Abstract
The changes in total phenolics, flavonoids, tannins, valerenic acid, and antioxidant activity were assessed in 25 populations of Valeriana jatamansi sampled from 1200 to 2775 m asl and four habitat types of Uttarakhand, West Himalaya. Significant (p < 0.05) variations in total phenolics, flavonoids, valerenic acid, and antioxidant activity in aerial and root portions and across the populations were observed. Antioxidant activity measured by three in vitro antioxidant assays, i.e., 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic) (ABTS) radical scavenging, 2,2′-diphenyl-1-picryylhydrazyl (DPPH) free radical scavenging, and ferric-reducing antioxidant power (FRAP) assays, showed significant (p < 0.05) differences across the populations. However, no clear pattern was found in phytochemicals across the altitudinal range. Among habitat types, (pine, oak, mixed forest, and grassy land), variation in phytochemical content and antioxidant activity were observed. Equal class ranking, neighbor-joining cluster analysis, and principal component analysis (PCA) identified Talwari, Jaberkhet, Manjkhali, and Khirshu populations as promising sources with higher phytochemicals and antioxidant activity. The results recommended that the identified populations with higher value of phytochemicals and antioxidants can be utilized for mass multiplication and breeding program to meet the domestic as well as commercial demand.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Naturally occurring biologically active compounds in plants are known for their (i) potential disease-inhibiting capabilities; (ii) ability to adapt in adverse environmental conditions; (iii) resistance to pathogens and pests; and (iv) controlling factors for taste, color, aroma, etc. [1]. Considering their effectiveness in combating several diseases, studies on plant-derived natural products are receiving greater attention in different systems of medicine [2], and Himalayan medicinal plants are being considered the best prospect/option.
Valeriana jatamansi Jones (family Valerianaceae, commonly known as “Tagar” or “Indian valerian”) is an important plant of the genus Valeriana that remains a subject of interest due to its extensive use in traditional and modern medicines, variability in morphology, reproductive behavior, and source of active ingredients, i.e., valerenic acid. The species is dioecious, perennial, polygamous, or occasionally polygamo monocious and grows wild in temperate Himalayan region at 1000–3000 m above sea level (asl). Traditionally, the species is used in the treatment of several diseases like sleeping disorders, obesity, epilepsy, insanity, snake poisoning, and eye and skin problems [3]. In addition, the species is source of various secondary metabolites, e.g., valepotriates [4], dihydrovaltrate [5], linarin-isovalerianate [6], sesquiterpenoids, valerenic acid derivatives (valerenic acid, acetoxy valerenic, and hydroxyvalerenic acid) [7], 6-methylapigenin and hesperidin [8], valerenic acid [9], phenolics, and flavonoids [10]. Among these, valerenic acid and valeopotriates are often used for drug preparation [9, 11]. Besides, the species is reported to be used as a substitute of V. officinalis, one of the widely used medicinal plants of the genus [11].
It has been reported that environmental factors are known to influence the quality and quantum of secondary metabolites [12, 13]. For example, increase in altitude showed a decreasing trend in berberine content of Berberis asiatica [13]. In contrast, phenolic content increased with increasing altitude in Hedychium spicatum [14], etc. These studies are an indicative of the fact that responses of a species for secondary metabolites do affect by environmental factors. Studies on these aspects are not systematically investigated in V. jatamansi. Earlier studies on the variation in morphological and genetic diversity of V. jatamansi suggest that environmental and genetic factors influence the diversity of the plant and its chemical constituents [15, 16]. However, a systematic investigation on the response of the species for its secondary metabolites and antioxidant activity across altitudinal range and habitat conditions is poorly known. This is important because the species grow in different habitat conditions at wide altitudinal range, where microclimatic conditions like temperature, moisture regime, and soil nutrients varied considerably [17].
All these factors are reported to have direct bearing with secondary metabolites of a plant species [18]. Thus, the present study attempts to investigate the level of chemical constituents (valerenic acid, total phenolic, flavonoid, and tannin contents) and antioxidant activity in V. jatamansi collected from different habitat conditions (oak, pine, oak-mixed, and grassy land) across altitudinal range. The results of this study will help in identification of promising populations, suitable habitat condition, and altitude for harnessing potential of the species.
