Asteriscus graveolens (Asteraceae) is a medicinal herb, used in Algeria to treat diabetes, hypertension, pain, fever, inflammation and gastrointestinal diseases. Doxorubicin (DOX) is an antibiotic antineoplastic drug used to treat many types of cancers; unfortunately, its antitumor activity links toxic effects to several organs including the heart, liver and testis. The appropriate mechanism of its organotoxicity is linked to free reactive oxygen species (ROS) generation and oxidative stress induction. In this study, the antioxidant and protective role of A. graveolens and vitamin E (Vit E) against DOX-induced hepatic and testicular toxicity was assessed. Thirty-five rats were distributed equally into five groups and orally administered with n-butanol extract of A. graveolens (75 mg/kg bw) or Vit E (100 mg/kg bw) for 10 days in the absence or presence of a single intraperitoneal injection of DOX (15 mg/kg bw). The results revealed that DOX toxicity induced a significant elevation in the liver serum marker enzymes and lipid profile levels (cholesterol, triglycerides and LDL). In addition, DOX-induced hepatic and testicular oxidative injury was indicated due to a significant increase of malondialdehyde levels along with a noticeable reduction of the antioxidant system. A. graveolens and Vit E treatment might improve the biochemical and histopathological changes induced by DOX. A. graveolens has antioxidant and hypolipidemic properties and it can reduce DOX-induced oxidative damage in the liver and testis. A. graveolens showed a similar protective effect of Vit E against DOX damage due to the presence of an abundant amount of phenolics such as flavonoids. This protection is mediated by their direct free-radical scavenging activity and their ability to prevent DOX depletion of the hepato-testicular antioxidant defense systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. INTRODUCTION
Doxorubicin (DOX) remains one of the clinically effective anticancer drugs. Despite its therapeutic value, DOX has significant toxic effects on various organs such as the heart [1], liver, lung, kidney, blood cells and testes [2]. Oxidative stress, inflammation and apoptosis are the most frequently proposed mechanisms by which DOX exerts its anticancerous or cytotoxic effects in healthy tissues [3]. The main toxic effect of DOX occurs via the overproduction of reactive oxygen species (ROS) via different mechanisms. One of them is a decline in cellular antioxidant defense expression. Another mechanism may involve triggering a Fenton-type reaction by cheating intracellular iron producing highly reactive and toxic hydroxyl radicals (•OH). The third mechanism involves redox cycles leading to the persistent production of the superoxide anion and secondary ROS (e.g. H2O2, •OH, and peroxynitrite) [4]. Many strategies have recently been investigated to minimize the side effects of DOX, including combined therapy with natural bioactive compounds such as vitamins (E, C, A, carotenoids), flavonoids, polyphenols and herbal antioxidants [2].
Asteriscus graveolens (Forsk) Less. (Syn. Bubonium graveolens, Odontospermum graveolens or Nauplius graveolens) is an endemic medicinal plant of the Asteraceae family, mainly distributed in southwestern Algeria and southeastern Morocco [5]. In traditional medicine, the species is used to treat diabetes, inflammation, stomach and intestinal diseases, headache, diuretic, hypotensive and depurative. A. graveolens shows interesting biological properties such as antioxidant, cytotoxic against different human cancer cell lines [6], anticancer [7], antibacterial, antileishmanial [8], antifungal [9] and protection of the skin tissues from damage caused by toxins in environmental pollution [10]. Phytochemical analyses of A. graveolens have revealed the presence of phenols, flavonoids, flavonols, tannins [8], humulene derivatives [10], monoterpenes and sesquiterpenes [11]. A new acyclic sesquiterpene [7,12-dihydroxy-6,7-dihydro-5,(6)E-dehydronerolidol] and sesquiterpene germacranolide lactone derivatives [9α-hydroxy-11α,13-dihydroparthenolide-9-O-α-D-glucopyranoside and 9β-hydroxy-11α,13-dihydroparthenolide-9-O-α-D-glucopyranoside] were isolated from polar extracts of the aerial parts of A. graveolens. Ethyl acetate and n-butanol extracts and compounds of A. graveolens showed a significant concentration-dependent inhibitory effect on the growth of Human Colon Carcinoma (HCT116) and Human Colorectal Adenocarcinoma (DLD1) [11].
Despite the high potential of A. graveolens to date, no study has been conducted to evaluate the in vivo protective effect on DOX toxicity. This research aimed to evaluate the protective effects of A. graveolens butanolic extract and vitamin E against DOX-induced hepato-testicular toxicity.
