Abstract
In vitro propagation of a medicinally important plant, Althaea officinalis, has been achieved through somatic embryogenesis. Somatic embryos (globular to torpedo-shaped embryos) were induced on Murashige and Skoog’s (MS) medium augmented with various concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D, 5.0, 10.0, 15.0, 20.0, and 25.0) alone or combined with N6-benzylaminopurine (BA, 0.1, 0.5, 1.0, 1.5, and 2.0 μM). These were directly formed from the cut ends and subsequently spread on the whole surface of internodal explants. For embryo maturation, torpedo embryos were transferred on a medium containing different levels of BA (0.1, 0.5, or 1.0 μM) and abscisic acid (ABA) (0.5, 1.0, or 1.5 μM) or α-naphthalene acetic acid (NAA) (0.1, 0.5 or 1.0 μM). Among the different concentrations tested, 0.5 μM BA along with 1.0 μM ABA was found most effective, on which a highest yield (58.0%) with an optimum number (35.0) of mature embryos (cotyledonary stage) was observed after 2 weeks of transfer. Germination of cotyledonary embryos into plantlets with 68% were observed on ½ MS medium. Histological and scanning electron microscopical (SEM) studies proved that the regenerated structures were somatic embryos and not shoot primordia. Plants grew vigorously when transferred to a greenhouse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Althaea officinalis L. (Malvaceae), also known as “official healer,” is a potent source of drugs against a variety of ailments as asthma, cough, peptic ulcer, gastritis, kidney stone, and intestinal problems [1]. Roots and leaves are generally used to cure common cold and also consumed in case of lipemia or inflammation of nasal and oral cavities [2, 3]. A wide variety of biologically active constituents such as asparagine, althein, flavonoids (quercetin, kaempferol), flavonol glycosides, pectin, phenolic acid, and others has been found in different parts of the plant [4]. The flowers and young leaves are used as famine foods in France and are often added to salads or vegetables. Further, it also provides cosmetic products, helping to soften the skin. Despite having a huge beneficial value, only few reports are available on an efficient culture system for regeneration [5, 6].
Advanced biotechnology provides a set of new approaches to accelerate the development and deployment of genetically improved genotypes. Among the other advanced biotechnological approaches, somatic embryogenesis has become a potential tool which provides an efficient clonal propagation system.
Somatic embryogenesis is a process in which a bipolar structure, resembling a zygotic embryo, develops from a non-zygotic cell without vascular connection with the original tissue [7]. In addition, direct mode of somatic embryogenesis has a lesser probability of genetic variation in spite of other propagation methods [8]. Induction of somatic embryos directly from the splant tissue is the most desirable approach because it appears to be associated with the cytological and genetic stability of regenerated plantlets [9].
Plant regeneration through direct somatic embryogenesis has been accounted for in different plant species including Phalaenopsis amabilis [10], Panax japonicus [11], Cymbidium bicolor [12], Anethum graveolens [13], and Sapindus mukorossi [14]. However, current study deals with a method for the development of high-frequency direct somatic embryogenesis from internodal explants and its confirmation using histological and scanning electron microscopical (SEM) studies. It is a first report on somatic embryogenesis in A. officinalis L.
Materials and Methods
Plant Materials and Explant Preparation
Internodal (IN, 1.0 cm) explants excised from 6-month-old A. officinalis maintained at the Botanical Garden of the University at Aligarh were thoroughly washed in running tap water for 30 min to remove adherent particles, immersed in 1% (w/v) solution of Bavistin, a fungicide for 20 min, treated with 5% (v/v) Teepol solution for 15 min, and rinsed with sterile distilled water followed by surface sterilization in 0.1% (w/v) mercuric chloride (HgCl2) solution under sterile conditions for 3 min and finally rinsed five times with sterile distilled water.
Media and Culture Conditions
In the whole experiment, a Murashige and Skoog (MS) [15] medium supplemented with 3% (w/v) sucrose, 0.8% (w/v) agar, and plant growth regulators at different concentrations was used. pH 5.8 was adjusted with 1 N NaOH before autoclaving at121 °C for15 min. The medium was poured out into 25 × 150-mm test tubes (Borosil, India) and covered with a non-absorbent cotton plug. All the cultures were maintained at 24 ± 2 °C under a 16/8-h photoperiod with a photosynthetic photon flux density (PPFD) of 50 μmol m−2 s−1 provided by cool white fluorescent light (40 W, Philips, India) with 55–60% relative humidity.