Materials and Methods
Study Area and Sampling
The samples (aerial and root portions of V. jatamansi) were collected during February 2009 from 25 populations of V. jatamansi in Uttarakhand (West Himalaya) region, India, representing diverse habitat conditions and altitudinal range (Table 1). Plants (four numbers) were randomly collected and divided in two portions (aerial and root portions) were dried in hot air oven at 40 °C and grounded to fine powder using Grinder mill (Macro Scientific, India). Immediately after grounding, the samples were processed for further analysis of total phenolics, flavonoids, and tannin estimation. Botanical identity of the species authenticated from Botanical Survey of India (BSI), Dehradun, and accession number of each population was obtained. Voucher specimens were deposited at herbaria of BSI and G.B. Pant Institute of Himalayan Environment and Development (GBPIHED), Kosi Katarmal, Almora, Uttarakhand, India (Table 1). Samples were collected from different altitudes (1201–1500, 1501–1800, 1801–2100, 2101–2400, and >2401 m asl) and habitat types (pine, oak, mixed forest, and grassy land) for investigating chemical constituents and antioxidant activity.
Extract Preparation for Phytochemical Analysis
Dried powder (50 mg) of each portion (aerial and root) extracted from each population was mixed separately with 25 ml 80 % (v/v) methanol, stirred gently (12 h), and sonicated (22 °C, 10 min; Model-ANIS 09001, Toshiba, New Delhi, India). The extract was centrifuged (10,000 rpm; 15 min, 22 ± 1 °C), and supernatants were collected, filtered, and stored at 4 °C prior to use for chemical analysis within 24 h.
Estimation of Total Phenolic, Flavonoid, and Tannin Contents
Total phenolic (TP), flavonoid (TF), and total tannin (TT) contents were determined following the methods used in earlier reports [10, 19].
Valerenic Acid and Antioxidant Activity
Valerenic acid was estimated using the method of Upton [20] in the ethanolic extract of aerial and root portions. Powdered material (10 mg) of plant portion (aerial and root) of each population was extracted in ethanol, and valerenic acid content was determined using external standard in a HPTLC system (Camag), equipped with an automatic TLC sampler (Linomat 5), TLC scanner 3, and integrated software WinCATS version 1.3.4 [21]. The antioxidant activity was determined using different in vitro methods such as 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic) (ABTS), 2,2-diphenyl-1-picryylhydrazyl (DPPH), and ferric-reducing antioxidant power (FRAP) method [10]. All the assays carried out in triplicates and average values were considered.
Prioritization and Elite Identification
Toward prioritization of suitable population for higher chemical constituents and antioxidant activity, value of each attributes in a population was classified into equal classes. The classification was done by subtracting the minimum value from the maximum and divided by 5 (Tables A1 and A2). These data were further subjected for principal component analysis (PCA) for identification of the promising populations using SPSS software. In the PCA, data set consisted of a 25 × 8 matrix (for phytochemicals) and 25 × 6 matrix (for antioxidants), where the results obtained for each parameter were adopted as columns and the Valeriana populations (samples) were used as the rows. Analyses were based on correlations and variances were computed as SS/(n − 1). Eigen values higher than 1.0 were adopted to explain the projection of the assays on the factor plane. All the data were auto scaled before the analysis, which means that each column data matrix was mean-centered and scaled to unit variance and avoid the effect of different scales of the variables [22].
Statistical Analysis
Analysis of variance and significant difference among means were tested using the Duncan’s multiple-range test by one-way ANOVA with SPSS program. Correlation coefficients (r) and coefficients of determination (R 2) were calculated using Microsoft Excel 2007. The independent sample (unpaired) t test was used to determine the significant difference in altitudinal range and habitat types. Toward prioritization of suitable population for phytochemical content and antioxidant activity, each population was classified into equal classes based on phytochemical content and antioxidant activity (sum of aerial and root portions). Neighbor-joining (NJ) cluster analysis was performed using PAST (Paleontological Statistics Software Package for Education and Data Analysis) packages [23] for classifying distant populations of V. jatamansi based on all studied secondary metabolites. PCA was performed using SPSS software version 16 to identify the major population groups with higher activity.
Results
Phytochemical Assessment
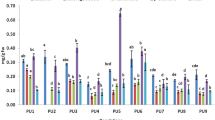
Significant (p<0.05) variation in total phenolics and flavonoids in aerial and root portions of V. jatamansi across all populations was observed (Table 2). Munsyari population exhibited significantly higher total phenolics (aerial − 25.23 ± 0.03 mg; root portion 27.54 ± 0.06 mg GAE/g dw) as compared to other populations. Flavonoid contents were significantly (p < 0.05) higher (aerial portion 32.67 ± 0.34 mg; root portion 29.91 ± 0.47 mg QE/g dw) in the Jaberkhet and Talwari populations. In case of total tannins, significant (p<0.05) variations were recorded in aerial portion; however, no significant variations were recorded in TT content in root portion (Table 2). The tannin content was significantly (p < 0.05) higher (aerial portion 8.09 ± 0.04) in the Ukhimath population as compared to other populations. Significant variation (p < 0.05) was detected in valerenic acid in aerial and root portions of V. jatamansi across all studied populations (Table A3). Significantly higher valerenic acid content was in the Katarmal (aerial portion 0.57 ± 0.04 %) and Joshimath populations (root portion 1.80 ± 0.12 %). Similarly, aerial portion of Dolti and root portion of Dwali also showed significantly higher valerenic acid content than the other populations. However, valerenic acid could not be detected in the aerial portion of some studied populations (Table A3).