2. MATERIALS AND METHODS
2.1. Chemicals
Vitamin C, doxorubicin, glutathione (GSH), thiobarbituric acid (TBA), 5,5-dithio-bis-2-nitrobenzoic acid (DTNB), trichloroacetic acid, and bovine serum albumin (BSA) were all purchased from Sigma (St. Louis, Missouri, USA). Vitamin E was purchased from pharmacies in the form of chewable tablets under the trade name API E ALPHA TOCOPHEROL (Api laboratory, Constantine, Algeria), each containing 100 mg of vitamin E. All other chemicals were of analytical grade.
2.2. Plant materials
Asteriscus graveolens (Forsk.) DC. was collected from the Daya of Bechar in southwest Algeria (31°37′0″ N, 2°13′02 W) and identified by M. Benabdelhakem Director of the Nature Preservation Agency, Bechar. Algeria. A voucher specimen AGA0510-BEC-ALG-52 was deposited in the Herbarium of the VARENBIOMOL research unit, Constantine 1 University.
2.3. Extraction procedure
Air-dried leaves and flowers (1580 g) of A. graveolens were macerated at room temperature with ethanol (EtOH)-H2O (70:30, v/v) for 24 h, three times. After filtration, the filtrate was concentrated under reduced pressure and dissolved in H2O (632 mL) by magnetic stirring, then maintained at 4°C overnight for maximum precipitation of chlorophyll. After filtration, the resulting solution was extracted with chloroform (CHCl3), ethyl acetate (EtOAc) and n-butanol (n-BuOH), respectively. The organic phases were dried with sodium sulfate (Na2SO4), filtered and concentrated in a vacuum to obtain the following extracts: CHCl3 (7.67 g), EtOAc (6.14 g) and n-BuOH (15.18 g). In this study, we used the n-BuOH extract which represents the highest yield (0.96 %) compared to CHCl3 (0.48%) and EtOAc (0.38%) extracts.
2.4. Analysis of total phenol and flavonoid content
The total phenolic content of the extract was determined by the modified Folin Ciocalteu method [12]. The results are expressed as μg gallic acid equivalents (GAE)/mg dry extract. Total flavonoid content was estimated using the colorimetric aluminum chloride assay described by Amrani, et al. (2017) [13]. Total flavonoid content was calculated as μg quercetin equivalents (QE)/mg dry extract.
2.5. 2,2-Diphenyl-1-picrylhydrazyl assay
The in vitro antioxidant activity of the extract was determined against 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical, as described by Amrani, et al. (2019) [14]. Different volumes of n-butanol extract (2.5 to 300 μl) or vitamin C (standard antioxidant) were added to 3 ml of 0.004% DPPH methanolic solution. The absorbance was measured at 517 nm after 30 min incubation in the dark. The antioxidant activity is expressed as the percentage of free radical inhibition of the extract and vitamin C and calculated using the following formula:
The IC50 values (the concentration of antioxidants, which eliminate 50% of DPPH radicals) were defined for samples.
2.6. Lipid peroxidation inhibition assay
Lipid peroxidation in egg yolk was determined via malondialdehyde assay (MDA) according to the method described by Amrani, et al. (2019) [14]. Fresh egg yolks were homogenized in ice-cold PBS (20 mM, pH 7.4) and centrifuged at 4000 rpm for 20 minutes. A 1 ml aliquot of the supernatant was incubated with the extract or vitamin C in the presence of FeSO4 (0.07 M) for 1 hour at 37°C. After cooling, 1 mL of 20% trichloroacetic acid and 1.5 mL of 1% thiobarbituric acid were added, and the mixture was then heated at 100° C for 15 minutes. The absorbance was measured at 532 nm after centrifugation at 4000 rpm for 20 minutes. Lipid peroxidation (LPO) inhibitory activity (%) was calculated using the following formula:
2.7. Drug and plant dose preparation
A single dose of 15 mg/kg body weight of DOX was injected into rats during the experimental period. The choice for DOX administration was chosen from the previous study [15].
To evaluate the preventive effects of A. graveolens against hepatic testicular toxicity induced by DOX, a dose of 75 mg/kg Bw of A. graveolens extract equivalent to 1/20 LD50 of extract [16] was selected for the present investigation. Vitamin E (100 mg/kg) was used as a positive control based on our earlier research that showed the hepatoprotective effect [17].