Initiation of Somatic Embryo and Plantlet Regeneration
Internodal segments excised from a mature plant were inoculated on the MS medium augmented with various concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D, 5.0, 10.0, 15.0, 20.0, and 25.0 μM) for initiation of somatic embryogenesis. For further differentiation (globular to torpedo stage), an optimum concentration of 2,4-D, along with various concentrations of 6-benzyladenine (BA, 0.1, 0.5, 1.0, 1.5, and 2.0 μM), was used. For germination, torpedo embryos were transferred to liquid MS medium containing different concentrations of BA (0.1, 0.5, or 1.0 μM) with abscisic acid (ABA, 0.5, 1.0, or 1.5 μM) or in combination with α-naphthalene acetic acid (NAA) (0.1, 0.5, or 1.0 μM). The 10-week-old cotyledonary embryos were transferred to another set of liquid MS (MS, ½, 1/3, or ¼) medium without a plant growth regulator. Germination of somatic embryos and their conversion into plantlets were recorded after 1 week of transfer.
Hardening and Acclimatization
After removal from the culture medium, young plantlets were washed gently under running tap water and shifted to thermocol cups bearing sterile vermiculite, soilrite (Keltech Energies Pvt. Ltd., India), or garden soil covered with transparent polythene bags. The plantlets were incubated in a growth chamber at 25 °C and a relative humidity of 55–60%. The light was provided with cool fluorescent tube light (16 h photoperiod; 50 μmol m−2 s−1 Philips, India) and watered every 2 days with half-strength MS salt solution (without vitamins) for 2 weeks. Polythene membranes were removed after 4 weeks in order to acclimatize plantlets, and after 8 weeks, they were transferred to pots containing normal garden soil and a mixture of garden and sandy (1:1) soil, maintained in a greenhouse.
Histological Studies of Somatic Embryos
Fixation and Storage of Plant Material
The explants with different developmental stages were fixed in FAA solution containing formalin/glacial acetic acid/alcohol (70%) in the ratio of 4:6:90 (v/v). The fixed samples were stored in 70% alcohol.
Embedding, Sectioning, and Staining
A standard method of paraffin embedding [16] was followed for histological studies. Ethanol-xylol series was used for dehydration and infiltration. The plant material to be sectioned was kept in a vacuum oven at 60 °C for 15 min for complete infiltration. Sections (longitudinal and transverse) of 10 μM thickness were cut through a Spencer 820 microtome (American Optical Corp. Buffalo, New York) and, obtaining paraffin ribbons, were passed using a series of deparafinizing solutions and stained in safaranin and Fast Green solutions. Permanent slides were made by using Canada balsam. The sections were examined under a light microscope (Olympus CH20i, Japan).
Scanning Electron Microscopy for the Study of Embryogenesis
Scanning electron microscopy (SEM) was performed using the method described by Vasil and Vasil [17]. Embryogenic tissue samples for SEM were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.0) for 4 h at 4 °C. Dehydration was occurred via a graded ethanol series (50, 70, 90, 100%) sequentially for 30 min each with two changes. The tissues were then passed through a sequential series of absolute alcohol and isoamyl alcohol (3:1, 2:2, 1:3) with 30 min in each. Finally, tissues were kept in isoamyl alcohol for 30 min. The tissue was then dried in a critical point dryer (HCP-2, Hitachi) and coated with gold and paladium in an ion coat (IB-2, Giko Engineering Co, Japan). The samples were examined under a scanning electron microscope JSM 6510LV-JEOL-SEM, and photographs were taken at different magnifications.
Statistical Analysis
The experiments were conducted in three replicates independently. Completely randomized block design (RBD) was used to test the effects of different concentrations of cytokinins and auxins. Data were analyzed statistically using one-way analysis of variance (ANOVA). The significance of differences among means were established by Duncan’s multiple range test (DMRT) at P = 0.05 using SPSS Ver. 16 (SPSS Inc., Chicago, USA). The results are expressed as mean ± SE of three experiments.
Results
Somatic Embryogenesis
Induction of Somatic Embryogenesis
When internodal segments (1.0 cm) (Fig. 1a) were cultured on the MS basal medium without hormonal supplementation, there was no response even after 4 weeks of incubation. However, when the medium was augmented with different concentrations of 2,4-D (5.0–25.0 μM), there appeared small clusters of protuberances on the cut edges of the explants after an incubation period of 5–6 weeks (Fig. 1b). Subsequently, these structures spread to the whole surface of explants and got differentiated into globular-shaped embryos after 6 weeks of culture (Table 1). Embryos were round, elongated, and creamish in appearance (Fig. 1c).
