Abstract
Diabetes mellitus (DM) is considered as a complex metabolic disease because it affects the metabolism of glucose and other metabolites. Although many diabetes studies have been conducted in animal models throughout the years, the pathogenesis of this disease, especially between lean diabetes (ND + STZ) and obese diabetes (OB + STZ), is still not fully understood. In this study, the urine from ND + STZ, OB + STZ, lean/control (ND), and OB + STZ rats were collected and compared by using 1H NMR metabolomics. The results from multivariate data analysis (MVDA) showed that the diabetic groups (ND + STZ and OB + STZ) have similarities and dissimilarities for a certain level of metabolites. Differences between ND + STZ and OB + STZ were particularly noticeable in the synthesis of ketone bodies, branched-chain amino acid (BCAA), and sensitivity towards the oral T2DM diabetes drug metformin. This finding suggests that the ND + STZ group was more similar to the T1DM model and OB + STZ to the T2DM model. In addition, we also managed to identify several pathways and metabolism aspects shared by obese (OB) and OB + STZ. The results from this study are useful in developing drug target-based research as they can increase understanding regarding the cause and effect of DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is one of the non-communicable diseases (NCDs) that caused more than 1.5 million deaths in 2012 [1]. It was estimated that 171 million people worldwide were diagnosed with DM in 2000, and this number is expected to increase to 366 million by 2030 [2, 3]. DM is generally characterized by a high glucose content in blood or hyperglycemia, which can cause long-term complications in the targeted cells (e.g., β-cells) and organs. Thus, it often causes other metabolic syndrome diseases such as cardiovascular disease (CVD), hypertension, and microcirculatory disorder [3, 4]. Generally, DM can be categorized into two types based on the requirement for insulin, where type 1 (T1DM) is insulin-dependent DM, and type 2 (T2DM) is non-insulin-dependent DM [5]. T1DM is an autoimmune disease, where the body itself (immune system) destroys the insulin-producing β-cells of the pancreas, whereas T2DM is more likely a combination of insulin insensitivity, inadequate function of the β-cell mass, and inactive lifestyle, such as obesity [4, 6].
For a better understanding of DM, many studies have been conducted on animal models throughout the years [7–10]. Both spontaneous or genetically modified models and non-spontaneous models (experimentally induced diabetes model) are currently available, in which non-spontaneous/experimentally induced diabetes models are much preferred due to their lower cost, ease of maintenance, and close relationship to human diabetes [3, 11]. For the non-spontaneous diabetes model, the diabetic condition can be induced by diet/nutrition (e.g., high sucrose and high fat content diet), chemically (i.e., streptozotocin (STZ) or alloxan), surgically (by removing organs such as pancreas), or by any combination of these methods [9, 10]. STZ is a broad-spectrum antibiotic and a powerful alkylating agent that is known to induce DM by selectively destroying the pancreatic β-cells that produce insulin, a hormone that regulates the glucose levels in the body [3, 11]. Therefore, the STZ-induced diabetic rat model has been used widely in diabetic studies, where it provides additional information on the mechanisms and complications that are associated with DM. A high dose of STZ (50–60 mg/kg) has been used for inducing the T1DM model, while a low dose of STZ (15–35 mg/kg) together with a modified diet, such as a high fat-fed diet (Hfd), is used to mimic the obese-diabetic state in humans or T2DM [11, 12]. Although numerous diabetic studies have been conducted in the STZ-induced rat model, there is still confusion about these models, especially whether the high-dose STZ-induced rat can represent the T1DM model or low-dose STZ-induced with modified diet as the T2DM model [13]. Hence, it is important to investigate this matter as it can provide useful information, especially in the drug-targeted research field.
Recently, the metabolomics approach has been applied in diabetic studies to diagnose disease progression, discovering biomarkers and responses towards treatment [3, 11]. There are multiple analytical tools available for metabolomics studies, such as GCMS, LCMS, FTIR, and nuclear magnetic resonance (NMR). However, NMR is more preferred as it has easy sample preparation and it is more robust and non-destructive [3, 11]. By using NMR-based metabolomics, any changes in metabolites can be detected, and this information is crucial as it can provide a view of how the biochemical pathways work [3, 11]. Studies conducted by Zhang et al. [11], Diao et al. [3], and Zhao et al. [14] managed to highlight several metabolites and pathways that are affected in diabetic rats. However, Zhao et al. [14] noted that the strain might affect the separation as the control group (Wistar rats) is a different strain from the diabetic rats (GK rats). No previous study has focused on comparing between lean diabetic rats (ND + STZ) and obese diabetic rats (OB + STZ). Therefore, this study aimed to identify disturbed metabolic pathways in STZ-induced diabetic rats, ND + STZ and OB + STZ, using the 1H NMR-based metabolomics approach. In addition, we also highlighted the similarities and dissimilarities in terms of metabolites in these models and also the relationship between obesity and DM.
Materials and Methods
Solvents and Chemicals
Non-deuterated potassium phosphate monobasic (KH2PO4), sodium deuterium oxide (NaOD), trimethylsilylpropionic acid-d4 sodium salt (TSP), deuterium oxide (D2O), diethyl ether, and a standard drug (metformin) were purchased from Merck (Darmstadt, Germany). Streptozotocin (STZ), carboxymethyl cellulose (CMC), and sodium azide, were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Experimental Design for In Vivo Study
Approval for the animal study was obtained from the Institutional Animal Care and Use Committee (IACUC) of the Faculty Medicine and Health Sciences, Universiti Putra Malaysia (IACUC No. UPM/FPSK/PADS/BRUUH/00490). Five-week-old male Sprague-Dawley rats, weighing 100–150 g, were used in the study. The rats were housed individually in plastic cages with stainless steel covers and put in acclimatization conditions for 1 week at room temperature (26–28 °C) under a 12-h dark/12-h light cycle. During the acclimatization period, the rats were fed with normal rat chow and tap water ad libitum.
