Abstract
Diabetes has been cited as the most challenging health problem in the twenty-first century. Accordingly, it is urgent to develop a new type of efficient and low-toxic antidiabetic medication. Since vanadium compounds have insulin-mimetic and potential hypoglycemic activities for type 1 and type 2 diabetes, a new trend has been developed using vanadium and organic ligands to form a new compound in order to increase the intestinal absorption and reduce the toxicity of vanadium compound. In the current investigation, a new organic vanadium compounds, vanadyl rosiglitazone, was synthesized and determined by infrared spectra. Vanadyl rosiglitazone and three other organic vanadium compounds were administered to the diabetic mice through oral administration for 5 weeks. The results of mouse model test indicated that vanadyl rosiglitazone could regulate the blood glucose level and relieve the symptoms of polydipsia, polyphagia, polyuria, and weight loss without side effects and was more effective than the other three organic vanadium compounds including vanadyl trehalose, vanadyl metformin, and vanadyl quercetin. The study indicated that vanadyl rosiglitazone presents insulin-mimetic activities, and it will be a good potential candidate for the development of a new type of oral drug for type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a chronic systemic metabolic disease which results from the interaction of genetic and environmental factors [1]. A continuous high blood glucose caused by the defect of insulin secretion and insulin resistance is the main characterization of this disease [2, 3]. Thus, a series metabolism disorders of fat, protein, and carbohydrate would occur, and probably result in serious complications and sequelae, such as arteriosclerosis, capillary lesion, nephropathy, cardiopathy, and cataract [4, 5]. As we all know, diabetes could be mainly classified to four kinds, including type 1 diabetes, type 2 diabetes, gestational diabetes, and other diabetes. Recently, a clinical data showed that 2∼3 % women would catch diabetes during the gestation period, and nearly 35 % pregnant women might appear symptoms of gestational diabetes, these women would possibly develop to type 2 diabetes [6]. Particularly, more than 90 % of all diabetic people were type 2 diabetes [7]. Type 2 diabetes has become a major risk factor to people’s health. Moreover, the rapid economic growth and changes in lifestyle contribute to the development of this disease. It is reported that the financial losses caused by diabetes and related complications will add up to $557.7 billion in China from 2005 to 2015 [8]. Various treatments have been available for type 2 diabetes patients; however, traditional antidiabetic drugs could not fundamentally cure the disease due to its complex disease process; consequently, the sufferers have to take a lifelong treatment. In order to ease the pain of the patients and improve the quality of their lives, it is significant to develop a new kind of drug with higher efficacy and less side effects.
Vanadium is a transition metal, and it has a number of oxidation states between −1 and +5. As a micronutrients, vanadium was mainly provided by the daily diet including soybean oil, olive oil, peanut oil, mushrooms, shellfish, spinach, gelatin, and skim milk powder, and the requirement of vanadium for human body is about 100 μg day−1 [9]. Vanadium could be distributed in various organs and tissues after absorption, such as liver, kidney, heart, muscle, and skeleton [10]. Although excessive accumulation of vanadium may induce a disorder of the cardiovascular system, degeneration of the renal tubules, congestion in the liver, and intestinal inflammation, moderate vanadium exhibit beneficial impact. Specifically, it has been reported that vanadium derivatives showed biological and pharmacological properties. In particular, vanadium compounds have been demonstrated to be effective to treat asthma, dermatosis, and cardiovascular disease. They also showed a positive effect on decreasing the glucose level [11].
Sodium vanadate, an inorganic vanadium compound, has been demonstrated to be a potent stimulator of glucose transport and promote oxygenolysis in adipocyte, also inhibit the hydrolysis of adipose [12]. It was also reported to lower the plasma glucose [13]. These results showed an insulin-like activity of vanadium. In addition, vanadyl sulfate hydrate could also prevent cardiopathy, cataract, and other complications that associated with diabetes. However, there are limitations for inorganic vanadium compounds to be applied to clinical therapy because of their toxic effects.