Antioxidant Activity
Antioxidant activity of V. jatamansi (aerial and root portions) measured by ABTS, DPPH, and FRAP assays showed significant difference (p < 0.05) across the populations (Table A3). In case of aerial portion, maximum (8.63 ± 0.06 mM AAE/100 g) activity was recorded in the Didihat population and minimum (2.75 ± 0.16 mM AAE/100 g dw) in the Malyadaur population using ABTS assay. Root portion of the Katarmal population exhibited maximum ABTS activity (8.36 ± 0.00 mM AAE/100 g) as compared to other populations (Table A3). Talwari population exhibited the maximum (16.80 ± 0.03 mM AAE/100 g dw) antioxidant activity in aerial portion by DPPH assay. Similarly, root portion of the Didihat population exhibited the maximum antioxidant activity (17.53 ± 0.04 mM AAE/100 g dw). The population from Didihat showed significantly higher (15.23 ± 0.09 mM AAE/100 g dw) antioxidant activity in aerial portion using FRAP assay. In case of root portion, the Ukhimath population exhibited significantly higher (12.71 ± 0.04 mM AAE/100 g dw) antioxidant activity as compared to other populations of V. jatamansi.
Variation in Phytochemicals and Antioxidant Activity Across Altitudinal Range and Habitat Types
Analysis using independent sample t test at different altitude level revealed the following (Table 3 and Table A4): (i) significant difference in total phenolics of root portion at 1201–1500 and 2101–2400 m asl (t = 2.618, p < 0.05) and 1201–1500 and 2101–2400 (t = 4.323, p < 0.05), (ii) significant difference in antioxidant activity in the aerial portion using ABTS assay at 1501–1800 and 2101–2400 m asl (t = 2.917, p < 0.05), DPPH assay in aerial portion at 1500–1800 and 1801–2100 (t = 2.497, p < 0.05) and 1501–1800 and 2101–2400 (t = 3.682, p < 0.05), and FRAP assay at 1201–1500 and 1501–1800 (t = 2.904, p < 0.05) and 1501–1800 and 2101–2400 (t = 3.585, p < 0.05). Similarly, only samples collected from grassy and pine habitat exhibited significant difference (t = −2.325, p = 0.045) in FRAP activity in aerial portion. Other studied attributes among habitat type did not show any significant difference; however, level of chemical content in different habitats varied (Fig. 1a–c). Pine forest habitat was found to be the best for valerenic acid (VA) content, mixed-oak forest for TP, and grassy land for total flavonoids and total tannins. Similarly, pine forest for antioxidant activity of aerial portion and grassy land habitat for root portion using ABTS and pine forest FRAP and DPPH emerged suitable habitats (Fig. 2a–c).
Relationship Among Phytochemicals and Antioxidant Activity
No significant (p < 0.05) relationship was found between altitude and studied phytochemical parameters using the Pearson’s correlation coefficient analysis in both plant portion (Tables 4 and 5). However, a significant (p < 0.05) positive relationship of TP and TF contents in aerial portion (r = 0.429, p < 0.05) was observed. Similarly, ABTS activity exhibited significantly positive relationship with DPPH (r = 0.840, p < 0.01) and FRAP activity (r = 0.811, p < 0.01) in aerial portion, while DPPH activity showed positive relationship with FRAP activity (r = 0.519, p < 0.05) in the aerial portion (Table 4).
TF in root portion showed a positive correlation with TT content (r = 0.519, p < 0.05). ABTS activity also showed a positive relationship with DPPH (r = 0.482, p < 0.05) and FRAP activity (r = 0.637, p < 0.01). Significant positive relationship was also found in DPPH activity with FRAP activity (r = 0.763, p < 0.01) in the root portion of V. jatamansi (Table 5).