Obesity Induction
Following acclimatization, the rats were divided randomly into two groups in which the lean/control group (ND) was fed with normal rat chow/normal diet (Nd) from the Gold Coin, Malaysia, and the other group, obese (OB), with a modified high-fat diet (Hfd). This Hfd contained 49% fat, 32% carbohydrate, and 19% protein for the total energy, which consists of fat from ghee (milk fat) and corn oil. The Nd contained 14% fat, 61% carbohydrate, and 25% protein from the total energy. These diets were continously given throughout this study, even after the induction of obesity (week 12). The final number of rats for ND was n = 10, and for OB, it was n = 8.
Diabetes Induction
After the induction of obesity, some of the ND and OB rats were then randomly chosen and injected with STZ intravenously (ND + STZ and OB + STZ). For this purpose, STZ was freshly prepared in citrate buffer (0.1 M, pH 4.5). The dosage of STZ for the lean diabetic group (ND + STZ) was 60 mg/kg, while for the obese diabetic group (OB + STZ), it was 25 mg/kg [9, 10]. Three days after the injection, the blood glucose was measured by tail prick using a glucometer (Accu-Chek Performa, Roche, Mannheim, Germany). The final number of rats for both the ND + STZ and OB + STZ groups was n = 5 after separating half of the rats out for metformin sensitivity test.
Sensitivity Towards Oral T2DM Drugs
To test their sensitivity towards the T2DM oral antidiabetic drug metformin, the rats from the lean diabetic group (ND + STZ) and the obese diabetic group (OB + STZ) were then again randomly divided into another group: treated lean diabetic (ND + STZ + MET, n = 6) and treated obese diabetic (OB + STZ + MET, n = 5). Metformin (150 mg/kg) was administered for 4 weeks. The lean/control (ND), obese (OB), and non-treated diabetes group (ND + STZ and OB + STZ) were given 1 mL of 0.03% (w/v) CMC.
Blood and Urine Collection
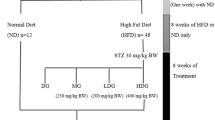
Urine was collected at weeks 12, 13, and 17 by placing the rats individually in plastic metabolic cages with urine bottles containing sodium azide (0.1%). The blood was collected into sterile tubes through retro orbital (week 12 and week 13) and cardiac puncture (week 17), after anesthetizing the rats with diethyl ether. Serum samples for the biochemical test were obtained by high-speed centrifugation at 6000×g for 10 min at 4 °C. All the urine and serum samples were labeled carefully and kept at −80 °C until further used. A summarized experimental diagram is shown in Fig. 1.
Biochemical Analyses
For biochemical analyses, the serum glucose, total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were measured using an automatic biochemical analyzer (Hitachi 902, Roche, Germany) [3]. The values are expressed as the mean ± SD.
Biofluid Preparation and NMR Measurements
Frozen urine samples were thawed and centrifuged at 3000 rpm before the supernatant was used. In microcentrifuge tubes, 0.40 mL of the supernatant was mixed with 0.20 mL phosphate buffer solution (0.308 g of KH2PO4, in 25 mL of D2O, pH 7.4, containing 0.1% TSP). All samples were vortexed for 1 min to obtain a homogenous mixture. The mixture (0.5 mL) was transferred to an NMR tube and subjected to 1H NMR analysis. The 1H NMR measurements were performed using a 500-MHz Varian INOVA NMR spectrometer (Varian Inc., CA, USA), functioning at a frequency of 499.91 MHz and maintained at 26 °C. The NMR analysis was performed for all of the urine samples. For the urine samples, a standard water-suppressed one-dimensional NMR was obtained using the PRESAT sequence. The acquisition time of each 1H NMR spectrum was 3.53 min, which consisted of 64 scans. The identification of the metabolites was assisted by the information from the software and online databases (Chenomx Profiler and Human Metabolome Database (HMDB): http://www.hmdb.ca/ [15]) as well as from the literature data.
Data Processing and Multivariate Data Analysis
The phasing and baseline corrections were conducted using the Chenomx software (v. 5.1, Alberta, Canada) with a consistent setting for all sample spectra. Phasing and baseline corrections were performed manually on each spectrum, and TSP was set as the reference peak at δ 0.00. Integration which resulted in 231 integral regions per spectrum was conducted on the urine sample spectral region from δ 0.50 to δ 10.0 ppm. The regions containing the water (δ 4.57–4.95) and urea (δ 5.55–5.95) were excluded from all the spectra. However, in NMR spectra for the urine at weeks 13 and 17, we attained another dataset by excluding the glucose region (δ 3.15–3.24, δ 3.31–3.52, δ 3.63–3.9, δ 5.15–5.24), and each of these points was normalized again to the sum row to compensate for the variation in the total volumes.
The multivariate data analysis (MVDA) was conducted on the preprocessed NMR dataset with the SIMCA-P software (v. 14.0, Umetrics, Umea, Sweden). First, principal component analysis (PCA) was performed to visualize and discriminate the groups according to their metabolites, thus giving the general idea within the dataset. The partial least square discriminant analysis (PLS-DA) and orthogonal partial least square discriminant analysis (OPLS-DA) models of the NMR spectral data were also carried out for class discrimination and potential biomarker selection [16]. Potential biomarkers in PLS-DA were determined based on the combination of loading scatter plot and variable importance in the projection (VIP) values using a VIP >1 [17], while in OPLS-DA, the determination of potential metabolites was made based on the selection from the S-plot and loading column plot [18].