It has been reported that organic vanadium compounds presented a better lipophilicity and absorbability [14]. Thus, it has become a highlight to synthesize various organic vanadium compounds. Bis (3-hydroxy-2-methyl-4-pyronato) oxovanadium (IV) (BMOV) is one of the organic vanadium complexes that have been well studied. Its therapeutic effect was twice higher than vanadyl sulfate, and the plasma glucose could be restored to normal level at the first day of administration [15, 16]. On the other hand, vanadyl acetylacetonate showed a higher insulin-like activity compared with inorganic vanadium salts and BMOV, without significant toxic effects [17].
As organic vanadium compounds presented enormous advantages, we attempted to develop a new kind of organic vanadium compounds. In the current investigation, we synthesized vanadyl rosiglitazone, an organic vanadium compound, and compared its hypoglycemic effect with three other organic vanadium complexes including vanadyl trehalose, vanadyl metformin, and vanadyl quercetin.
Methods
Materials
All chemical reagents are at analytical grade. Vanadyl sulfate was obtained from Alfa Aesar (Tianjin); trehalose, metformin, rosiglitazone, and quercetin were acquired from Ximake Biotech (Tianjin); ethanol and methanol were got from Tianjin chemical reagent factory; citric acid and streptozotocin were purchased from Sigma (USA).
Animals
Three-week-old male Kunming (KM) mice (weighing 16.50–20.30 g) were provided by the Laboratory Animal Center of the Academy of Military Medical Sciences of China (animal license number SCXK-2007-005). Mice were housed in a room with a 12:12-h artificial light cycle, a temperature of 20 °C ± 2 °C, and a humidity of 50 ± 5 %. The mice in normal control group were fed a standard diet. The rest of the mice were fed a high-fat and high-sucrose diet (food composition 18 % lard, 20 % sucrose, 3 % whole milk powder, 4 % yolk, 55 % basic feed). All procedures were conducted in accordance with the guidelines of the Chinese Council on Animal Care as well as the University of Nankai Animal Care Committee.
The Synthesis of Vanadyl Rosiglitazone
Rosiglitazone (0.15 g) was dissolved in 60 mL ethanol and then heated in water bath in a flask equipped with a reflux unit. A liquor of vanadyl sulfate (1.29 mmol, 50 mL) was mixed into the flask. Finally, dark green solid was formed. Separate the solid after cooling down and wash it three times with water and ethanol, respectively. The vanadyl rosiglitazone powder was acquired after dried in a vacuum desiccator.
The Synthesis of Vanadyl Trehalose
The synthesis of vanadyl trehalose was carried out in the light of the method by Barrio et al. [18]. The solution of vanadyl sulfate (2 mmol, 20 mL) was dropped to an aqueous solution of trehalose (4 mmol, 10 mL). The pH of the solution was adjusted to 13 by an addition of NaOH (2 M). The brown miscible liquid was sealed for 12–24 h at room temperature. Upon the addition of absolute ethanol, a microcrystalline powder was formed. Filter and wash the solid with absolute ethanol. Dry and store the hygroscopic sodium salt in a desiccator.
The Synthesis of Vanadyl Metformin
The synthesis of vanadyl metformin was achieved based on the method of Woo et al. [19]. Vanadyl sulfate trihydrate (0.115 M, 30 mL) was added dropwise to the solution of metformin (1.23 M, 7.5 mL) under a stream of argon. The mixture turned from gray to brown when NaOH (2 M) was added and adjusted pH value to 11. Green sediment was filtered out after stirring and washed with water and ether, then dried and stored the powder in an oven.
The Synthesis of Vanadyl Quercetin
The synthesis of vanadyl quercetin was conducted according to the method of Ferrer et al. [20]. An aqueous solution of vanadyl sulfate (1.72 mmol, 30 mL) was mixed to a solution of quercetin (0.2 g quercetin dissolved in 50 mL methanol). After refluxing for 3 h, a green solid precipitated. The product was filtered out and washed with methanol, lastly dried overnight in an oven.
Structure Analysis
IR spectra was recorded in KBr pellets with a Fourier transform infrared (JASCO FTIR-4100) spectrophotometer.