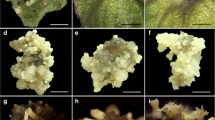
Plant regeneration via direct somatic embryogenesis. a Internodal explants. b Protrusion of direct embryos at the cut surface of explants on the same medium. c Cluster of globular somatic embryos on MS + 10.0 μM 2,4-D after 6 weeks of culture. d, e Heart-shaped embryo differentiated on MS + 10.0 μM 2,4-D + 1.0 μM BA after 7 weeks of culture (arrow). [Bar = 1 cm for a; 0.2 mm for b; 0.5 mm for c–e]. f, g Torpedo stage embryos with distinct shoot and root poles on MS + 10.0 μM 2,4-D + 1.0 μM BA after 8 weeks of culture (arrow). h, i Somatic embryos at the cotyledonary stage on liquid MS + 0.5 μM BA + 1.0 μM ABA after 2 weeks of transfer (arrow). j Germinated embryos with elongated shoots and roots on liquid ½ MS medium after 1 week of transfer. [Bar = 1 mm for f, g; 1 cm for h–j]. k Somatic embryos at different development stages. l Somatic embryos derived from well-developed small plantlets. m Acclimatized plantlet in sterile soilrite. [Bars = 1 cm]
Among the different concentrations of 2,4-D tested, 10.0 μM 2,4-D gave the maximum (70.4%) production of globular embryos (Fig. 1c), while, at higher concentration, the frequency was considerably reduced (11.6%) (Table 1). Prolonged culture in 2,4-D containing medium did not support further differentiation.
Combined Effect of Auxin and Cytokinin on Somatic Embryo Differentiation
When globular embryos (along with explants) were shifted to a medium supplemented with 10.0 μM 2,4-D along with a range of BA (0.1–2.0 μM), further differentiation could be recognized with regard to color and texture. These embryos passed through several stages of development resembling their zygotic counterparts, as heart-shaped after 7 weeks (Fig. 1d, e and torpedo after 8 weeks (Fig. 1f, g). During their development, somatic embryos progressively accumulated chlorophyll pigments and turned light green and were distinguishable from non-embryogenic tissues.
Among tested combinations, BA (1.0 μM) along with 10.0 μM 2,4-D showed the highest frequency (69.0%) with maximum (40.4 ± 0.50) number of heart-shaped embryos after 7 weeks of culture (Fig. 1d, e). While in another 1 week of incubation, 37.0 ± 0.70 torpedo embryos in 65.4% of cultures were formed (Table 2; Fig. 1f, g). Increasing concentration of BA, beyond optimal concentration, inhibited further differentiation of embryos. In addition, on the same medium, torpedo embryos did not attain cotyledonary stage (maturation) even after 10 weeks of incubation but showed an increased root pole growth without developing shoot apex (Fig. 1g).
Effect of Liquid Medium on Somatic Embryo Maturation
Isolated torpedo embryos were shifted to liquid MS medium containing different strengths of BA (0.1–1.0 μM) along with ABA (0.5–1.5 μM) or a combination with NAA (0.1–2.0 μM) to facilitate embryo maturation, whereas the residual tissue with embryos at early developmental stages were recultured on fresh medium (2,4-D + BA). Frequency of mature embryos depended on BA along with ABA. However, removal of 2,4-D was compulsory for the maturation of embryos at this stage. On media containing different levels of BA and ABA, a variation in conversion of torpedo embryos into mature embryos was noticed. Among the different concentrations tested, 0.5 μM BA along with 1.0 μM ABA was found most effective, on which a highest yield (58.0%) with an optimum number (35.0 ± 0.57) of mature embryos (cotyledonary stage) was obtained after 2 weeks of transfer (Table 3; Fig. 1h, i), while BA (0.1 μM) along with 0.5 μM ABA was found futile for conversion into mature embryos. Mature somatic embryos had two cotyledons, which differ in size and shape (Fig. 1i).
In addition, existence of NAA to the maturation medium with BA and ABA significantly reduced the regeneration frequency as compared to the medium with BA and ABA alone. This effect was straightforwardly associated to the NAA concentration, such that at the distinctive concentration tried (0.1–2.0 μM NAA), plant recovery was stifled to a rate of 20.3% at 2.0 μM NAA (Table 4).
Germination and Conversion into Plantlets
Growth of somatic embryos continued when cotyledonary stage embryos were transferred on hormone-free MS liquid medium. The effect of various strengths of MS (MS, ½, 1/3, or ¼) basal medium on conversion and germination is presented in Fig. 2. Highest (68%) response with maximum number (33.3 ± 0.88) of germinated embryos (small plantlets) was obtained on ½ MS medium after 1 week of transfer (Figs. 1j and 2). Merely embryos demonstrating both root and shoot were considered as germinated emblings or plantlets (Fig. 1k, l).