Statistical Analysis
Significant differences in basal parameters and metabolite changes were determined using statistical analysis software, SPSS 16.0. Statistical analysis using one-way ANOVA and Tukey’s post hoc test were performed, where p < 0.05 was considered as statistically significant. All data were presented as mean ± standard deviation (SD). The quality of PLS-DA and OPLS-DA models was expressed by using the goodness-of-fit R 2 and Q 2, whereas for the validity, the model was tested using permutation test (100 cycle), misclassification probability test, and ANOVA of cross-validated residuals (CV ANOVA) [17, 18].
Results
Basal Parameters (Weight and Biochemical)
Basal parameters include weight and clinical chemistry data as shown in Supplementary Fig. S1. Obese rats (OB) showed an increase in weight after 12 weeks of Hfd and after induction of diabetes. Rat weight began to decrease as the weeks progressed especially in diabetes groups: obese diabetes (OB + STZ) and lean diabetes (ND + STZ). Meanwhile, in clinical chemistry, the diabetes group, especially the diabetes groups (OB + STZ), showed increased levels of serum glucose level, LDL, TC, and TG after diabetes induction in week 13. In addition, changes in TC and TG can be seen in the obese group (OB) as early as week 12. All of these results suggested that diabetes induction/progression affected the weight and metabolisms (lipid and carbohydrate) in the diabetes group.
1H NMR Spectral Analysis of Urine Samples
Figure 2 shows a comparison of the general 1H NMR spectra of urinary samples from normal (ND), obese (OB), and STZ-induced diabetic samples, lean diabetic (ND + STZ) and obese diabetic (OB + STZ) rats. The urinary spectra were assigned according to Chenomx NMR Suite 5.1 (Chenomx Inc., Edmonton, Canada), HMDB databases, and published assignments. A total of 27 metabolites (excluding water and TSP) from various groups of endogenous metabolites ranging from amino acids such as leucine, taurine, and lysine; organic anions such as formate, acetate, allantoin, and tricarboxylic cycle (TCA); and intermediates such as succinate, citrate, and 2-oxoglutarate were identified (Supplementary Table S1). Note that exceptional levels of glucose can be observed in the STZ-induced diabetic group as compared to the ND group spectra (Fig. 2).
Representative of 500 MHz 1H NMR spectra of urine samples for normal (ND), obese (OB), lean diabetic (ND + STZ), and obese diabetic (OB + STZ) rats. Labels: 1, TSP; 2, isoleucine; 3, lactate; 4, leucine; 5, lysine; 6, acetate; 7, acetoacetate; 8, succinate; 9, 2-oxoglutarate; 10, citrate; 11, dimethylamine; 12, N,N-dimethylglycine; 13, creatine; 14, carnitine; 15, taurine; 16, methanol; 17, creatinine; 18, kynurenine; 19, trigonelline; 20, 1-methylnicotinamide; 21, water; 22, allantoin; 23, urea; 24, tryptophan; 25, N-phenylacetylglycine; 26, hippurate; 27, formate; 28, 3-hydroxybutryrate (3-HB); and 29, glucose
Multivariate Analysis of Spectral Data
Obesity Development
Multivariate data analysis (MVDA) was conducted on the normalized NMR spectrum dataset to identify the metabolic perturbations associated with DM. Firstly, the unsupervised method represented by PCA was conducted on the 1D 1H NMR urine spectra after the 12th week of Hfd feeding for a general overview of the sample (sample) distribution (Supplementary Fig. S2). A PCA with a goodness of fit, R 2 X (cum) = 0.68 and Q 2 (cum) = 0.34, was achieved. From the overall overview, there was a trend from the separation of those two groups. For further and clearer separation, we conducted another MVDA method which is OPLS-DA. A distinctive discrimination can be seen between the OB group and the ND group from OPLS-DA on the 12th week (Supplementary Fig. S3), with the goodness-of-fit R 2 X (cum) = 0.56, R 2 Y (cum) = 0.98, and Q 2 (cum) = 0.94. This model was considered as a good model because the goodness of fit, R 2 Y (cum) and Q 2 (cum), were >0.9, and the difference between R 2 Y (cum) and Q 2 (cum) was <0.3, indicating that each of the subjects equally and uniformly contributed to the observed group separation [19]. In addition, the validity of the model using permutation test, CV ANOVA, and misclassification table also showed that the model was valid (Supplementary Figs. S4 and S5 and Tables S2 and S3). This result was in agreement with the previous body weight result from week 12, which proves that obesity induction was successful. The clear discrimination in OPLS-DA indicates that diet plays important roles in the development of obesity.
Justification of Model by Sensitivity Test Towards Metformin
To identify which type of diabetes model (T1DM or T2DM) obese diabetes and lean diabetes resemble to, a sensitivity test towards the T2DM oral drug metformin was conducted on the diabetes group for 4 weeks (after the induction of diabetes at week 13). The OB + STZ and ND + STZ rats were divided into two groups where they were tested with metformin. The OB + STZ metformin-treated group was labeled as OB + STZ + MET, while the ND + STZ group treated with metformin was labeled as ND + STZ + MET. The PLS-DA was first performed on urine spectrum data for week 17, where we achieved a good model with the goodness-of-fit R 2 X (cum) = 0.93, R 2 Y (cum) = 0.63, Q 2 (cum) = 0.54, and validation test (Supplementary Figs. S6, S7, S8, S9, S10, S11, and S12 and Tables S4 and S5).
As illustrated in Supplementary Fig. S4, a clear separation from PC1 can be seen where the non-diabetic groups ND and OB were separated from the diabetic groups. This separation was due to the high concentration of glucose in the urine. However, there was a movement of individuals in the OB + STZ + MET group in which two out of five rats shifted from the negative to the positive side of PC1, where the non-diabetic group (OB) was located. The movement of individuals (rats) from OB + STZ + MET shows that metformin managed to lower the glucose level in the obese diabetic rats similarly to the non-diabetic OB but it did not manage to revert the condition in OB + STZ + MET back to ND. Metformin is a biguanide oral antihyperglycemic agent that is used alone or in conjunction with another therapy to treat T2DM [20]. It functions by decreasing hepatic glucose production and improves insulin sensitivity by encouraging glucose uptake by the muscle [21]. Generally, the only therapy available for T1DM is insulin, either from insulin injection or from insulin pump therapy [22]. Therefore, this result suggests that OB + STZ + MET is sensitive towards the drugs compared to ND + STZ + MET, which also indicates that the obese diabetic model (OB + STZ) is more similar to T2DM.