Identification of Promising Source (Chemotypes) and Elite Identification
Neighbor-joining cluster analysis was performed to prioritize the populations and identify promising individuals/populations based on phytochemicals and antioxidant activity in aerial and root portions (Fig. 3a, b). Cluster analysis based on all studied attributes grouped the populations into two major clusters, with one cluster joining those V. jatamansi populations having higher value of phytochemicals [(cluster I, population no. 2–16 (78.56–107.28 total of all phytochemicals)] and the second cluster joining those populations with a low phytochemical content [(cluster II, population no. 6–13 (62.16–68.45 phytochemical content; Fig. 3a)]. Similarly, neighbor-joining cluster analysis of antioxidant activity determined by all three assays grouped all populations into three major clusters, with one cluster joining the V. jatamansi populations which maintained higher levels of antioxidant activity (population no. 6–2; cluster no II), while remaining two cluster joining those populations with low levels of antioxidant activity (Fig. 3b). Based on neighbor-joining cluster analysis, populations 16 (Jaberkhet) and 7 (Talwari) can be prioritized for harboring maximum phytochemicals and populations 14 (Ukimath), 6 (Majkhali), and 8 (Khirshu) for maximum antioxidant activity.
Promising source(s) based on phytochemicals and antioxidant activity among different populations were identified using equal group classification method. However, shortcoming of equal group categorization (identification of various populations based on different components in higher value class) is further resolved using the PCA. For example, the Munsyari population fall in class 5 based on total phenolic content, while the Talwari, Gwaldam, and Jaberkhet populations fall in class 5 based on total flavonoids. Similarly, Joshimath population exhibited higher value of valerenic acid and fall in the class 5 category; however, 19 populations fall in class 5 based on total tannins. PCA categorized all the populations in two major chemotypes. Chemotype I includes the populations from Khirshu and Munsyari, which showed almost similar higher level of all phytochemicals, while chemotype II includes all remaining populations of V. jatamansi (Fig. 4a). In addition, PCA-based analysis of phytochemicals exhibited the three first principal components, representing 98.65 % of the total variability. Figure 4a shows a classical representation of the scores on PC1 and PC2 (95.83 % of total variance). Similarly, PCA-based analysis of antioxidant activity revealed that three first principal components represented 92.56 % of the total variability. Figure 4b shows a classical representation of the scores on PC1 and PC2 (85.63 % of total variance). PCA classified all 25 populations into two major groups; group I contains the Khirshu population while group II contains all remaining 24 populations of V. jatamansi (Fig. 4b).
Discussion
A marked intraspecific variability in the phytochemical content and antioxidant activity among plant samples has been reported for different species of the region including V. jatamansi. Causes of such variation are generally attributed either to (i) genetic character of source population/plant [19], (ii) growing season/development stages of plant [24], (iii) effect of light intensity [25], (iv) altitude of plant origin [26], and (v) microclimatic condition of the area [27]. In the present study, sample collected from different localities (25 populations) with diverse altitudinal range (1215–2775 m asl), varying habitat (oak, pine, mixed-oak, and grassy land), and environmental conditions along with different genetic background of each individual (sample) might be responsible for such variation. Such types of studies are also available elsewhere. For example, growing season found to be responsible for variations in phenolic mass fraction among different cultivars of blueberry [28] highlights that intensity in glasshouse condition is reported to increase total phenolics, flavonoids, and antioxidant activities in Zingiber officinale [25] latitude of plant origin that influenced the tannin and glycyrrhizin contents of the licorice (Glycyrrhiza glabra, Leguminosae). Anthocyanin content is influenced by soil pH and electrical conductivity [27]; seasonal changes in the shoots of Mentha longifolia are reported to have influence on dry matter, fiber, fat, protein, net free energy (NFE), nitrogen-free extractable substances (NFESs), macronutrients (Na, N, Ca, K, and P) and micronutrients (Zn, Mg, Fe, and Cu), and alkaloid, flavonoid, and phenolic contents [29]; and growth stages are reported to influence phenolics and antioxidant activity in H. spicatum [14]. Moreover, phytochemical diversities in Echinacea angustifolia populations from different natural geographic areas were found to be significantly associated with latitudinal variation [30]. Similar reports are also available on other species like Eucommia ulmodies [31] and Centella asiatica [32]. Similarly, significant variations were recorded in total phenolics, flavonoids, proline, malondialdehyde, H2O2, and antioxidant activity of Achillea millefolium, A. nobilis, and A. filipendulina growing under four irrigation regimes [33]. In addition, the effect of initial temperature treatment on phytochemicals and antioxidant activity was also studied in Azadirachta indica [34].