Construction of Type 2 Diabetic Mice Model
The study was performed after the mice were allowed to acclimate for 1 week. Eight mice were selected randomly as the normal control group and fed with a standard diet. The rest of the mice were fed with a high-fat and high-sugar diet for 4 weeks. After 4 weeks, all of the mice except for the normal control group were injected with STZ (100 mg kg−1 body weight, BW) intraperitoneally after fasting for 12 h, while the normal control mice were given equal volume of citrate buffer. Fasting blood glucose (FBG) was measured after 1 month, the mice of FBG >11.1 mmol L−1 were considered as type 2 diabetic mice [7, 21, 22].
Group and Drug Delivery
The type 2 diabetic mice were divided into the following six groups: the vanadyl rosiglitazone group (n = 8), the vanadyl trehalose group (n = 8), the vanadyl metformin group (n = 8), the vanadyl quercetin group (n = 8), the positive control group (n = 8, treated with rosiglitazone), and the negative control group (n = 8, treated with saline). All drug candidates were administered by oral gavage in a volume of 0.1 mL/10 g BW at a dose of 80 mg kg−1 BW.
Measurement of Body Weight, Food Consumption, and Drink Level
General clinical appearance and mortality of all groups were observed daily. Food and water consumption and body weight of all groups were also examined weekly.
Examination of Blood Glucose
The FBG levels were measured via tail vein blood by using a glucometer (ONETOUCH UltraEasy) weekly.
Statistical Analysis
The results were presented as means ± standard deviation (X ± SD). Statistical analysis was performed with the one-way analysis of variance (ANOVA) test of SPSS 17.0 for Windows and Duncan’s new multiple range test. Differences were considered to be statistically significant when p < 0.05 and p < 0.01.
Results
Synthesis and Infrared Spectroscopic Analysis of Vanadyl Rosiglitazone
The vanadyl rosiglitazone, an atrovirens powder, was synthesized as described above.
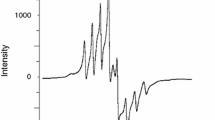
Infrared (IR) spectra of rosiglitazone and vanadyl rosiglitazone are shown in Fig. 1a, b. There were significant differences between rosiglitazone and vanadyl rosiglitazone in the spectra. The absorption peak of N-H of rosiglitazone located at 3416 cm−1. The location of 2937, 1330, and 1311 cm−1 represented the stretching vibration and bending vibration of methyl and methylene in rosiglitazone. The carbonyl group of acylamide in rosiglitazone was located at 1694 cm−1. The location of thioester of rosiglitazone was at 1738 cm−1. In rosiglitazone, the characteristic absorption peaks of aromatic ring skeleton were 1611 and 1510 cm−1. In addition, the peak at 1246 cm−1 represented the aryl ether in rosiglitazone. On the other hand, the absorption peak at 3503 cm−1 in vanadyl rosiglitazone described the vibration absorption of oxygen hydrogen bond, which demonstrated the generation of oxhydryl in the complex. The disappearance of the peak at 3416 cm−1 in vanadyl rosiglitazone identified the participation in the complex reaction of N-H. The bands at 1414, 1465, 1511, and 1615 cm−1 were indicative of the aromatic ring skeleton, and the band at 1246 cm−1 represented aryl ether in vanadyl rosiglitazone. The locations of these groups were nearly the same as that in rosiglitazone, illustrating that aromatic ring skeleton and aryl ether were absent from the complexion with vanadyl sulfate. A vibration absorption peak of V=O presented at 978 cm−1, indicating the involvement of vanadyl in the complex. Thus, a new compound, vanadyl rosiglitazone, was formed. Some of the main chemical bonds corresponded with the wave number are summarized in Fig. 1c, d.
Synthesis of Vanadyl Trehalose, Vanadyl Metformin, and Vanadyl Quercetin
The vanadyl trehalose, vanadyl metformin, and vanadyl quercetin were synthesized as described above. In addition, their infrared spectroscopic had been studied [18–20].
Changes of Physiological Indexes After the Construction of Mice Model
Body Weight
Before the construction, the body weight of mice with high-fat and high-sugar diet (averagely 35.23 ± 0.28 g) was much higher than the normal control group (averagely 30.55 ± 0.37 g). There was a reversal in the trend of weight on the first week after the injection of STZ. The average weight of diabetic mice was 38.15 ± 0.17 g, while the normal control group showed 42.52 ± 0.33 g (Fig. 2a). There was an obvious decline of the body weight in the diabetic group.