Metabolite Variation Between Obese, Lean Diabetic, and Obese Diabetic After Diabetes Induction
To see the metabolic link in the ND, OB, OB + STZ, and ND + STZ groups, the PLS-DA was performed on the urinary data after induction of diabetes at week 13. A distinctive cluster was formed, separating the non-diabetic group from the diabetic group in PC1. As expected, the separation between these groups was mainly due to the higher content of glucose and the other metabolites that overlapped with the glucose region such as taurine, carnitine, and N-phenylacetylglycine in the diabetic group. However, it is difficult to identify the other metabolites in the STZ-induced diabetic group as the glucose signals (including other metabolites that overlap with the glucose region) suppress the other metabolite signals in the NMR spectrum. To improve the identification of new potential metabolites in the diabetic group, we decided to exclude the glucose region in the new NMR spectrum dataset. To avoid biases or ambiguous results due to the influence of the glucose region, we also decided to exclude the metabolites that overlapped with the glucose region, such as taurine, carnitine, and N-phenylacetylglycine, from the selection of potential biomarkers.
The PLS-DA was then performed on the new normalized dataset (Fig. 3a), where the goodness of fit shows the values for R 2 X (cum) = 0.61, R 2 Y (cum) = 0.71, and Q 2 (cum) = 0.58. This model is considered a good model, in terms of its goodness of fit (R 2 Y (cum) >0.7) and good predictive ability (Q 2 (cum) >0.5). A permutation test with 100 permutation cycles was also conducted to validate the model, and the result demonstrated model validity with the R 2 Y-intercept <0.3–0.4 and Q 2 Y-intercept <0.05 (Supplementary Figs. S13, S14, S15, and S16). In addition, misclassification probability test and CV ANOVA were also conducted to validate the PLS-DA model (Supplementary Tables S6 and S7). The model exhibited a clear cluster that separated ND rats from OB, ND + STZ, and OB + STZ. Interestingly, we also noted a trend in OB rats, where few of the OB rats overlapped with OB + STZ at the upper part of the positive side in PC1, and three out of ten rats from OB + STZ were nearer to ND + STZ rats (lower part of the positive side in PC1). As illustrated in the loading scatter plot (Fig. 3b), endogenous metabolites from the TCA cycle such as 2-oxoglutarate, citrate, succinate, and others, including hippurate, dimethylamine (DMA), and tryptophan (TRP), were higher in ND than in other groups. Meanwhile, metabolites such as creatinine were high in OB rats that are located at the upper part of the negative side of PC1, and metabolites such as allantoin, leucine, lysine, lactate, and acetate were high in the overlapped group of OB and OB + STZ. Lastly, the metabolites that affected the clustering in ND + STZ and some of the OB + STZ rats were trigonelline, 3-hydroxybutyrate (3-HB), kynurenine (KYN), and acetoacetate (AcAc).
To further validate the identified potential biomarkers, only the metabolites with VIP ≥1 were selected from PLS-DA (Fig. 3). From Table 1, only 15 metabolites including glucose were identified as potential biomarkers from ND, OB, ND + STZ, and OB + STZ, and their relative concentrations were compared. We noted that several metabolites showed the same trend in OB and OB + STZ rats (leucine, allantoin, and trigonelline) and also between the OB and diabetic groups, ND + STZ and OB + STZ (2-oxoglutarate, citrate, succinate, lysine, TRP, DMA, and hippurate), when compared to the ND. In the comparison between ND + STZ and OB + STZ, metabolites such as allantoin, creatinine, leucine, and lysine were significantly higher (p < 0.05) in OB + STZ rats compared to ND + STZ rats, while metabolites such as hippurate, 3-HB, and trigonelline were higher in ND + STZ rats than in OB + STZ rats. In addition, ND + STZ and OB + STZ also had no significant difference (p > 0.05) between them in the amounts of certain metabolites such as glucose, citrate, TRP, and AcAc.
Discussion
After induction of DM by STZ, changes in metabolite levels were observed, especially for ND + STZ and OB + STZ. Both groups shared similarities in some of the metabolite changes, which indicate that both groups shared some similar features, especially in biochemical traits (e.g., glucose). In addition, the closeness of OB and OB + STZ rats in PLS-DA suggested the association or link of obesity with diabetes. This may denote that the dietary effect plays a large role in diabetes progression [23]. Figure 4 shows the summarized altered metabolites compared to the ND group and the possible pathways and aspects of metabolism involved using MetaboAnalyst 3.0 [24].
Summary of metabolite changes (a) in obese (OB), lean diabetic (ND + STZ), and obese diabetic (OB + STZ) groups as opposed to normal (ND), where metabolites name indicated in bold are significantly increased and those non bold significantly decreased in the respective group. Numbers represent the region metabolites shared by the group; 1, OB, ND + STZ, and OB + STZ; 2, ND + STZ and OB + STZ; and 3, OB and OB + STZ. b Simplified schematic of some of the disturbed pathways as identified by MetaboAnalyst 3.0
Metabolites Involved in Carbohydrate Metabolism
In this study, a high accumulation of glucose in both ND + STZ and OB + STZ was observed. In addition, we also found a lower level of TCA intermediates, such as 2-oxoglutarate, citrate, and succinate, in the OB and diabetic groups. These findings were in agreement with the previous dietary effect studies [25, 26] and diabetic studies [3, 14], where they found elevated glucose levels and lower levels of 2-oxoglutarate and citrate in obese Goto-Kakizaki and STZ-induced diabetic rats. Glucose buildup in the urine can be contributed by the breakdown of non-carbohydrate substrates (e.g., pyruvate, lactate, and glycerol) via gluconeogenesis, the degradation of glycogen via glycogenolysis, or the breakdown of carbohydrates from the diet [14]. Moreover, glycosuria also indicates impairment in insulin function and sensitivity, as high glucose levels should trigger the release of insulin that is supposed to lower the glucose level, which did not happen in this case.