2.8. Experimental Animals
Healthy Male Wistar albino rats weighing 180–220 g were purchased from the Pasteur Institute (Algiers, Algeria). The animals were kept in carefully controlled conditions (25±3°C and 12 h light/dark cycles) with free access to standard rodent food and water ad libitum.
The in vivo experimental protocol was approved by the institutional project committee (PRFU, D01N01UN250120180009). The experiments were conducted per international guidelines for the care and use of laboratory animals as published by the US National Institute of Health (NIH), publication No. 85 – 23, revised in 1985.
Rats were housed seven per cage and randomly divided into five groups. Group 1: Control rats or untreated rats. The animals received water for 10 days, with a single dose of saline intraperitoneally (i.p.) on the 8th day. Group 2: Ext, rats received n-butanol extract (75 mg/kg, by gavage) for 10 consecutive days. Group 3: DOX, rats received a single DOX injection (15 mg/kg, i.p.). Group 4: Ext + DOX, the rat received n-butanol extract (75 mg/kg, by gavage) for 10 consecutive days and a single DOX injection on day 8. Group 5: Vitamin E + DOX, rats received vitamin E (100 mg/kg by gavage) for 10 consecutive days and a single injection of DOX on the 8th day.
Rats were sacrificed at the end of the experiment 24 hours after the last dose of extract or vitamin E and 72 hours after doxorubicin treatment. Blood samples were collected intravenously and serum was separated by centrifugation (3000 g for 10 min) and stored in a –-20°C refrigerator for subsequent analysis. Liver and testicular tissues were carefully removed, washed with ice-cold saline to remove any blood, spotted on filter paper, weighed and homogenized with ice-cold KCl 1.15%. The homogenates (20%) were centrifuged and the supernatants were refrigerated at –80°C until the assay was carried out.
2.8.1. Serum and tissue biochemical assays
The serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), Cholesterol LDL, Cholesterol HDL, total cholesterol and triglycerides kits were provided by Spinreact (Spain). All were measured spectrophotometrically (UV-1280 UV-Vis Spectrophotometer, Europe) following the manufacturer’s recommended protocols. Hepatic and testicular malondialdehyde lipid peroxidation biomarker) was determined colorimetrically using the method of Uchiyama and Mihara (1978) [18]. Reduced glutathione (GSH) content in hepatic and testicular tissues was measured according to the method described by Elman (1959) [19] using Elman’s reagent. This method is based on the reactive cleavage of 5,5′-dithiobis-(2-nitrobenzoic acid) via a sulfhydryl group, resulting in a yellow color with an absorbance maximum at 412 nm.
Glutathione peroxidase (GPx) activity in hepatic and testicular tissues was assessed according to the method of Flohe and Gunzler (1984) [20]. The activity was expressed as μmol of GSH oxidized per minute per milligram of protein. Protein concentrations were estimated colorimetrically using the method of Lowry, et al. (1951) [21]. Bovine serum albumin was used as a standard.
2.8.2. Histopathology
Rat organs (livers and testes) were preserved in 10% buffered formaldehyde. Tissue samples were embedded in paraffin and 5-ìm sections were cut using a rotary microtome and stained with hematoxylin and eosin. Histopathological evaluation was made by an expert pathologist.
2.9. Statistical analysis
Results are expressed as mean ± SD. Assessment of these results was performed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test using GraphPad Prism version 5.01. Statistical significance was accepted at the level of p < 0.05.
3. RESULTS
3.1. DPPH radical scavenging activity
DPPH free radical scavenging activity of n-butanol extract of A. graveolens is shown in Fig 1. The results reveal that the percentage of inhibition of DPPH radicals was concentration dependent. The n-butanol extract showed maximum activity of 91.86 ± 0.50% at 50 μg/ml, whereas the positive standard, vitamin C, exhibited 97.51 ± 0.67 % inhibition. From Table 1 it is evident that the n-butanol extract of A. graveolens has significant free radical scavenging activity. Vitamin C has greater scavenging activity (IC50 = 5.4 ± 0.11μg/mL) than n-butanol extract (IC50 = 26.67 ± 0.18 μg/mL). Earlier studies with the plant A. graveolens indicate good antioxidant and radical scavenging properties [8, 22].