Well-developed plantlets were transferred to pots containing sterile soilrite for 4 weeks (Fig. 1m) and were kept in a growth room. Morphologically, plants were normal in appearance and show the development of new leaves in field condition where about 60% of plants survived and exhibited normal growth.
Histologial and Scanning Electron Microscopical Studies of Somatic Embryos
Somatic embryogenesis was confirmed by the histological and scanning electron microscopical studies.
Histological Study of Somatic Embryos
A histological study of the embryogenic tissue was carried out after 6 weeks of somatic embryo initiation. A study revealed that the somatic embryos emerged simultaneously at various formative stages. The somatic embryos started their development at the stage of globular to cotyledonary shape. Initially, small protuberance (pro-embryonic structure) appeared at the cut end of explants (Fig. 3a) and after rapid division, these protruding outgrowths switched to globular embryos (Fig. 3b, c).
a Histological section showing the formation of protuberances on the cut surface of explants (arrow). b, c Globular embryo formation with suspensor-like structure (arrow). d Bipolar heart-shaped embryo with vascular traces (arrow). [Bars = 100 μm]. e Torpedo stage embryo. f Longitudinal section of germinated embryo with distinct cotyledon, root pole, and vascular bundle (arrow). g Photomicrographs showing mature embryos with shoot tip and root tip on the surface of explant (arrow). [Bars = 100 μm]
At the time of somatic pro-embryonic structure to globular embryo, a suspensor-like structure appeared connecting the globular embryos to parent explants (Fig. 3b). Globular embryos continued to increase in size and progressed to heart-shaped with a separate lobe and closed vascular bundles within 7 weeks of culture (Fig. 3d). No vascular connection was seen between a somatic embryo and surface cells of explants. The heart stage embryo further elongated and developed into torpedo stage embryo (Fig. 3e) and ultimately formed small plantlets with distinct cotyledonary leaves, shoot apical meristem, and root pole in liquid medium (Fig. 3f). In all samples observed, there was a distinct limiting epidermis surrounding the somatic embryos with no visible vascular connection to the parent explant (Fig. 3g).
Scanning Electron Microscopical Analysis
Other than histological observation, SEM analysis provides more informative task during embryo development. At the beginning of culture, SEM revealed that a small swelling appeared on expanded internodal explants after 4 weeks on MS medium supplemented with 2,4-D. At 5 weeks, these swelling-like structures solely converted into oval or oblong cells. After 6 weeks, these cells became turgid and formed a pre-embryo-like structure characterized by granular extracellular materials (ECMs, Fig. 4a).
Scanning electron microscopic (SEM) study of direct somatic embryogenesis. a Pre-embryonic cells with extracellular matrix (ECM) (arrow). b Globular embryos developed on the surface of explants. c Heart-shaped embryo with distinct notch (arrow). [Bar = 100 μm for a; 500 μm for b, c]. d, e Torpedo-shaped somatic embryos showing elongated root with shoot pole (arrow). f Cotyledonary stage of somatic embryo with two cotyledons. [Bars = 200 μm]
As time increased, these pre-embryos gradually switched to globular embryos (Fig. 4b). Globular embryos were high in number but varied in shape (round to elongate) and produced continuously. Embryos got bunched together in a common mass and could not be easily detached from the parental tissue. The continuous proliferation of these embryos led to the multicellular, three-lobed heart-shaped structures, where the shoot pole (cotyledon initial) was separated from the root pole without extracellular matrix (Fig. 4c). These heart-shaped embryos proceeded into torpedo-shaped embryos (Fig. 4d, e). The extracellular matrix was gradually degraded as the embryos grew to its maturity. On transferring to liquid medium, these torpedo-shaped embryos could be characterized by well-differentiated cotyledons (Fig. 4f).
Discussion
The ability to induce somatic embryos in tissue culture opens new vistas which were not available in plantlet regeneration via organogenesis. The advantages of somatic embryogenesis are the simultaneous development of root and shoot systems. Somatic embryogenesis paves a direct role in clonal propagation [18]. The development of somatic embryos (either direct or indirect) differs according to plant growth regulators. Perhaps, this occurs due to the differences in susceptibilities of different tissues to external stimuli such as pH, carbon source, and other stress [19]. In vitro somatic embryogenesis depends on plant genetic potential for embryogenesis, competency of some cells for receiving endogenous or exogenous signal triggering the initiation of embryogenesis, and finally commitment for mature embryo production [20].
In A. officinalis, somatic embryogenesis was induced at cut edges or on the surfaces of internodal sections by using an exogenous phytohormone, 2,4-D. Development of somatic embryos at the cut surface of explants may be due to the increase in embryogenic competence of wounded tissue due to changes at the level of endogenous growth regulators [21].