Measurements of body weight, drink level, food consumption, and blood glucose before and after the construction of diabetic mice model. a Body weight; b drink level; c food consumption; d blood glucose (1, normal control before model construction; 2–10, different individuals after model construction). Data are the means ± standard deviation (n = 8). *p < 0.05, compared with before construction; **p < 0.01, compared with before construction
Food Ingestion and Drink Level
The drink level of the normal control group maintained a relative stable state, ranging from 7.60 ± 0.21 to 6.90 ± 0.47 mL. However, significant changes could be observed in the diabetic mice (p < 0.01), ranging from 7.03 ± 0.25 to 10.14 ± 1.47 mL (Fig. 2b).
The food ingestion showed a similar trend as the drink level. In the normal control group, the mean consumption ranged from 12.05 ± 0.19 to 12.60 ± 0.46 g, while the high-fat diet group ranged from 13.90 ± 0.45 to 16.80 ± 0.16 g (Fig. 2c).
Change of Plasma Glucose
Average plasma glucose level of mice was about 16.40 ± 5.08 mmol L−1 in the high-fat diet group mice, significantly higher than the regular diet group which remained at 5.04 ± 0.12 mmol L−1 (Fig. 2d).
Physical Condition of Mice
Mice of normal control group kept growing on the body weight with a normal degree of the drink level and urination. On the contrary, the diabetic mice exhibited polydipsia, polyphagia, polyuria, and weight loss.
Physiological Index Changes After Treatment of Organic Vanadium Compounds
Effect on Body Weight of Diabetic Mice
The body weight of normal control group showed a modest rise. Neither vanadyl rosiglitazone nor the other three organic vanadium compounds displayed sufficient improvement on the body weight when compared with the normal control (p < 0.01). There was no significant difference between vanadyl rosiglitazone and positive control group; on the other hand, the body weight of the other three organic vanadium compounds groups was markedly lower than the positive control group (P < 0.01). However, all the four organic vanadium treatment groups had an increase on the body weight in comparison with the negative group, especially the vanadyl rosiglitazone group (P < 0.01) (Fig. 3a).
Effects of organic vanadium compounds on physiological indexes after the 5-week treatment. a Body weight; b drink level; c food consumption; d blood glucose. The numbers at X-axis represent the different treatments: 1, normal control; 2, negative control; 3, positive control; 4, vanadyl rosiglitazone; 5, vanadyl trehalose; 6, vanadyl metformin; and 7, vanadyl quercetin (the same as the order of the columns from left to right). Data are the means ± standard deviation (n = 8). Results were analyzed by one-way ANOVA and Duncan’s new multiple range test. The numbers in Tn column represent the corresponding treatments to the X-axis. The different lowercase letters express the significant difference at p < 0.05 level, and the different majuscules express significant difference at p < 0.01 level
Effects of Organic Vanadium Compounds on Food Ingestion and Drink Level
Though still higher than the normal control group, the drink level was availably declined after the 5-week gavage of vanadyl rosiglitazone and the other three organic vanadium compounds in comparison with the negative control group (p < 0.01). Apparently, vanadyl rosiglitazone was the one which was the most effective as there were no significant differences comparing with positive control (p < 0.01) (Fig. 3b). Otherwise, the drink level of normal control group was smooth and steady. On the other hand, when compared with the negative control, the food consumption level had been decreased after the treatment of vanadyl rosiglitazone and the other three organic vanadium compounds (p < 0.01). Moreover, the food intake of vanadyl rosiglitazone group was similar to the positive control at the end of the fifth week (p < 0.01). Furthermore, no significant differences were found between the vanadyl rosiglitazone and normal control group (p < 0.01). Nevertheless, the normal control group showed an increasing trend (Fig. 3c).
Effects of Organic Vanadium Compounds on Blood Glucose
The blood glucose level of negative control was much higher than the normal control group (p < 0.01).