The mean values for total phenolics, flavonoids, tannins, and antioxidant activity measured by all three assays in V. jatamansi were considerably higher as compared to earlier reports [10, 19, 35]. Similarly, the reported values in this species were higher than the value (0.34 mg/g GAE dw) in root portion of other related species of the family, e.g., Nardostachys jatamansi and V. officinalis [32]. Antioxidant activity of the essential oil and root extract derived from V. jatamansi using DPPH and FRAP assays suggests potential of the species as natural source of antioxidant [35, 36]. Higher amount of phytochemicals in root portion as compared to aerial portion in V. jatamansi corresponds with earlier reports for the same species [10]. Although studies are also available on valerenic acid quantification with respect to V. jatamansi [11], present study is not in agreement with the earlier study where valerenic acid was not reported in the samples collected from the western Himalaya.
Altitude plays an important role in the accumulation of secondary metabolites in plants. For example, altitudinal differences in flowering heads of three members of the species Crepis capillaris, Hieracium pilosella, and Hypochaeris radicata showed a positive correlation with the altitude of the growing site and the contents of flavonoid and phenolic acids [37]. In the present study, altitude did not show any significant relationship with the phytochemical content and antioxidant activity; however, higher TP, TF, and TT (aerial portion) and TP (root portion) at 2101–2400-m zone and TF (in root portion) at >2401 m were recorded. Observations from the population at higher altitude are considered obvious largely on account of higher exposure to UV radiation, lower temperature, and stressed conditions that induce production of secondary metabolites [38, 39].
Environmental factors affect the production of biologically active constituents in plants and animals at different habitat conditions. For example, the study on yeast revealed that a variety of physical and chemical stressors, including temperature, hypoxia, reactive oxygen species, and osmotic shock, triggered relatively similar changes in expression of approximately 900 genes, which represents approximately 14 % of the yeast genome [40]. Variations in phytochemical content across habitat conditions in different species of medicinal plants like Ocimum sanctum [41] and Pteridium arachnoideum [42] are also reported. In the present study, pine forest and mixed forest emerged as suitable habitat.
Strong positive relationship (p < 0.05 and p < 0.01) of antioxidant assays suggested the suitability of the assays used in this study. Relationship between tannin content and ABTS and DPPH assays in V. jatamansi suggests the role of tannin content in generating antioxidant activity [29, 39]. Although several reports exist on highly positive relationship between total phenols and antioxidant activity in many plant species, non-significant relationship between total phenolic content and antioxidant activity was determined in this study. It is possible that antioxidant activities in the target species may be attributed to some other compounds than the phenolics, flavonoids, and tannins. Although significant differences were recorded in total phenolics, tannins and FRAP activity (in aerial portion), and tannin content (in root portion) among different altitudinal ranges, no correlation was found between the altitudes, phytochemicals, and antioxidant activity. These results are not in agreement with Rawat et al. [40] who showed increased phytochemical content in H. spicatum with the increase in altitude.
Based on NJ cluster analysis, populations Jaberkhet and Talwari were identified for harboring maximum phytochemicals and Ukimath, Majkhali, and Khirshu for maximum antioxidant activity. The NJ cluster analysis is generally used for the creation of phenograms, but it can also be used for the identification of best methods from a set of multiple procedures involving multiple variables [42]. Population with high phytochemical and antioxidant activity identified using NJ cluster analysis can be potential candidates in the breeding programs. PCA is a multivariate analysis technique that was employed to identify promising sources based on the phytochemical and antioxidant activity analysis. This is a frequently employed statistical analysis method and has been successfully applied to analytical results, both for individual compounds and component combinations [43]. All phytochemicals and antioxidant attributes were considered to identify the principal components. Generally, the two first principal components (PCs) are sufficient to explain the maximum variation in all original data. In the present study, two-dimensional score plot in the space defined by PC1 and PC2 shows that the distribution of samples does not follow a pattern that depends on PC2 (Fig. 4a, b). Earlier studies on V. jatamansi based on essential oil composition identified two chemotypes [44] and three chemotypes [15]. Chemotypic variability in various other species based on essential oil composition have also been reported, e.g., Origanum vulgare [45], Hippophae rhamnoides [46], Lychnophora ericoides [47], Liptospermum scorparium [48], Anemopsis californica [49], and Artemisia dracunculus [50]. However, identification of chemotypes based on the active compound, valerenic acid, and other antioxidant phytochemicals is reported for the first time. Based on the phytochemicals and antioxidant activity, Munsyari (P22) and Doongiri (P18) populations were found to be most promising sources. These sources can further be explored for focusing particular group of chemical content and obtaining the desired phytochemicals. Also, the compound specific population can be promoted for harnessing the potential of species.