In addition, the specific role of PGRs, especially auxin, has been considered to be conducive in somatic embryo formation. Auxins (natural as well as synthetic, NAA, 2,4-D) have been shown to act like a molecular glue binding to its TIR1 receptor and promoting ubiquitin-dependent degradation of auxin/IAA repressor proteins, activating the auxin response elements [22, 23]. The efficacy of 2,4-D in somatic embryogenesis has been well documented in many plant species [24, 25].
Conversion from globular to torpedo stage is a critical stage in somatic embryogenesis. MS medium containing 2,4-D alone was not sufficient for further differentiation (heart to torpedo stage). The association of 2,4-D and BA was essential for transition of embryos from heart and torpedo stages. Development of somatic embryos using 2,4-D along with BA has been accounted for in a number of plant species like Phellodendron amurense [26] and C. bicolor [12]. Torpedo embryos were separated from the mother explants in the same medium but did not attain the maturity in the same medium. Auxin removal from culture medium is a necessary step to inactivate genes or synthesis of its products for embryo maturation as suggested by Zimmerman [27].
Continuing exposure of 2,4-D in the medium showed delay in maturation, which has also been reported in Heracleum candicans [28]. The physical state of medium (liquid or solid) also plays an essential role for somatic embryogenesis [29]. In solid medium, gelling agents like agar reduce water potential and uptake of macro- and microelements for growing cells/tissues [30], but in liquid medium, cells efficiently and easily uptake inorganic and organic supplements and oxygen which are better nutrients for somatic embryo maturation and germination [31]. Keeping this view in mind, torpedo embryos were transferred onto a liquid MS medium having BA and ABA (without 2,4-D), where 0.5 μM BA and 1.0 μM ABA were found to be optimum for the maturation of torpedo embryos (cotyledonary stage). Similar to our study, liquid culture has also been shown to be beneficial for somatic embryogenesis in Nigella sativa [32], Lilium longiflorum [33], and Gymnema sylvestre [34].
In addition, ABA has been shown to modulate many physiological processes in plants including induction of dormancy, embryo maturation, inhibition of guard cell opening, and adaptation to environmental stress [35, 36]. Combined effect of BA and ABA was critical in the maturation of somatic embryos as reported in chickpea [37] and Desmodium motorium [38].
To increase the embryo maturation, a different range of NAA (0.1–1.0 μM) along with optimal concentration of BA (0.5 μM) and ABA (1.0 μM) was also tried. Although a combination of auxin and cytokinin has played a vital role for embryo maturation in different plant species including Psoralea corylifolia [39] and Cassia angustifolia [40], in A. officinalis, addition of NAA was not found effective for somatic embryo maturation. Thus, our results are similar to earlier findings in Gentiana straminea [41] and pigeonpea [42].
The success of micropropagation via somatic embryogenesis depends on the maturation and their conversion into plantlets [43]. According to Chalupa [44], germination frequencies of somatic embryos are considerably affected by the prior maturation treatment. Fully differentiated and mature (cotyledonary stage) somatic embryos were transferred to ½ MS liquid medium where they developed into complete plantlets. PGR-free ½ MS medium appeared to be a permissible stimulus for plantlet formation. Comparable to our results, hormone-free ½ MS medium appeared to be beneficial for germination and conversion of somatic embryos into plantlets in Eleutherococcus senticosus [45], P. amurense [26], and G. straminea [41].
Histological Study of Somatic Embryos
Histological studies of somatic embryos revealed that the developmental process was embryogenic and not organogenic in nature. Histological sections of embryos (globular to cotyledonary stage) confirmed that these embryos differentiated from the pre-determined cells, which were attached to the mother surface during initiation and became detached at later stages of development.
The morphological and histological observations proved that somatic embryogenesis was direct, without the production of callus.
Scanning Electron Microscopy
Scanning electron microscope analysis revealed the developmental changes during embryo formation (globular-torpedo shaped) on the surface of explants. Initially, the pre-embryogenic determined cell was characterized by the deposition of granular material which was followed by a thin fibrillar network representing an extracellular matrix (ECM). Samaj et al. [46] reported that the ECM has emerged as an important morpho-regulator during somatic embryogenesis and also plays an active role in plant regeneration. As is observed that ECM appeared only on certain regions and missing on other regions indicates that it is perhaps due to the contraction of the ECM layer caused by the critical point dryer (CPD) during the sample preparation. Presence of the extracellular matrix at the surface of the pre-embryogenic tissue was observed in Drosera rotundifolia [47], Cichorium intybus [48], and Murraya koenigii [49]. Occurrence of the extracellular matrix in confined developmental stage points out its important role in fixing cell position and in morphogenesis before the protodermis formation [50]. Further, it can act as a morphological marker on the surface of regenerating competent cells during direct embryogenesis [50].