The antioxidant properties of vegetables and fruits are significantly correlated with flavonoids, a class of secondary plant phenols [23, 24]. The flavonoid content of the A. graveolens n-butanol extract, in terms of quercetin equivalents, was 134.58 ± 5.02 μg/mg QE, and total phenols was 154 ± 11.23 μg/mg GAE. This finding is consistent with results obtained from the free radical scavenging activity. The total content of the phenols and the flavonoidsof the previously investigated A. graveolens were comparable to the results presented here [8].
3.2. Lipid peroxidation inhibition
Figure 2 clearly shows that A. graveolens butanolic extract and vitamin C (standard antioxidant) inhibited LPO in a dose-dependent manner. The vitamin C produced greater inhibition (IC50 = 20.64 ± 2.33 μg/ml) as compared to the extract (IC50 = 467.5 ± 8.92 μg/ml). n-Butanol extract of A. graveolens demonstrated moderate anti-lipid peroxidative effects.
3.3. Effects of A. graveolens on biochemical parameters
The levels of liver enzymes (AST and ALT) in serum are shown in Fig. 3. DOX-treated rats had significantly increased (p < 0.01 and p < 0.001, respectively) serum levels of these enzymes (134.61 ± 11.02 and 195.21 ± 31.15 U/L for AST and ALT) compared to the control group (103.10 ± 14.41 and 45.99 ± 7.2932 U/L for AST and ALT, respectively). It was interesting to note that the Vit E pretreated rats showed an important decrease (p < 0.05 and p < 0.01, respectively) in the level of these enzymes (105.05 ± 8.13 and 112.00 ± 15.01 U/L for AST and ALT, respectively) when compared to rats treated with DOX. n-Butanol extract of A. graveolens exhibited the same protective effect of Vit E against DOX hepatotoxicity (102.05 ± 11.62 and 127.39 ± 19.04 U/L for AST and ALT, respectively).
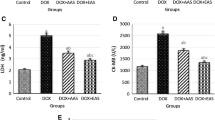
There was no significant difference in the level of CH, TG, LDL and HDL in the A. graveolens treated group compared to the control group. There was a significant increase (p < 0.001) in the level of CH, TG and LDL and no significant change in the level of HDL in the DOX-treated group when compared to the control group. There was a significant decrease in the level of CH (p < 0.05), TG (p < 0.05) and LDL (p < 0.001) in the Vit E+DOX-treated group when compared to the DOX-treated group (Fig. 4). A. graveolens exhibited the same protective effect of Vit E in DOX-induced-altered lipid parameters (LDL, CH and TG level). Fig. 5 demonstrates that treatment of rats with DOX induced significant increases (p < 0.001) in liver (7.15 ± 0.61 nmol MDA equivalents/g tissue) and testis (0.66 ± 0.055 nmol MDA equivalents/g tissue) lipid peroxidation levels compared to the control group (2.11 ± 0.29 nmol MDA equivalents/g liver and 0.24 ± 0.023 nmol MDA equivalents/g testis). However, the pretreated rats with n-butanol extract of A. graveolens reduced the lipid peroxidation level significantly (p < 0.001) to 3.54 ± 0.4 nmol MDA equivalents/g liver and 0.32 ± 0.02 nmol MDAequivalents per gram testis in hepatic and testicular tissues, respectively. Vit E exhibited the same protective effect as A. graveolens in DOX-induced lipid peroxidation in the testis and a better protective effect than A. graveolens in the liver (p < 0.01). No significant change was noticed in the MDA level, in the A. graveolens group, compared to the control. Fig. 6 presents the effects of DOX on the level of total glutathione (GSH). It is remarkable that DOX treatment induced a significant decline in the liver and testis GSH level (p < 0.001) (15.17 ± 0.66 μmol/g liver and 1.41 ± 0.07 μmol/g testis, respectively) compared to control rats (18.81 ± 0.26 μmol/g liver and 2.90 ± 0.12 μmol/g testis, respectively). However, the administration of n-butanol extract of A. graveolens and Vit E caused a significant increase in the GSH level (p < 0.001) as compared to DOX-administered rats where the GSH content both in the liver and testis returned to its normal value. No significant change was noticed at the GSH level in the A. graveolens group compared to that of the control. DOX administration recorded high significant depletion in GPx activity both in the liver (p < 0.01) and testis (p < 0.001) (13.17 ± 0.24 μmol GSH/mg protein and 4.4 ± 0.6 μmol GSH/mg protein, respectively) compared to control rats (18.61 ± 0.3 μmol GSH/mg protein and 8.8 ± 0.3 μmol GSH/mg protein, respectively) (Fig. 7). Groups treated with A. graveolens and Vit E returned the GPx activity in both the liver and testis to its normal value.