Though there are fluctuations on the trend of the blood glucose, vanadyl rosiglitazone exerted a remarkable influence and reduced the blood glucose significantly lower than the positive control at the end of the treatment (p < 0.01). Besides, the other three organic vanadium compounds showed a similar effect as the positive control. Moreover, the blood glucose level of vanadyl rosiglitazone group was the closest to the normal control in comparison with the other organic vanadium compounds (P < 0.01) (Fig. 3d). In addition, the blood glucose level did not rebound within a week after the drug withdrawal (data not shown).
Effects of Organic Vanadium on the Physical Condition of Mice
There was an improvement on the physical condition after the gavage with vanadyl rosiglitazone and the other three organic vanadium compounds, especially the vanadyl rosiglitazone. Moreover, there was no treatment-related mortality. All animals were killed by cervical decapitation under anesthesia at the end of the observation period, and there were no injuries observed in all organs after examined carefully for macroscopic abnormalities.
Discussion
It has been reported that the protective effects of vanadium on the pancreas islet B cells contribute to the insulin-mimetic activity of vanadium complexes [23]. Vanadium facilitates a series processes including glucose transport and metabolism, lipid synthesis, and protein synthesis in different cell types [24–28]. Besides, vanadate could conduce to the stimulation to the glucose uptake, glycogen synthase, glycolysis, and glucose oxidation in skeletal muscle [29–33]. The use of organic ligands could increase the lipophilicity of vanadium, improving its poor absorption in the gastrointestinal tract, and thereby decreasing the dose of vanadium required to produce its corresponding effects [14].
Diabetes is characterized by obvious polydipsia, polyphagia, polyuria, and weight loss. In the present study, we synthesized and detected the structure attribute of vanadyl rosiglitazone through organic and coordination chemistry method (Fig. 1). Vanadyl rosiglitazone and three other organic vanadium compounds were delivered to the mouse model through oral administration for 5 weeks. All diabetic mice except the negative control group had shown a relief of the symptoms through the 5-week treatment. Vanadyl rosiglitazone presented more effective on reducing the food and water consumption and suppressing the weight loss than the other three organic vanadium compounds used in this study (Fig. 3a–c). Correspondingly, the glucose-lowering ability of vanadyl rosiglitazone was not only better than the other three organic vanadium compounds but also a bit more effective than the positive control (Fig. 3d). All these data indicated that vanadyl rosiglitazone displayed insulin-mimetic effects and had an antidiabetic potential.
During the treatment, the mice kept a tendency of getting well. No side effects like diarrhea, nose bleeding, and dehydration, which usually caused by inorganic vanadium compounds, had been found in the treatments. Moreover, the blood glucose maintained on a stable level after a drug withdrawal (data not shown). This may due to the better lipophilicity and absorbability of organic vanadium compounds. The experimental evidence indicated that the vanadyl rosiglitazone could be a good potential candidate for therapeutic applications in the treatment of diabetes owing to its characteristics of insulin-mimetic and no side effects. However, further study is required to elucidate the mechanism of vanadyl rosiglitazone action.
Conclusion
In summary, we have synthesized vanadyl rosiglitazone, an organic vanadium compound, which presents insulin-mimetic activities. Vanadyl rosiglitazone could regulate the blood glucose level and relieve the symptoms of polydipsia, polyphagia, polyuria, and weight loss without obvious side effects at the same time. This may have provided an experimental basis for the development of a new type of oral drug for type 2 diabetes.
References
Canivell, S., & Gomis, R. (2014). Diagnosis and classification of autoimmune diabetes mellitus. Autoimmunity Reviews, 13, 403–407.
Whiting, D. R., Guariguata, L., Weil, C., & Shaw, J. (2011). IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Research and Clinical Practice, 94, 311–321.
Honardoost, M., Sarookhani, M. R., Arefian, E., & Soleimani, M. (2014). Insulin resistance associated genes and miRNAs. Applied Biochemistry and Biotechnology, 174, 63–80.
Pinhas-Hamiel, O., & Zeitler, P. (2007). Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet, 369, 1823–1831.