However, as the time progressed, the pre-embryogenic cell transforms to form globular embryos. The surface of the globular stage as well as subsequent stages (heart, torpedo) of embryos was smooth and free from deposition of the ECM. Disappearance of material in the mature somatic embryos refers that the deposition of extracellular materials on the surface of any cell determines the formation of pre-embryo [47].
Conclusions
The present study describes the successful experiment of direct somatic embryogenesis which includes initiation, proliferation, and development of somatic embryos and their germination pattern under in vitro conditions using internode explants of A. officinalis. Somatic embryogenesis was confirmed with light microscopy and SEM analysis. Therefore, this useful protocol can be applied for the micropropagation and for future genetic transformation studies in Altheae plants.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxy acetic acid
- ABA:
-
Abscisic acid
- BA:
-
6-Benzyladenine
- MS:
-
Murashige and Skoog (1962)
- NAA:
-
α-Naphthalene acetic acid
- SE:
-
Somatic embryo
References
Anonymous. (2003). The wealth of India. A dictionary of Indian raw materials and industrial products (Vol. 1, pp. 207–208). New Delhi: Council of Scientific and Industrial Research.
Sutovska, M., Nosalova, G., Sutovsky, J., Franova, S., Prisenznakova, L., & Capek, P. (2009). Possible mechanisms of dose-dependent cough suppressive effect of Althaea officinalis rhamnogalacturonan in guinea pigs test system. International Journal of Biological Macromolecules., 45, 27–32.
Hage-Sleiman, R., Mroueh, M., & Daher, C. R. (2011). Pharmacological evaluation of aqueous extract of Althaea officinalis flower grown in Lebanon. Pharmaceutical Biology, 49, 327–333.
Blumenthal, M., Goldberg, A., & Brinckmann, J. (2000). Herbal medicine: expanded commission E monographs (pp. 244–248). Austin: American Botanical Council.
Naz, R., & Anis, M. (2012). Acceleration of adventitious shoots by interaction between exogenous hormone and adenine sulphate in Althaea officinalis L. Applied Biochemistry and Biotechnology, 168, 1239–1255.
Naz, R., Anis, M., & Aref, I. M. (2015). Management of cytokinin–auxin interactions for in vitro shoot proliferation of Althaea officinalis L.: a valuable medicinal plant. Rend Fis Acc Lincei, 26, 323–334.
Morel, A., Trontin, J. F., Corbineau, F., Lomenech, A. M., Beaufour, M., Reymond, I., et al. (2014). Cotyledonary somatic embryos of Pinus pinaster Ait. most closely resemble fresh, maturing cotyledonary zygotic embryos: biological, carbohydrate and proteomic analyses. Planta, 240, 1075–1095.
Merkle, S. A., Bailey, R. L., Pauley, B. A., Neu, K. A., Kim, M. K., Rugu, C. L., & Montello, P. M. (1997). Somatic embryogenesis from tissues of mature sweetgum trees. Canadian Journal of Forest Research, 27, 959–964.
Pedroso, C. M., & Pais, M. S. (1995). Factors controlling somatic embryogenesis. Plant Cell, Tissue and Organ Culture, 43, 147–154.
Chen, J. T., & Chang, W. C. (2006). Direct somatic embryogenesis and plant regeneration from leaf explants of Phalaenopsis ambilis. Biologia Plantarum, 50, 169–173.
You, X. L., Han, J. Y., & Choi, Y. E. (2007). Plant regeneration via direct somatic embryogenesis in Panax japonicas. Plant Biotechnological Reports, 1, 5–9.
Mahendran, G. V., & Bai, V. N. (2012). Direct somatic embryogenesis and plant regeneration from seed derived protocorms of Cymbidium bicolor Lindl. Scientia Horticulturae, 135, 40–44.
Dhir, R., Shekhawat, G. S., & Alam, A. (2014). Improved protocol for somatic embryogenesis and calcium alginate encapsulation in Anethum graveolens L.: a medicinal herb. Applied Biochemistry and Biotechnology, 173, 2267–2278.
Singh, R., Raj, M. K., & Kumari, N. (2015). Somatic embryogenesis and plant regeneration in Sapindus mukorossi Gaertn. from leaf-derived callus induced with 6-benzylaminopurine. Applied Biochemistry and Biotechnology, 177, 498–510.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays for tobacco tissue cultures. Physiologiae Plantarum, 15, 473–497.