Protective role of A. graveolens (75 mg/kg) and Vit E (100 mg/kg) against DOX (15 mg/kg, i.p.) induced hepato-testicular toxicity effects on lipid peroxidation (MDA concentration) in rat livers and testes. Data are reported as means ± SD. a: group compared to the control group, b: group compared to the DOX group. c: Ext+DOX group compared to Vit E +DOX group. (*p < 0.05; **p < 0.01; ***p < 0.001).
Protective role of A. graveolens (75 mg/kg) and Vit E (100 mg/kg) against DOX (15 mg/kg, i.p.) induced hepato testicular toxicity. Effect on the GSH level in rat livers and testes. Data are reported as means ± SD. a: group compared to the control group, b: group compared to the DOX group. (*p < 0.05; **p < 0.001).
Protective role of A. graveolens (75 mg/kg) and Vit E (100 mg/kg) against DOX (15 mg/kg, i.p.) induced hepato-testicular toxicity. Effect on the glutathione peroxidase (GPx) activity in rat livers and testes. Data are reported as means ± SD. a: group compared to the control group, b: group compared to the DOX group. (*p < 0.05; **p < 0.01; ***p < 0.001).
3.4. Histopathologic examination of testis tissue
Hematoxylin and eosins were used to stain testis tissue slides for histopathological analysis, as shown in Fig. 8. The histological appearances of testicular tissues from the control and A. graveolens groups were normal. The histopathological alterations such as necrosis and distorted seminiferous epithelium with impaired spermatogenesis, interstitial necrosis and congestion were observed in the DOX group. A. graveolens or Vit E and DOX treatment showed normal testicular architecture.
Cross sections of testicular structure in rats treated with DOX and/or A. graveolens (75 mg/kg), DOX and Vit E (100 mg/kg) (H&E). (A, B) – Photomicrographs of the testicular section from control and A. graveolens treated groups show normal features of the seminiferous epithelium, lumen, Leydig cells and interstitial tissue. (C, D, E, F) – A photomicrograph of the testicular section from a DOX-treated rat shows distorted seminiferous epithelium with impaired spermatogenesis, necrosis, and venous congestion in the interstitial region (G, H) – A photomicrograph of a testicular section from a DOX + A. graveolens and DOX +Vit E-treated shows nearly normal seminiferous epithelium (G×400).
3.5. Histopathologic examination of liver tissue
Histopathological investigation of the livers from the DOX treated group showed dilated and congested portal tracts, inflammatory cell infiltrations and necrosis of hepatocytes (Fig. 9 C, D, E). Post-administration of A. graveolens or Vit E attenuated the hepatic injuries and uniform hepatocyte morphology close to the control group, as seen in Fig. 9 G, H. No abnormal morphological alterations were discovered in the control and A. graveolens-treated groups (Fig. 9 A, B).
4. DISCUSSION
Many strategies have recently been investigated to minimize the side effects of DOX. Previous investigations suggested that natural products could be included in combination with DOX to reduce toxic side effects and drug resistance without affecting the antitumor activity of DOX. Tayeh, et al. (2018) and Tayeh & Ofir (2018) reported that A. graveolens extracts are cytotoxic to cancer cells but not to noncancerous cells and can synergize with doxorubicin without reducing cytotoxicity to cancer cells [25, 26]. In this concept, this study is the first to explore the protective role of A. graveolens against DOX-induced alteration in the biochemistry and histopathology of hepatic and testis tissues. Our results highlighted the fact that the administration of DOX (15 mg/kg body weight) significantly induced oxidative injury and reduced the activities of antioxidant enzymes in the liver and testes. On the other hand, we have shown that A. graveolens and Vit E protect against oxidative injuries induced by DOX.