Conclusions
The present study suggests that screening of multilocational plants/populations is essential for optimizing suitable sources for higher phytochemicals and antioxidant activity. The aerial and root portions of V. jatamansi showed that higher phytochemicals and antioxidant activity indicate that the whole plant can be utilized to harness its potential. The results of the study support the fact that species is a promising source of various phytochemicals and antioxidant activity and found comparable with some commercially available plant species, e.g., Origanum spp. and tea. Also, the species can be a substitute of V. officinalis, one of the highly commercial species across the globe. The present study revealed that isolation of active constituents and their impact on various health improvement/control of free radical-mediated disorder through in vitro studies is needed. As the population at 1501–1800 m asl and pine forest is the best source for total phenolic content and antioxidant activity, mixed type habitat is best for other phtytochemicals; thus, the cultivation on these sites is recommended. Higher amount of phytochemicals and antioxidant activity in the Munsyari and Doonagiri populations could be used as an elite stock for mass multiplication and to meet the market demand for target species. Further, the species has potential to enhance the income of Himalayan people; therefore, breeding program on this species needs to be initiated and material for the same can be used from the identified promising sources.
References
Schmidt, B., Ribnicky, D. M., Poulev, A., Logendra, S., Cafalu, W. T., & Raskin, I. (2008). A natural history of botanical therapeutics. Metabolism, Clinical and Experimental, 57, S3–S9.
KIT (2003). Cultivating a healthy enterprise. In Bulletin 350 Royal Tropical Institute, Amsterdam, The Netherlands.
Jugran, A.K. (2013). Fingerprinting phytochemical and genetic variations in Valeriana jatamansi and V. himalayana in Uttarakhand. Ph. D. Thesis, in Biotechnology submitted to Kumaun University, Nainital, Uttarakhand India.
Becker, H., & Chavadeoi, S. (1985). Valepotriate production of normal and colchicine-treated cell suspension cultures of Valeriana wallichii. Journal of Natural Products, 48, 17–21.
Bounthanh, C., Bergmann, C., Beck, J. P., Hagg-Berrurier, M., & Anton, R. (1981). Valepotriates, a new class of cytotoxic and antitumor agents. Planta Medica, 41, 21–28.
Thies, P. W. (1968). Linarin-isovalerianate, a currently unknown flavonoid from Valeriana wallichii. Planta Medica, 16, 363–371.
Ron, B. H., Willis, C. V., Bone, K., & Morgan, M. (2000). Herbal products: active constituents, mode of action and quality control. Nutrition Research Reviews, 13, 47–77.
Marder, M., Viola, H., Wasowski, C., Fernandez, S., Medina, J. H., & Paladini, A. C. (2003). 6-methylapigenin and hesperidins: new Valeriana flavonoids with activity on the CNS. Pharmacology and Biological Behavior, 75, 537–545.
Singh, N., Gupta, A. P., Singh, B., & Kaul, V. K. (2006). Quantification of valeric acid in Valeriana jatamansi and Valeriana officinalis by HPTLC. Chromatographia, 63, 209–213.
Bhatt, I. D., Dauthal, P., Rawat, S., Gaira, K. S., Jugran, A., Rawal, R. S., & Dhar, U. (2012). Characterization of essential oil composition, phenolic content, and antioxidant properties in wild and planted individuals of Valeriana jatamansi Jones. Scientia Horticulturae, 136, 61–68.
Singh, R. D., Gopichand Meena, R. L., Sharma, B., Singh, B., Kaul, V. K., & Ahuja, P. S. (2010). Seasonal variation of bioactive components in Valeriana jatamansi from Himanchal Pradesh, India. Industrial Crops and Products, 32, 292–296.
Gautier, H., Diakou-Verdin, V., Bénard, C., Reich, M., Buret, M., Bourgaud, F., Poessel, J. L., Caris-Veyrat, C., & Génard, M. (2008). How does tomato quality (sugar, acid, and nutritional quality) vary with ripening stage, temperature, and irradiance? Journal of Agriculture and Food Chemistry, 56, 1241–1250.
Andola, H. C., Gaira, K. S., Rawal, R. S., Rawat, M. S. M., & Bhatt, I. D. (2010). Habitat dependent variation in berberine content of Berberis asiatica Roxb. Ex. DC. In Kumaon, western Himalaya. Chemistry and Biodiversity, 7, 415–420.
Rawat, S., Bhatt, I. D., & Rawal, R. S. (2011). Total phenolic compounds and antioxidant potential of Hedychium spicatum Buch. Ham. ex D. Don in West Himalaya, India. Journal of Food Componant Analysis, 24, 574–579.