Stratton, I. M., Adler, A. I., Neil, H. A., Matthews, D. R., Manley, S. E., Cull, C. A., Hadden, D., Turner, R. C., & Holman, R. R. (2000). Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ, 321, 405–412.
Hassan, H. A., & El-Gharib, N. E. (2015). Obesity and clinical riskiness relationship: therapeutic management by dietary antioxidant supplementation—a review. Applied Biochemistry and Biotechnology, 176, 647–669.
Liu, Y., Gao, Z., Guo, Q., Wang, T., Lu, C., Chen, Y., Sheng, Q., Chen, J., Nie, Z., Zhang, Y., Wu, W., Lv, Z., & Shu, J. (2014). Anti-diabetic effects of CTB-APSL fusion protein in type 2 diabetic mice. Marine Drugs, 12, 1512–1529.
Morabia, A., & Abel, T. (2006). The WHO report “preventing chronic diseases: a vital investment” and us. Sozial- und Präventivmedizin, 51, 74.
Roy, S., Majumdar, S., Singh, A. K., Ghosh, B., Ghosh, N., Manna, S., Chakraborty, T., & Mallick, S. (2015). Synthesis, characterization, antioxidant status, and toxicity study of vanadium-rutin complex in Balb/c mice. Biological Trace Element Research, 166, 183–200.
Boden, G., Chen, X., Ruiz, J., van Rossum, G. D., & Turco, S. (1996). Effects of vanadyl sulfate on carbohydrate and lipid metabolism in patients with non-insulin-dependent diabetes mellitus. Metabolism, 45, 1130–1135.
Shechter, Y., Goldwaser, I., Mironchik, M., Fridkin, M., & Gefel, D. (2003). Historic perspective and recent developments on the insulin-like actions of vanadium; toward developing vanadium-based drugs for diabetes. Coordination Chemistry Reviews, 237, 3–11.
Heyliger, C. E., Tahiliani, A. G., & McNeill, J. H. (1985). Effect of vanadate on elevated blood glucose and depressed cardiac performance of diabetic rats. Science, 227, 1474–1477.
Collins, F. S., Green, E. D., Guttmacher, A. E., & Guyer, M. S. (2003). A vision for the future of genomics research. Nature, 422, 835–847.
Wang, J., Yuen, V. G., & McNeill, J. H. (2001). Effect of vanadium on insulin sensitivity and appetite. Metabolism - Clinical and Experimental, 50, 667–673.
Caravan, P., Gelmini, L., Glover, N., Herring, F. G., Li, H. L., McNeill, J. H., Rettig, S. J., Setyawati, I. A., Shuter, E., Sun, Y., Tracey, A. S., Yuen, V. G., & Orvig, C. (1995). Reaction chemistry of BMOV, bis(maltolato)oxovanadium(IV)—a potent insulin mimetic agent. Journal of the American Chemical Society, 117, 12759–12770.
Yuen, V. G., Orvig, C., & McNeill, J. H. (1995). Comparison of the glucose-lowering properties of vanadyl sulfate and bis(maltolato)oxovanadium(IV) following acute and chronic administration. Canadian Journal of Physiology and Pharmacology, 73, 55–64.
Poucheret, P., Verma, S., Grynpas, M. D., & McNeill, J. H. (1998). Vanadium and diabetes. Molecular and Cellular Biochemistry, 188, 73–80.
Barrio, D. A., Williams, P. A., Cortizo, A. M., & Etcheverry, S. B. (2003). Synthesis of a new vanadyl(IV) complex with trehalose (TreVO): insulin-mimetic activities in osteoblast-like cells in culture. Journal of Biological Inorganic Chemistry, 8, 459–468.
Woo, L. C. Y., Yuen, V. G., Thompson, K. H., McNeill, J. H., & Orvig, C. (1999). Vanadyl-biguanide complexes as potential synergistic insulin mimics. Journal of Inorganic Biochemistry, 76, 251–257.
Ferrer, E. G., Salinas, M. V., Correa, M. J., Naso, L., Barrio, D. A., Etcheverry, S. B., Lezama, L., Rojo, T., & Williams, P. A. (2006). Synthesis, characterization, antitumoral and osteogenic activities of quercetin vanadyl(IV) complexes. Journal of Biological Inorganic Chemistry, 11, 791–801.