First, phytochemical screening revealed that A. graveolens is rich in bioactive products, including polyphenols and flavonoids, which have significant antioxidant activity. According to preliminary results, Using the DPPH radical scavenging capacity, it was established that A. graveolens possesses an immense scavenging activity. Indeed, the presence of these bioactive compounds in the form of polyphenols and flavonoids in the extract of A. graveolens may be responsible for the detected antioxidant potency. In addition, we assessed the effect of A. graviolens n-butanol extract on egg yolk homogenate in the presence of FeSO4, a ROS-generating system. Lipid peroxidation in the egg yolk by FeSO4 is a net result of iron-mediated hydroxyl radicals and can be achieved by scavenging hydroxyl radicals or chelating the iron ions involved in initiating the Fenton reaction [27]. Both n-butanol extracts from A. graveolens and standard vitamin C inhibited ferrous sulfate-induced lipid peroxidation in egg yolk homogenates in a concentration-dependent manner. There was a significant difference in the IC50 values of both the n-butanol extract of A. graveolens aerial part and vitamin C, which is about 467.5 ± 8.92 μg/mL and 20.64 ± 2.33 μg/mL, respectively (Table 1). The n-butanol extract of A. graveolens has less lipid peroxidation inhibitory activity than vitamin C (Table 1). The inhibition of lipid peroxidation exhibited by the extract may be attributed to the presence of polyphenols, flavonoids and tannins that have metal chelating and hydroxyl radical scavenging properties [8, 22].
Regarding the in vivo aspect, a DOX single intraperitoneal (i.p.) injection at a dose of 15 mg/kg b.w. was selected as the toxic dose to induce testicular toxicity [26] and hepatocellular damage [29].
Our results showed that administration of DOX induced significant diminutions in the level of GSH and the activity of GPx, but produced significant increases in the levels of MDA and biochemical levels of LDL, cholesterol, TG, AST, and ALT. These results are in line with recent studies carried out by Mecheri et al. (2018, 2019) [17, 30] who showed significant elevations in the serum levels of biomarkers for liver injury (such as AST, ALT), and markers of oxidative stress after DOX administration to female Wistar albinos rats. Our findings were also in accordance with the results of some other studies [31, 32], which confirmed that DOX has deleterious effects on the liver and testis tissues because it increases the peroxidation of lipid membrane and alters the antioxidant systems.
DOX is known to have several deleterious effects on different experimental organ systems [3], whereby it decreases the level of GSH and inhibits antioxidant enzymes [33]. In addition, DOX contributes to the accumulation of free radicals, NO, and superoxide, which react together to form the highly reactive product peroxynitrite (ONOO-), a chemical that can damage cell membranes [34].
Diverse experimental studies have been conducted combining DOX with natural antioxidants to protect healthy tissues from free radicals without reducing their anti-tumor capacity [2]. In this context, medicinal plants have been used for their richness in bioactive compounds. A. graveolens is one of the most well-known plants because of traditional knowledge of its medicinal properties and various therapeutic effects, according to the biological and pharmacological potential of A. graveolens [6, 8].
The extract from A. graveolens and Vit E significantly improved the DOX-induced alterations: all measured parameters (oxidative stress parameters, levels of LDL, CH, TG, AST and ALT in plasma and histology) were restored to near-normal values (control). Components of A. graveolens appear able to counteract the DOX-induced production of aggressive oxidants or to impair the mechanisms by which these oxidants damage key molecules within tissues.
The antioxidant properties of A. graveolens essential oil [35], sesquiterpene lactone and flavonoids, are all known to be effective radical scavengers [22]. The observed reduction in the levels of lipid peroxidation in A. graveolens-treated animals is in part due to its ability to scavenge the hydroxyl and peroxyl radicals [6, 36].
The present study revealed that DOX injection caused testicular damage associated with oxidative stress injury, as demonstrated by lipid and protein oxidation causing MDA production and depletion of sulfhydryl content. These disorders are associated with antioxidant deficiencies. Previous studies showed that the DOX-treated rats exhibit a higher oxidative stress effect linked to a significant decrease in sperm quality parameters [37, 38].
Testicles are designed to be more sensitive to oxidative stress as a result of the abundance of polyunsaturated fatty acids in this environment [39, 40]. In this context, DOX may disrupt the antioxidant system, leading to increased production of ROS [41, 42]. In this regard, it was found that A. graveolens treatment induced an increase in sperm concentration (Fig. 8), which can be ascribed to the antioxidant effects of A. graveolens, which is responsible for its protective effect against the toxicity of DOX. Thus, the testes are protected by various antioxidants which belong to the phytochemical component of the extract.
The biochemical data was supported by a histopathological report that showed severe hepatic testicular necrosis, with congestion and inflammation in DOX-injected rats. A. graveolens and Vit E shows that well-preserved hepatic testicular tissues were the indication of hepato-testicular protection.