Johansen, D. A. (1940). Plant microtechnique. New York: Mc Graw-Hill Book Co. Inc..
Vasil, V., & Vasil, I. K. (1984). Preparation of cultured tissues for scanning electron microscopy. In I. K. Vasil (Ed.), Cell culture and somatic cell genetics of plants (Vol. I, pp. 738–743). Orlando: Academic Press.
Kanita, A., & Kothari, S. I. (2002). High efficiency adventitious shoot bud formation and plant regeneration from leaf explants of Dianthus chinensis L. Scientia Horticulturae, 96, 205–212.
Nishiwaki, M., Fujino, K., Koda, Y., Masuda, K., & Kikuta, Y. (2000). Somatic embryogenesis induced by the simple application of abscisic acid to carrot (Daucus carota L.) seedlings in culture. Planta, 211, 756–759.
Feher, A. (2005). Why somatic plant cells startto form embryos? In A. Mujib & J. Samaj (Eds.), Somatic embryogenesis (pp. 85–101). Berlin, Heidelberg: Springer.
Ivanova, A., Velcheva, M., Denchev, P., Atanassov, A., & Van Onckelen, H. (1994). Endogenous hormone levels during direct somatic embryogenesis in Medicago falcata. Physiologiae Plantarum, 92, 85–89.
Guilfoyle, T. J., & Hagen, G. (2007). Auxin response factors. Current Opinion in Plant Biology, 10, 453–460.
Tan, X., Calderon-Villalobos, L. I. A., Sharon, M., Zheng, C. X., Robinson, C. V., Estelle, M., & Zheng, N. (2007). Mechanism of perception by the TIR1 ubiquitin ligase. Nature, 446, 640–645.
Prange, A. N. S., Serek, M., Bartsch, M., & Winkelmann, T. (2010). Efficient and stable regeneration from protoplasts of Cyclamen coum Miller via somatic embryogenesis. Plant Cell Tissue and Organ Culture, 101, 171–182.
Chai, M., Jia, Y., Chen, S., Gao, Z., Wang, H., Liu, L., Wang, P., & Hou, D. (2011). Callus induction, plant regeneration, and long-term maintenance of embryogenic cultures in Zoysia matrella [L.] Merr. Plant Cell Tissue and Organ Culture, 104, 187–192.
Azad, M. A. K., Yokota, S., Begum, F., & Yoshizawa, N. (2009). Plant regeneration through somatic embryogenesis of a medicinal plant, Phellodendron amurense Rupr. In Vitro Cellular Developmental Biology–Plant, 45, 441–449.
Zimmerman, J. L. (1993). Somatic embryogenesis: a model for early development in higher plants. Plant Cell, 5, 1411–1423.
Wakhlu, A. K., & Sharma, R. K. (1998). Somatic embryogenesis and plant regeneration in Heracleum candicans wall. Plant Cell Reports, 7, 866–869.
Raemakers, C. J. J. M., Jacobsen, E., & Visser, R. G. F. (1995). Secondary somatic embryogenesis and application in plant breeding. Euphytica, 81, 93–107.
Scholten, H. J., & Pierik, R. L. M. (1998). Agar as a gelling agent: chemical and physical analysis. Plant Cell Reports, 17, 230–235.
Mujib, A., Ilah, A., Aslam, J., Fatima, S., Siddiqui, Z. H., & Maqsood, M. (2012). Catharanthus roseus alkaloids: application of biotechnology for improving yield. Plant Growth Reg., 68(2), 111–127.
Elhag, H., El-Olemy, M. M., & Al-Said, M. S. (2004). Enhancement of somatic embryogenesis and production of developmentally arrested embryo in Nigella sativa L. Horticultural Science, 39, 321–323.
Nhut, D. T., Hanh, N. T. M., Tuan, P. Q., Nguyet, L. T. M., Tram, N. T. H., Chinh, N. C., Nguyen, N. H., & Vinh, N. D. (2006). Liquid culture as a positive condition to induce and enhance quality and quantity of somatic embryogenesis of Lilium longiflorum. Scientia Horticulturae, 110, 93–97.
Ahmed, A. B. A., Rao, A. S., & Rao, M. V. (2009). Somatic embryogenesis and plant regeneration from cell suspension culture of Gymnema sylvestre (Retz) R. Br. Ex Roemer & Schultes. KMITL Science Technology Journal, 9, 18–26.
Davies, W. J., & Jones, H. G. (1991). Abscisic acid: physiology and biochemistry (pp. 39–52). Oxford: Bios.
Senger, S., Mokc, H. P., Conrad, U., & Manteuffel, R. (2001). Immunomodulation of ABA function affects early events in somatic embryo development. Plant Cell Reports, 20, 112–120.