Li, W., Zhang, M., Gu, J., Meng, Z. J., Zhao, L. C., Zheng, Y. N., Chen, L., & Yang, G. L. (2012). Hypoglycemic effect of protopanaxadiol-type ginsenosides and compound K on type 2 diabetes mice induced by high-fat diet combining with streptozotocin via suppression of hepatic gluconeogenesis. Fitoterapia, 83, 192–198.
Tu, P., Li, X., Ma, B., Duan, H., Zhang, Y., Wu, R., Ni, Z., Jiang, P., Wang, H., Li, M., & Zhu, J. (2015). Liver histone H3 methylation and acetylation may associate with type 2 diabetes development. Journal of Physiology and Biochemistry, 71, 89–98.
Cam, M. C., Rodrigues, B., & McNeill, J. H. (1999). Distinct glucose lowering and beta cell protective effects of vanadium and food restriction in streptozotocin-diabetes. European Journal of Endocrinology, 141, 546–554.
Jackson, T. K., Salhanick, A. I., Sparks, J. D., Sparks, C. E., Bolognino, M., & Amatruda, J. M. (1988). Insulin-mimetic effects of vanadate in primary cultures of rat hepatocytes. Diabetes, 37, 1234–1240.
Morita, T., Imagawa, T., Kanagawa, A., & Ueki, H. (1995). Sodium orthovanadate increases phospholipase A2 activity in isolated rat fat pads: a role of phospholipase A2 in the vanadate-stimulated release of lipoprotein lipase activity. Biological and Pharmaceutical Bulletin, 18, 347–349.
Maher, P. A. (1992). Stimulation of endothelial cell proliferation by vanadate is specific for microvascular endothelial cells. Journal of Cellular Physiology, 151, 549–554.
Barnes, D. M., Sykes, D. B., Shechter, Y., & Miller, D. S. (1995). Multiple sites of vanadate and peroxovanadate action in Xenopus oocytes. Journal of Cellular Physiology, 162, 154–161.
Hajjar, J. J., Fucci, J. C., Rowe, W. A., & Tomicic, T. K. (1987). Effect of vanadate on amino acid transport in rat jejunum. Proceedings of the Society for Experimental Biology and Medicine, 184, 403–409.
Nakai, M., Watanabe, H., Fujiwara, C., Kakegawa, H., Satoh, T., Takada, J., Matsushita, R., & Sakurai, H. (1995). Mechanism on insulin-like action of vanadyl sulfate: studies on interaction between rat adipocytes and vanadium compounds. Biological and Pharmaceutical Bulletin, 18, 719–725.
Duckworth, W. C., Solomon, S. S., Liepnieks, J., Hamel, F. G., Hand, S., & Peavy, D. E. (1988). Insulin-like effects of vanadate in isolated rat adipocytes. Endocrinology, 122, 2285–2289.
Shechter, Y., & Karlish, S. J. (1980). Insulin-like stimulation of glucose oxidation in rat adipocytes by vanadyl (IV) ions. Nature, 284, 556–558.
Tamura, S., Brown, T. A., Whipple, J. H., Fujita-Yamaguchi, Y., Dubler, R. E., Cheng, K., & Larner, J. (1984). A novel mechanism for the insulin-like effect of vanadate on glycogen synthase in rat adipocytes. Journal of Biological Chemistry, 259, 6650–6658.
McNeill, J. H., Yuen, V. G., Dai, S., & Orvig, C. (1995). Increased potency of vanadium using organic ligands. Molecular and Cellular Biochemistry, 153, 175–180.
Acknowledgments
This work is supported by the Key Technologies R&D Program of Tianjin (14ZCZDSY00013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, P., Dong, Z., Ma, B. et al. Effect of Vanadyl Rosiglitazone, a New Insulin-Mimetic Vanadium Complexes, on Glucose Homeostasis of Diabetic Mice. Appl Biochem Biotechnol 180, 841–851 (2016). https://doi.org/10.1007/s12010-016-2137-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2137-1