Higher lactate in urine also indicates disturbance of pyruvate metabolism, where pyruvate is converted to lactate instead of being converted to acetyl-CoA, resulting in lower TCA intermediates in the OB and diabetic groups. Theoretically, the conversion of pyruvate to acetyl-CoA was accomplished by the pyruvate dehydrogenase complex (PDC) and the pyruvate complex (PC) in the mitochondria under aerobic conditions [27]. However, in an anaerobic condition, lactate dehydrogenase (LDH) catalyzed the reduction of pyruvate to lactate [28].
It is unclear how cellular respiration affects diabetes progression in the OB and diabetic groups. However, it was previously found that the overexpression of the LDH enzyme, especially LDH-A, can lead to perturbation in β-cell mitochondria and insulin secretion [28]. It was also reported that PDC deficiency can impair glucose-stimulated insulin secretion in a β-cell-specific PDH deficiency (β-PDHKO) mouse model [27]. Therefore, the results suggested that high anaerobic respiration occurs in OB and diabetic rats, which leads to the conversion of pyruvate to lactate. However, further studies are needed with regard to correlates of anaerobic respiration, enzymes, and their effect on OB and diabetic rats.
Metabolites Involved in Lipid Metabolism
Acetate is one of the intermediary metabolites involved in glucose metabolism (glycolysis and gluconeogenesis), fatty acid β-oxidation, and glycerophospholipid metabolism [15]. Increases in the level of urine acetate in the OB and OB + STZ groups can be a sign of accumulation of phospholipids in the blood, which was also supported by a lipid profile test (LDL and TG) result of the serum biochemistry. A similar outcome has also been reported by Zhao et al. [29] and Diao et al. [3], where they found increased levels of lipid profiles after injection with STZ.
The accumulation of lipids in the body has been associated with reduced mitochondrial density and mitochondrial dysfunction in insulin-resistant individuals with T2DM [30]. This finding is also supported by the above results, where low levels of TCA intermediates were found in OB, ND + STZ, and OB + STZ. As mitochondria is one of the sites where ATP production happens via the TCA cycle, the results show that the diabetic condition not only causes lipid accumulation but also leads to the deterioration of the mitochondrial density and function that later affects energy metabolism.
Ketone bodies (3-HB, AcAc, and acetone) are the metabolic products of lipid metabolism in the liver mitochondria [31]. Higher 3-HB and AcAc production in ND + STZ as opposed to OB + STZ suggests that mitochondrial dysfunction and ketosis are more severe in ND + STZ than in OB + STZ. It is well known that ketosis usually occurs in T1DM rather than in T2DM [3, 11]. The accumulation of 3-HB and AcAc is due to the β-oxidation of free fatty acids (FFAs) that leads to the production of acetyl-CoA, which is later converted to ketone bodies [32]. Therefore, this result suggested that the metabolite characteristics of the ND + STZ group are more closely related to T1DM. This suggestion is also in line with the previous result where we observed that OB + STZ was more sensitive towards metformin than was ND + STZ. In summary, the diabetic condition affected not only glucose and energy metabolism but also lipid metabolism. Limited energy availability due to the lack of absorption of glucose in the skeletal muscle and tissues has caused the breakdown of fats via gluconeogenesis and fatty acid β-oxidation, thus resulting in the accumulation of lipids (TC and TG) and free fatty acids [3].
Metabolites Involved in Amino Acid Metabolism
Amino acids play crucial roles in cellular function, including the building blocks of proteins, participating in cellular growth, repairing damaged cells, and being involved in lipid synthesis [33]. From the result, a high level of amino acids such as leucine and lysine can be noted from OB and OB + STZ (p < 0.05). Previous cohort studies conducted by Newgard et al. [34] and Würtz et al. [33] have concluded that several aromatic and branched-chain amino acids (BCAAs), such as leucine and valine, could contribute to insulin resistance in obese and young adults. High levels of BCAAs can interfere with insulin signaling through the stimulation of mTOR-S6K1 (protein and enzyme complex) and the phosphorylation of insulin receptor substrate 1 protein (IRS-1) on its serine residues [35, 36]. Therefore, BCAAs have been considered as biomarkers for predicting DM risk in an obese individual [34].
In addition to BCAAs, changes in TRP metabolism can also be noted in both diabetic and OB rats. Low levels of TRP in OB and both diabetic groups indicate that most of the TRPs have been converted to KYN. In the body, 95% of the major metabolic pathways involved in TRP metabolism are KYN pathways. KYN then further goes through two different pathways, which are the kynurenine-kynurenine acid (KYN-KYNA) pathway and the kynurenine-nicotinamide adenine dinucleotide (KYN-NAD) pathway, where NAD was the end product of the KYN-NAD pathway [37]. Numerous studies have reported that the accumulation of endogenous metabolites or kynurenines from KYN pathways can cause cell damage to the organism, which includes neurological disorders, psychiatric disturbances, and systemic disorders such as hypertension, osteoporosis, lipid metabolism disorder, and anemia [37, 38]. Among them, kynurenines such as 3-hydroxyanthranilic acids (3-HAAs) and quinolinic acids (QAs) have been known to induce apoptosis, and a high accumulation of l-kynurenine and QA has been linked to renal insufficiency [39, 40].