Ghanti, S. K., Sujata, K. G., Rao, M. S., & Kishor, P. V. K. (2010). Direct somatic embryogenesis and plant regeneration from immature explants of chickpea. Biologia Plantarum, 54, 121–125.
Devi, C. B., & Narmathabai, V. (2011). Somatic embryogenesis in the medicinal legume Desmodium motorium (Houtt.) Merr. Plant Cell Tissue and Organ Culture, 106, 409–418.
Sahrawat, A. K., & Chand, S. (2001). Continuous somatic embryogenesis and plant regeneration from hypocotyls segments of Psoralea corylifolia L., an endangered and medicinally important fabaceae plant. Current Science, 81, 1328–1331.
Agrawal, V., & Sardar, P. R. (2007). In vitro regeneration through somatic embryogenesis and organogenesis using cotyledons of Cassia angustifolia Vahl. In Vitro Cellular Developmental Biology–Plant, 43, 585–592.
He, T., Yang, L., & Zhao, Z. (2011). Embryogenesis of Gentiana straminea and assessment of genetic stability of regenerated plants using inter simple sequence repeat (ISSR) marker. African Journal of Biotechnology, 10, 7604–7610.
Anbazhagan, V. R., & Ganapathi, A. (1999). Somatic embryogenesis in cell suspension cultures of pigeonpea (Cajanus cajan). Plant Cell Tissue and Organ Culture, 56, 179–184.
Rout, G. R., & Nanda, R. M. (2005). Protocol of somatic embryogenesis in Acacia Arabica (Lamk) Willd. In S. Jain & P. Gupta (Eds.), Protocol for somatic embyogenesis in woody plants (pp. 401–412). Netherlands: Springer.
Chalupa, V. (2005). Protocol of somatic embryogenesis: penunculate oak (Quercus robur L) and sessile oak (Quercus petraea/Matt./Liebl.). In P. Gupta & S. Jain (Eds.), Protocol for somatic embryogenesis in woody plants (pp. 369–378). Netherlands: Springer.
Choi, Y. E., Kim, J. W., & Yoon, E. S. (1999). High frequency of plant production via somatic embryogenesis from callus or cell suspension cultures in Eleutherococcus senticosus. Annals of Botany, 83, 309–314.
Samaj, J., Baluska, F., Bobak, M., & Volkmann, D. (1999). Extracellular matrix surface network of embryogenic units of friable maize callus contains arabinogalactan-proteins recognized by monoclonal antibody JIM4. Plant Cell Reports., 18, 369–374.
Samaj, J., Bobak, M., Blehova, A., Kristin, J., & Auxtova Samajova, O. (1995). Developmental SEM observations on an extracellular matrix in embryogenic calli of Drosera rotundufolia and Zea mays. Protoplasma, 186, 45–49.
Chapman, A., Blervacq, A. S., Tissier, J. P., Delbreil, B., Vasseur, J., & Hilbert, J. L. (2000a). Cell wall differentiation during early somatic embryogenesis in plants. I. Scanning and transmission electron microscopy study on embryos originating from direct, indirect, and adventitious pathways. Canadian Journal of Botany, 78, 816–823.
Paul, S., Dam, A., Bhattacharyya, A., & Bandyopadhyay, T. K. (2011). An efficient regeneration system via direct and indirect somatic embryogenesis for the medicinal tree Murraya koenigii. Plant Cell Tissue and Organ Culture, 105, 271–283.
Bobak, M., Samaj, J., Pretova, A., Blehova, A., Hlinkova, E., Ovecka, M., Hlavacka, A., & Kutarnova, Z. (2004). The histological analysis of in direct somatic embryogenesis on Drosera spathulata Labill. Acta Physiologiae Plantarum, 26, 353–361.
Acknowledgements
Research support from the Department of Science and Technology (DST) and the University Grants Commission (UGC), New Delhi, Government of India, under the DST-FIST-II (2012–2017) and UGC-DRS II (2016–2021) Programs, respectively, is greatly acknowledged. The authors extend their appreciation to the International Scientific Partnership Program (ISPP) at King Saud University for partial assistance. RN is grateful to the University Grants Commission, New Delhi, for the award of Dr. DS Kothari Postdoctoral Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(JPEG 79 kb)
Rights and permissions
About this article
Cite this article
Naz, R., Anis, M. & Alatar, A.A. Embling Production in Althaea officinalis L., Through Somatic Embryogenesis and Their Appraisal via Histological and Scanning Electron Microscopical Studies. Appl Biochem Biotechnol 182, 1182–1197 (2017). https://doi.org/10.1007/s12010-016-2391-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2391-2