Sundaresan, V., Shani, G., Verma, R. S., Padalia, R. C., Mahrotra, S., & Thul, S. T. (2012). Impact of geographic range on genetic and chemical diversity of Indian Valerian (Valeriana jatamansi) from northwestern Himalaya. Biochemical Genetics, 50, 797–808.
Jugran, A. K., Bhatt, I. D., Rawal, R. S., Nandi, S. K., & Pande, V. (2013). Patterns of morphological and genetic diversity of Valeriana jatamansi Jones in different habitats and altitudinal range of West Himalaya, India. Flora Morphology, Distribution, Functional Ecology of Plants, 208, 13–21.
Rawal, R. S., Pandey, B., & Dhar, U. (2003). Himalayan forest database—thinking beyond dominants. Current Science, 84, 990–994.
Andola, H. C., Rawal, R. S., & Bhatt, I. D. (2011). Comparative studies on the nutritive and anti-nutritive properties of fruits in selected Berberis species of West Himalaya, India. Food Research International, 44, 2352–2356.
Jugran, A., Rawat, S., Dauthal, P., Mondal, S., Bhatt, I. D., & Rawal, R. S. (2013). Association of ISSR markers with some biochemical traits of Valeriana jatamansi Jones. Industrial Crops and Products, 44, 671–676.
Upton, R. (1999). Commercial sources and handling. In R. Upton (Ed.), Valerian root, Valeriana officinalis, analytical, quality control and therapeutic monograph (pp. 6–7). Santa Cruz: American Herbal Pharmacopoeia.
Jugran, A. K., Bhatt, I. D., & Rawal, R. S. (2015). Identification of ISSR markers associated with valerenic acid and antioxidant activity in Valeriana jatamansi Jones in western Himalaya. Molecular Breeding, 35, 1–14. doi:10.1007/s11032-015-0241-5.
Cruz, A. G., Cadena, R. S., Alvaro, M. B. V. B., Sant’Ana, A. S., Oliveira, C. A. F., Faria, J. A. F., et al. (2013). Assessing the use of different chemometric techniques to discriminate low-fat and full-fat yogurts. LWT-Food Science and Technology, 50, 210–214.
Hammer, O., Harper, D. A. T., & Ryan, P. D. (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4, 1–9.
Rawat, S., Andola, A., Giri, L., Dhyani, P., Jugran, A., Bhatt, I. D., & Rawal, R. S. (2014). Assessment of nutritional and antioxidant potential of selected vitality strengthening medicinal plants. International Journal of Food Properties, 17, 703–712.
Ghasemzadeh, A., Jaafar, H. Z. E., Rahmat, A., Wahab, P. E. M., & Halim, M. R. A. (2010). Effect of different light intensities on total phenolics and flavonoids synthesis and anti-oxidant activities in young ginger varieties (Zingiber officinale Roscoe). International Journal of Molecular Sciences, 11, 3885–3897.
Alonso-Amelot, M. E., Oliveros-Bastidas, A., & Calcagno-Pisarelli , M. (2007). Phenolics and condensed tannins of high altitude Pteridium arachnoideum in relation to sunlight exposure, elevation, and rain regime. Biochemical Systematics and Ecology, 35, 1–7.
Oloumi, H., & Hassibi, N. (2011). Study the correlation between some climate parameters and the content of phenolic compounds in roots of Glycyrrhiza glabra. Journal of Medicinal Plant Research, 5, 6011–6016.
Dragovi-Uzelac, V., Savi, Z., Brala, A., Levaj, B., Kovaceveic, D. B., & Bisko, A. (2010). Evaluation of phenolic content and antioxidant capacity of blueberry cultivars (Vaccinium corymbosum L.) grown in the northwest Croatia. Food Technology and Biotechenology, 48, 214–221.
Ahmad, I., Ahmad, M. S. A., Ashraf, M., Hussain, M., & Ashraf, M. Y. (2011). Seasonal variation in some medicinal and biochemical ingredients in Mentha longifolia (L.) Huds. Pakistan Journal of Botany, 43, 69–77.
Binns, S. E., Arnason, J. T., & Baum, B. R. (2002). Phytochemical variation within populations of Echinacea angustifolia (Asteraceae). Biochemical Systematics and Ecology, 30, 837–854.
Tong, L., Wang, Y., Xiong, J., Cui, Y., Zhou, Y., & Yi, L. (2008). Selection and fingerprints of the control substances for plant drug Eucommia ulmodies Oliver by HPLC and LC–MS. Talanta, 76, 80–84.