Metabolites Involved in Gut Microbiota Metabolism
Recently, changes in the gut microbiota have been linked to obesity and diabetes progression [41]. Hippurate is a normal endogenous urinary metabolite that has long been associated with the gut microbial degradation of certain dietary components [42]. Meanwhile, trigonelline is a by-product of niacin metabolism (vitamin B3) that is later excreted in urine [15]. The urinary levels of hippurate and trigonelline in our study were significantly decreased in Hfd rats compared to Nd rats, ND and ND + STZ. It has been reported that the long-term consumption of Hfd, which is low in dietary fiber, can cause an alteration in the gut microbiome [43]. This finding is in agreement with Won et al. [41], where it was also noted that hippurate levels were significantly decreased in the leptin-deficient (ob/ob) mouse.
Metabolites Involved in Other Metabolisms
Creatinine and creatine are metabolized in the kidney, muscle, liver, and pancreas [15]. It is well known that creatinine and creatine are highly associated with each other as creatine is converted to creatinine before being transported out from the muscle and excreted in urine [44]. The decreased level of creatinine in the OB + STZ and ND + STZ groups also implies reduced levels of creatine in these groups. This decrease might be due to several factors, including creatine reabsorption by the kidney, cell leakage, and changes in the muscle mass and caloric intake (low protein diet) [28]. Previously, Diao et al. [3] and Zhao et al. [28] also found that STZ-induced diabetic rats have creatine depletion, and Zhao et al. [28] suggested that altered renal tubular function and morphology might be one of the causes. This suggestion was further supported by the increased urinary allantoin excretion in the diabetic rats, which indicates an increased glomerular filtration rate [45].
In addition to creatinine, other interesting metabolites that should be highlighted here include allantoin, the end product of the oxidation of uric acid from purine metabolism [46]. Allantoin has been considered one of the oxidative stress markers as it also can be produced through non-enzymatic processes, especially when the level of reactive oxygen species (ROS) is high [40]. High ROS have been associated with diabetes progression and kidney dysfunction [29]. In this study, increased allantoin level was observed in the OB rats followed by OB + STZ and ND + STZ. This result indicates that ROS production might be influenced by dietary effects as the rats fed with Hfd had higher allantoin levels than the Nd-fed rats.
A low concentration of DMA was observed in OB, OB + STZ, and ND + STZ and indicates perturbations in methylamine metabolism. Acting as osmoregulatory compounds, methylamines are produced from the degradation of dietary choline to trimethylamine (TMA) and di- and mono-amine metabolites by gut microbiota [47]. In addition, polyamines also can be produced from choline breakdown into betaine and dimethylglycine (DMG). Therefore, perturbations of methylamine can also reflect the choline availability in the body. The accumulation of liver fat has been observed in choline-deficient Hfd mice [48], and the increased serum lipid in the OB, OB + STZ, and ND + STZ groups seems to support this.
Conclusions
Metabolite changes in OB, ND + STZ, and OB + STZ vary from each other. After diabetes induction, metabolic disturbance can be seen in carbohydrate, amino acid, and lipid metabolism. In both OB + STZ and ND + STZ, metabolites such as glucose were found to be higher, while creatinine was lower. In comparisons between these two groups, we found that metabolites such as ketone bodies were higher in ND + STZ, and OB + STZ had more BCAA (leucine). It was also found that the OB + STZ group was sensitive towards a T2DM drug. All these results suggest that ND + STZ is similar to the T1DM model, and OB + STZ is similar to the T2DM model. In addition, several similarities in metabolites between OB, ND + STZ, and OB + STZ were also highlighted, such as acetate, DMA, TRP, lysine, hippurate, and TCA intermediates, which emphasizes the link between obesity and DM. The dietary effect also influenced the metabolite levels of OB and OB + STZ, where the metabolite concentration of BCAA was higher and trigonelline was lower compared to the other groups.
References
World Health Organization (2015) Diabetes: the cost of diabetes. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/. Accessed July 30.
World Health Organization (2002), Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus.
Diao, C., Zhao, L., Guan, M., Zheng, Y., Chen, M., Yang, Y., Lin, L., Chen, W., & Gao, H. (2014). Systemic and characteristic metabolites in the serum of streptozotocin-induced diabetic rats at different stages as revealed by a 1H-NMR based metabonomic approach. Molecular BioSystems, 10, 686–693.
Rubin, A. L. (2012) Diabetes for dummies. 2ed. Wiley.
Sebedio, J. L., Pujos-Guillot, E., & Ferrara, M. (2009). Metabolomics in evaluation of glucose disorders. Current Opinion in Clinical Nutrition and Metabolic Care, 12, 412–418.
Honardoost, M., reza Sarookhani, M., Arefian, E., & Soleimani, M. (2014). Insulin resistance associated genes and miRNAs. Applied Biochemistry and Biotechnology, 174, 63–80.
Rees, D. A., & Alcolado, J. C. (2005). Animal models of diabetes mellitus. Diabetic Medicine: A Journal of the British Diabetic Association, 22, 359–370.
Chatzigeorgiou, A., Halapas, A., Kalafatakis, K. and Kamper, E. (2009). The use of animal models in the study of diabetes mellitus. In vivo (Athens, Greece), 23, 245–258.
Srinivasan, K., & Ramarao, P. (2007). Animal models in type 2 diabetes research: an overview. The Indian Journal of Medical Research, 125, 451–472.
Kumar, R., Sharma, B., Tomar, N. R., Roy, P., Gupta, A. K., & Kumar, A. (2011). In vivo evaluation of hypoglycemic activity of Aloe spp. and identification of its mode of action on GLUT-4 gene expression in vitro. Applied Biochemistry and Biotechnology, 164, 1246–1256.
Zhang, S., Nagana Gowda, G. A., Asiago, V., Shanaiah, N., Barbas, C., & Raftery, D. (2008). Correlative and quantitative 1H NMR-based metabolomics reveals specific metabolic pathway disturbances in diabetic rats. Analytical Biochemistry, 383, 76–84.