We do not know currently which A. graveolens component is responsible for such protection, bearing in mind the complex composition of A. graveolens n-butanol extract. The HPLC analysis of n-butanol extract of A. graveolens revealed the presence of various phenolic compounds such as phenolic acid, trans-cinnamic acid, and a total of eleven flavonoids, namely myricetin and its O-glucoside derivative, luteolin and its glycosides (cynarosid, orientin and isoorientin); rutin (diglycoside of quercetin), hyperoside (3-O-galactoside of quercetin), and apigenin and its C-glycosylated derivatives (vitexin and iso-vitexin) [43]. Most of these compounds are known for their antioxidant and hepato-testicular protection properties and could have acted individually or synergistically to reduce the oxidative stress induced by DOX. In this respect, it was recently demonstrated that rutin and quercetin alone or in combination [44], apigenin [45] or luteolin [46] might have potential preventive effects against DOX-induced toxicity through inhibiting oxidative stress, inflammation, and apoptosis. Studies aimed at the identification of the bioactive components responsible for the protective effect are in progress.
CONCLUSION
This study, therefore suggests that A. graveolens and Vit E may be useful preventive agents against DOX-induced oxidative damage in the liver and testis, at least partly due to their antioxidant properties. Further study will be needed to ascertain the dose response for this plant. Future research should investigate the anti-tumor action of A. graveolens combined with DOX in animal models with malignant tumors to intensify the anti-tumor action of DOX, and at the same time to diminish its toxicity.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
Funding
The authors are thankful to DGRSDT and MESRS for financial support.
Animal and Human Consent
No humans were involved in the study. All the experiments conducted on animals were in accordance with the guidelines set by the Principles of Laboratory Animal Care by the National Institute of Health of the United States (NIH) publication number 85 – 23, revised 1985.
Acknowledgements
Declared none.
Author Contributions
Amira Mecheri: Performed experimental studies and wrote the original draft, Leila Hammoud contributed to the plant extraction procedure, Samia Belahcene and Nassima Boubekri contributed to the experimental studies, Mounir Kout performed the histological study, Fadila Benayache supervised the phytochemical study, Amel Amrani experimental design, writing (review and editing), statistical analysis of the data, validation and supervision.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
