 4147–4158. https://doi.org/10.1039/c2ob25174d.
Lykkesfeldt, J. (2007). Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clinica Chimica Acta, 380, 50–58. https://doi.org/10.1016/j.cca.2007.01.028.
Waseem, M., & Parvez, S. (2013). Mitochondrial dysfunction mediated cisplatin induced toxicity: modulatory role of curcumin. Food and Chemical Toxicology, 53, 334–342. https://doi.org/10.1016/j.fct.2012.11.055.
Bagchi, D., Swaroop, A., Preuss, H. G., & Bagchi, M. (2014). Free radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: an overview. Mutation Research, 768, 69–73. https://doi.org/10.1016/j.mrfmmm.2014.04.004.
Santos, U. P., Campos, J. F., Torquato, H. F., Paredes-Gamero, E. J., Carollo, C. A., Estevinho, L. M., de Picoli Souza, K., & Dos Santos, E. L. (2016). Antioxidant, antimicrobial and cytotoxic properties as well as the phenolic content of the extract from Hancornia speciosa Gomes. PloS One, 11, 1–19. https://doi.org/10.1371/journal.pone.0167531.
Campos, J. F., de Castro, D. T., Damião, M. J., Torquato, H. F. V., Paredes-Gamero, E. J., Carollo, C. A., Estevinho, L. M., de Picoli Souza, K., & Dos Santos, E. L. (2016). The chemical profile of Senna velutina leaves and their antioxidant and cytotoxic effects. Oxidative Medicine and Cellular Longevity, 2016, 1–12. https://doi.org/10.1155/2016/8405957.
Elberry, A. A., Abdel-Naim, A. B., Abdel-Sattar, E. A., Nagy, A. A., Mosli, H. A., Mohamadin, A. M., & Ashour, O. M. (2010). Cranberry (Vaccinium macrocarpon) protects against doxorubicin-induced cardiotoxicity in rats. Food and Chemical Toxicology, 48, 1178–1184. https://doi.org/10.1016/j.fct.2010.02.008.
Ahmed, F., & Urooj, A. (2012). Cardioprotective activity of standardized extract of Ficus racemosa stem bark against doxorubicin-induced toxicity. Pharmaceutical Biology, 50, 468–473. https://doi.org/10.3109/13880209.2011.613848.
Kumral, A., Giriş, M., Soluk-Tekkeşin, M., Olgaç, V., Doğru-Abbasoğlu, S., Türkoğlu, Ü., & Uysal, M. (2015). Effect of olive leaf extract treatment on doxorubicin-induced cardiac, hepatic and renal toxicity in rats. Pathophysiology, 22, 117–123. https://doi.org/10.1016/j.pathophys.2015.04.002.
Araújo, M. C. S., Farias, I. L., Gutierres, J., Dalmora, S. L., Flores, N., Farias, J., Cruz, I., Chiesa, J., Morsch, V. M., & Schetinger, M. R. C. (2012). Uncaria tomentosa-adjuvant treatment for breast cancer: clinical trial. Evidence-based Complementary and Alternative Medicine, 1, 1–8. https://doi.org/10.1155/2012/676984.
Acknowledgements
This work was supported by grants from Foundation to Support to Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Contributions
P.S.R., J.F.C., K.P.S., and E.L.S. conceived and designed the experiments; P.S.R., J.F.C., V.N.S., A.P.A.B., L.A.R., and K.P.S. performed the experiments; P.S.R., J.F.C., V.N.S., M.C.A., A.P.A.B., L.A.R., K.P.S., and E.L.S. analyzed the data; V.N.S., M.C.A., L.A.R., K.P.S., and E.L.S. contributed reagents/materials/analysis tools; P.S.R., J.F.C., L.A.R., K.P.S., and E.L.S. wrote the paper. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rocha, P.d.S.d., Campos, J.F., Nunes-Souza, V. et al. Antioxidant and Protective Effects of Schinus terebinthifolius Raddi Against Doxorubicin-Induced Toxicity. Appl Biochem Biotechnol 184, 869–884 (2018). https://doi.org/10.1007/s12010-017-2589-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2589-y