Srinivasan, K., Viswanad, B., Asrat, L., Kaul, C. L., & Ramarao, P. (2005). Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacological Research, 52, 313–320.
Skovsø, S. (2014). Modeling type 2 diabetes in rats using high fat diet and streptozotocin. Journal of Diabetes Investigation.
Zhao, L. C., Zhang, X. D., Liao, S. X., Wang, H. Y., Lin, D. H., & Gao, H. C. (2010). A metabonomic comparison of urinary changes in Zucker and GK rats. Journal of Biomedicine and Biotechnology, 1–6.
Wishart, D., Knox, C., Guo, A., Eisner, R., Young, N., Gautam, B., Hau, D., Psychogios, N., Dong, E., Bouatra, S., Mandal, R., Sinelnikov, I., Xia, J., Jia, L., Cruz, J., Lim, E., Sobsey, C., Shrivastava, S., Huang, P., Liu, P., Fang, L., Peng, J., Fradette, R., Cheng, D., Tzur, D., Clements, M., Lewis, A., De Souza, A., Zuniga, A., Dawe, M., Xiong, Y., Clive, D., Greiner, R., Nazyrova, A., Shaykhutdinov, R., Li, L., Vogel, H., & Forsythe, I. (2009). HMDB: a knowledgebase for the human metabolome. Nucleic Acids Research, 37, 603–610.
Eriksson, L., Johansson, E., Kettaneh-Wold, N., Wold, S., Trygg, J. and Wikstrom, C. (2006). Multi- and megavariate data analysis part I: basic principles and applications (2nd ed.) ed. Umetrics Academy (Appendix I), Sweden.
An, L., Shi, Q., & Feng, F. (2015). Metabolomics approach to identify therapeutically potential biomarkers of the Zhi-Zi-Da-Huang decoction effect on the hepatoprotective mechanism. RSC Advances, 5, 84048–84055.
Song, X., Wang, J., Wang, P., Tian, N., Yang, M., & Kong, L. (2013). 1H NMR-based metabolomics approach to evaluate the effect of Xue-Fu-Zhu-Yu decoction on hyperlipidemia rats induced by high-fat diet. Journal of Pharmaceutical and Biomedical Analysis, 78-79, 202–210.
Wheelock, A. M., & Wheelock, C. E. (2013). Trials and tribulations of ’omics data analysis: assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Molecular BioSystems, 9, 2589–2596.
Quaile, M. P., Melich, D. H., Jordan, H. L., Nold, J. B., Chism, J. P., Polli, J. W., Smith, G. A., & Rhodes, M. C. (2010). Toxicity and toxicokinetics of metformin in rats. Toxicology and Applied Pharmacology, 243, 340–347.
Inzucchi, S. E. (2002). Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA: The Journal of the American Medical Association, 287, 360–372.
Wallymahmed, M. (2006). Insulin therapy in the management of type 1 and type 2 diabetes. Nursing standard (Royal College of Nursing (Great Britain): 1987), 21, 50–56; quiz 58.
Khazrai, Y. M., Defeudis, G., & Pozzilli, P. (2014). Effect of diet on type 2 diabetes mellitus: a review. Diabetes Metabolism Research and Reviews, 30(1), 24–33.
Xia, J., Sinelnikov, I. V., Han, B. and Wishart, D. S. (2015). MetaboAnalyst 3.0 making metabolomics more meaningful. Nucleic Acids research, 43, 1–7.
Jiang, C. Y., Yang, K. M., Yang, L., Miao, Z. X., Wang, Y. H., & Zhu, H. B. (2013). A H NMR-based metabonomic investigation of time-related metabolic trajectories of the plasma, urine and liver extracts of hyperlipidemic hamsters. PloS One, 8, e66786.
Xu, J., Liu, C., Cai, S., Dong, J., Li, X., Feng, J., & Chen, Z. (2013). Metabolomic profilings of urine and serum from high fat-fed rats via 1H NMR spectroscopy and pattern recognition. Applied Biochemistry and Biotechnology, 169, 1250–1261.
Srinivasan, M., Choi, C. S., Ghoshal, P., Pliss, L., Pandya, J. D., Hill, D., Cline, G., & Patel, M. S. (2010). β-Cell-specific pyruvate dehydrogenase deficiency impairs glucose-stimulated insulin secretion. American Journal of Physiology - Endocrinology and Metabolism, 299, 910–917.
Ainscow, E. K., Zhao, C., & Rutter, G. A. (2000). Acute overexpression of lactate dehydrogenase-A perturbs beta-cell mitochondrial metabolism and insulin secretion. Diabetes, 49, 1149–1155.
Zhao, L., Gao, H., Lian, F., Liu, X., Zhao, Y., & Lin, D. (2011). 1H-NMR-based metabonomic analysis of metabolic profiling in diabetic nephropathy rats induced by streptozotocin. American Journal of Physiology- Renal Physiology, 300, 947–956.
Befroy, D. E., Petersen, K. F., Dufour, S., Mason, G. F., de Graaf, R. A., Rothman, D. L., & Shulman, G. I. (2007). Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes, 56, 1376–1381.
Dedkova, E. N., & Blatter, L. A. (2014). Role of beta-hydroxybutyrate, its polymer poly-beta-hydroxybutyrate and inorganic polyphosphate in mammalian health and disease. Frontiers in Physiology, 5, 260.
Filla, L. A., Yuan, W., Feldman, E. L., Li, S., & Edwards, J. L. (2014). Global metabolomic and isobaric tagging capillary liquid chromatography–tandem mass spectrometry approaches for uncovering pathway dysfunction in diabetic mouse aorta. Journal of Proteome Research, 13, 6121–6134.