References
Y. L. Lyu and L. F. Liu, Recent Advances in Cancer Research and Therapy, Elsevier Inc (2012), pp. 351 – 369.
R. Injac and B. Strukelj, Technol. Cancer Res. Treat., 7(6), 497 – 516 (2008).
A. Pugazhendhi, T. N. J. I. Edison, B. K. Velmurugan, et al., Life Sci., 200, 26 – 30 (2018).
H. Zhu, S. Sarkar, L. Scott, et al., React. Oxyg Species, 1(3), 189 – 198 (2016).
G. Cristofari, M. Znini, L. Majidi, et al., Chem. Biodivers., 9, 727 – 738 (2012).
B. E. Ziani, R. C. Calhelha, J. C. Barreira, et al., Ind. Crop Prod., 77, 582–589 (2015).
Z. Tayeh, N. Dudai, A. Schechter, et al., Int. J. Mol. Sci., 19, 2162 (2018).
F. Ramdane, R. Essid, K. Mkadmini, et al., J. Process Biochem., 56, 186 – 192 (2017).
M. Znini, G. Cristofari, L. Majidi, et al., Nat. Prod. Commun., 6, 1763 – 1768 (2011).
L. Hammoud, F. Leon, I. Brouard, et al., Tetrahedron Lett., 59(27), 2668 – 2670 (2018).
H. Achoub, T. Mencherini, T. Esposito, et al., Nat. Prod. Res., 23, 1 – 9 (2019).
V. L. Singleton and J. A. Rossi, Am. J. Enol. Vitic., 16, 144 – 158 (1965).
A. Amrani, O. Benaissa, N. Boubekrie, et al., JMES, 8, 4002 – 4006 (2017).
A. Amrani, A. Lahneche, O. Benaissa, et al., Braz. Arch. Biol. Technol., 62, e19170779 (2019).
T. O. Omobowale, A. A. Oyagbemi, U. E. Ajufo, et al., J. Diet. Suppl., 15(2),183 – 196 (2018).
M. Gad, H. Z. Hassouna, K. Mahmoud, et al., Egypt. J. Chem., 64, 3489 – 3498 (2021).
A. Mecheri, W. Benabderrahmane, A. Amrani, et al., Recent Pat. Food Nutr. Agric., 10(1), 70 – 75 (2019).
M. Uchiyama and M. Mihara, Anal. Biochem., 86, 271 – 278 (1978).
G. L. Ellman, K. D. Courtney, V. Andres, et al., Biochem. Pharmacol., 7, 88 – 95 (1961).
L. Flohe and W. A Gunzler, Methods Enzymol., 105, 114 – 121 (1984).
O. H. Lowry, N. J. Rosebrough, A. L. Farr, et al., J. Biol. Chem., 193(1), 265 – 275 (1951).
F. Haddouchi, T. M. Chaouche, and N. Halla, Phytotherapie (2016); doi:10.1007 / s10298 – 016 – 1086 – 8
J. A. Badmus, O. T. Adedosu, E. G. Adeleke, et al., Int. Scholarly Res. Not., 2014, 391692 (2014).
A. Amrani, A. Mecheri, C. Bensouici, et al., Biocatal. Agric. Biotechnol., 2019, 20101209 (2019).
Z. Tayeh, N. Dudai, A. Schechter, et al., Int. J. Mol. Sci., 19(8), 2162 (2018).
Z. Tayeh and R. Ofir., Int. J. Mol. Sci., 19(8), 2219 (2018).
M. Gangwar, M. K. Gautam, A. K. Sharma, et al., Sci. World J., 2014, 279451 (2014).
B. Farsani, S. Karimi, and E. Mansouri, J. Basic Clin. Physiol. Pharmacol., 30(1), 103 – 109 (2018).
E. Mansouri, A. Jangaran, and A. Ashtari, Bratisl. Lek. Listy., 118(5), 273 – 277 (2017).
A. Mecheri, A. Amrani, W. Benabderrahmane, et al., Phytotherapie, 16(S1), S22 – S31 (2018).
S. Rashid, N. Ali, S. Nafees, et al., Toxicol. Mech. Methods, 23(5), 337 – 345 (2013).
R. Djebbari, Y. Chemam, N. Boubekri, et al., IJPPR, 9(7), 903 – 910 (2017).
S. Hamlaoui, Y. Hamdi, F. Tannich, et al., Pharm. Chem. J., 56(9), 1199 – 1208 (2022).
A. A. Fouad, M. M. M. Refaie, M. I. Abdelghany, Toxicol. Mech. Methods, 29(1), 67 – 73 (2019).
H. Aouissi, N. Gourine, H. Wang, et al., Orient. Pharm. Exp. Med., 18(3), 217 – 223 (2018).
F. El-Ouady and M. Eddouks, Nat. Prod. J., 10, 459 – 466 (2019).
Y. Xin, Z. Q. You, H. Y. Gao, et al., Phytother. Res., 26(5), 716 – 721 (2012).
S. Türedi, E. Yuluğ, A. Alver, et al., Exp. Toxicol. Pathol., 67(3), 229 – 235 (2015).
R. J. Aitken and S. D. Roman, Oxid. Med. Cell Longev., 1, 15 – 24 (2008).
P. Badkoobeh, K. Parivar, S. M. Kalantar, et al., Iran. J. Reprod. Med., 11(5), 355 – 364 (2013).
R. Mohamed, R. A. Karam, H. A. Hagrass, et al., Gene, 561(1), 107 – 114 (2015).
Z. A. Ahmed, A. N. Abtar, H. H. Othman, et al., Drug Des., Dev. Ther., 13, 3321 – 3329 (2019).
F. Belhadi, S. Ouafi, N. Bouguedoura, Trop. J. Pharm. Res., 19 (9), 1895 – 1901 (2020).
O. M. Ahmed, M. H. Elkomy, H. I. Fahim, et al., Oxid. Med. Cell., 2022 (2022).
Q. Wu, W. Li, J. Zhao, et al., Biomed. Pharmacother., 137, 111308 (2021).
S. E. Owumi, D. O. Lewu, U. O. Arunsi, et al., Hum. Exp. Toxicol., 40(10), 1656 – 1672 (2021).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mecheri, A., Hammoud, L., Belahcene, S. et al. In Vitro Antioxidant Activity of Asteriscus Graveolens (Forsk.) and Its Protective Effect on Doxorubicin-Induced Hepatotoxicity and Testicular Oxidative Damage in Rats. Pharm Chem J 57, 1956–1966 (2024). https://doi.org/10.1007/s11094-024-03102-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-024-03102-4