Wurtz, P., Soininen, P., Kangas, A. J., Ronnemaa, T., Lehtimaki, T., Kahonen, M., Viikari, J. S., Raitakari, O. T., & Ala-Korpela, M. (2013). Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care, 36, 648–655.
Newgard, C. B., An, J., Bain, J. R., Muehlbauer, M. J., Stevens, R. D., Lien, L. F., Haqq, A. M., Shah, S. H., Arlotto, M., Slentz, C. A., Rochon, J., Gallup, D., Ilkayeva, O., Wenner, B. R., Yancy Jr., W. S., Eisenson, H., Musante, G., Surwit, R. S., Millington, D. S., Butler, M. D., & Svetkey, L. P. (2009). A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabolism, 9, 311–326.
Tremblay, F., Krebs, M., Dombrowski, L., Brehm, A., Bernroider, E., Roth, E., Nowotny, P., Waldhausl, W., Marette, A., & Roden, M. (2005). Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes, 54, 2674–2684.
Krebs, M., Brunmair, B., Brehm, A., Artwohl, M., Szendroedi, J., Nowotny, P., Roth, E., Furnsinn, C., Promintzer, M., Anderwald, C., Bischof, M., & Roden, M. (2007). The mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes, 56, 1600–1607.
Unluturk, U. and Erbas, T. (2015), in Tryptophan metabolism: implications for biological processes, health and disease: molecular and integrative toxicology (Engin, A. and Engin, A. B., eds.), Springer International Publishing, pp. 147–171.
Stone, T. W., & Darlington, L. G. (2007). Pharmacology of the kynurenine pathway. International Congress Series, 1304, 298–304.
Pawlak, D., Pawlak, K., Malyszko, J., Mysliwiec, M., & Buczko, W. (2001). Accumulation of toxic products degradation of kynurenine in hemodialyzed patients. International Urology and Nephrology, 33, 399–404.
Saito, K., Fujigaki, S., Heyes, M. P., Shibata, K., Takemura, M., Fujii, H., Wada, H., Noma, A., & Seishima, M. (2000). Mechanism of increases in l-kynurenine and quinolinic acid in renal insufficiency. American Journal of Physiology - Renal Physiology, 279, 565–572.
Won, E. Y., Yoon, M. K., Kim, S. W., Jung, Y., Bae, H. W., Lee, D., Park, S. G., Lee, C. H., Hwang, G. S., & Chi, S. W. (2013). Gender-specific metabolomic profiling of obesity in leptin-deficient ob/ob mice by 1H NMR spectroscopy. PloS One, 8, e75998.
Lees, H. J., Swann, J. R., Wilson, I. D., Nicholson, J. K., & Holmes, E. (2013). Hippurate: the natural history of a mammalian-microbial cometabolite. Journal of Proteome Research, 12, 1527–1546.
Kim, S. H., Yang, S. O., Kim, H. S., Kim, Y., Park, T., & Choi, H. K. (2009). 1H-nuclear magnetic resonance spectroscopy-based metabolic assessment in a rat model of obesity induced by a high-fat diet. Analytical and Bioanalytical Chemistry, 395, 1117–1124.
Wyss, M., & Kaddurah-Daouk, R. (2000). Creatine and creatinine metabolism. Physiological Reviews, 80, 1107–1213.
Briggs, J. P., Levitt, M. F., & Abramson, R. G. (1977). Renal excretion of allantoin in rats: a micropuncture and clearance study. American Journal of Physiology - Renal Physiology, 233, 373–381.
Liu, G., Yang, G., Fang, T., Cai, Y., Wu, C., Wang, J., Huang, Z., & Chen, X. (2014). NMR-based metabolomic studies reveal changes in biochemical profile of urine and plasma from rats fed with sweet potato fiber or sweet potato residue. RSC Advances, 4, 23749–23758.
Asatoor, A. M., & Simenhoff, M. L. (1965). The origin of urinary dimethylamine. Biochimica et Biophysica Acta, 111, 384–392.
Raubenheimer, P. J., Nyirenda, M. J., & Walker, B. R. (2006). A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes, 55, 2015–2020.
Acknowledgement
This work was supported by the Research University Grant from Universiti Putra Malaysia (Grant No. 9362200). The first author acknowledges the support from the Ministry of Education Malaysia for a scholarship under the MyBrain Science Scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Approval for the animal study was obtained from the Institutional Animal Care and Use Committee (IACUC) of the Faculty Medicine and Health Sciences, Universiti Putra Malaysia (IACUC No. UPM/FPSK/PADS/BRUUH/00490).
Electronic supplementary material
Figure S1
(DOCX 450 kb)
Figure S2
(DOCX 23 kb)
Figure S3
(DOCX 82 kb)
Figure S4
(DOCX 104 kb)
Figure S5
(DOCX 97 kb)
Figure S6
(DOCX 57 kb)
Figure S7
(DOCX 41 kb)
Figure S8
(DOCX 41 kb)
Figure S9
(DOCX 41 kb)
Figure S10
(DOCX 40 kb)
Figure S11
(DOCX 40 kb)
Figure S12
(DOCX 38 kb)
Figure S13
(DOCX 42 kb)
Figure S14
(DOCX 43 kb)
Figure S15
(DOCX 43 kb)
Figure S16
(DOCX 43 kb)
Table S1
(DOCX 14 kb)
Table S2
(DOCX 13 kb)
Table S3
(DOCX 13 kb)
Table S4
(DOCX 13 kb)
Table S5
(DOCX 14 kb)
Table S6
(DOCX 13 kb)
Table S7
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Abu Bakar Sajak, A., Mediani, A., Maulidiani et al. Metabolite Variation in Lean and Obese Streptozotocin (STZ)-Induced Diabetic Rats via 1H NMR-Based Metabolomics Approach. Appl Biochem Biotechnol 182, 653–668 (2017). https://doi.org/10.1007/s12010-016-2352-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2352-9