Devkota, A., Dall Acqua, S., Jha, P. K., & Innocenti, G. (2010). Variation in the active constituent contents in Centella asiatica grown in different habitats in Nepal. Botnica Orientalis- Journal of Plant Science, 7, 43–47.
Gharibi, S., Tabatabaei, B. E. S., Saeidi, G., & Goli, S. A. H. (2015). Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Applied Biochemistry and Biotechnology. doi:10.1007/s12010-015-1909-3.
Vats, S. (2015). Effect of initial temperature treatment on phytochemicals and antioxidant activity of Azadirachta indica A. Juss. Applied Biochemistry and Biotechnology. 1–9.
Surveswaran, S., Cai, Y., Corke, H., & Sun, M. (2007). Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chemistry, 102, 938–953.
Das, J., Mao, A. A., & Handique, P. J. (2011). Terpenoid composition and antioxidant activities of two Indian Valerian oils from the Khasi Hills of north-east India. Journal of Natural Products and Communication, 6, 129–132.
Kalim, M. D., Bhattacharya, D., Banerjee, A., & Chattopadhyay, S. (2010). Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complementary and Alternative Medicine, 10, 77 (http://www.biomedcentral.com/1472-6882/10/77).
Zidorn, C., Schubert, B., & Stuppner, H. (2005). Altitudinal difference in the contents of phenolics in flowering heads of three members of the tribe Lactuceae (Asteraceae) occurring as introduced species in New Zealand. Biochemical Systematics and Ecology, 33, 855–872.
Spitaler, R., Winkler, A., Lins, I., Yanar, S., Stippner, H., & Zidorn, C. (2008). Altitudinal variation of phenolic contents in flowering heads of Arnica montana CV. ARBO: a 3 year compassion. Journal of Chemical Ecology, 34, 369–375.
Rawat, S., Jugran, A., Giri, L., Bhatt, I. D., & Rawal, R. S. (2010). Assessment of antioxidant properties in fruits of Myrica esculenta: a popular wild edible species in Indian Himalayan region. Evidence based Complementary and Alternative Medicine. doi:10.1093/ecam/neq055.
Gasch, A. P., Spellman, P. T., Kao, C. M., Carmel-Harel, O., Eisen, M. B., Storz, G., Botstein, D., & Brown, P. O. (2000). Genomic expression programs in the response of yeast cells to environmental changes. Molecular Biology of the Cell, 11, 4241–4257.
Saitou, N., & Nei, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4, 406–425.
Selvam, K., Rajinikanth, R., Govarthanan, M., Paul, A., Selvankumar, T., & Sengottaiyan, A. (2013). Antioxidant potential and secondary metabolites in Ocimum sanctum L. at various habitats. Journal of Medicinal Plant Research, 7, 706–712.
Mathela, C. S., Sati, S., & Chanotiya, C. S. (2005). Comparative investigations of the leaf and root oils of Valeriana wallichii DC. from north western Himalaya. Journal of Essential Oil Research, 17, 408–409.
Mockute, D., Bernotiene, G., & Judzentiene, A. (2001). The essential oil of Origanum vulgare L. spp. vulgare growing wild in Vilnius district (Lithuania). Phytochemistry, 57, 65–69.
Tian, C., Nan, P., Chen, J., & Zhong, Y. (2004). Volatile composition of Chinese Hippophae rhamnoides and its chemotaxonomic implications. Biochemical Systematics and Ecology, 32, 431–441.
Curado, M. A., Carolina, B. A., Oliveira, J. G. J., Suzana, C. S., Seraphin, J. C., & Ferri, P. H. (2006). Environmental factors influence on chemical polymorphism of the essential oils of Lychnophora ericoides. Phytochemistry, 67, 2363–2369.
Douglas, M. H., Van Klink, J. W., Smallfield, B. M., Perry, N. B., Anderson, R. E., John-Stone, P., & Weaver, R. T. (2004). Essential oils from New Zealand manuka: triketone and other chemotypes of Leptospermum scoparium. Phytochemistry, 65, 1225–1264.
Medina, H. A., Omar Holguin, F., Micheletto, S., Goehle, S., Julian, A., & O’connell, M. A. (2008). Chemotypic variation of essential oils in the medicinal plant, Anemopsis californica. Phytochemistry, 69, 919–927.
Chauhan, R. S., Kitchlu, S., Ram, G., Kaul, M. K., & Tava, A. (2010). Chemical composition of capillene chemotypes of Artemissia dracunculus L. from northwest Himalaya. Industrial Crops and Products, 31, 546–549.
Acknowledgments
We thank Dr. P.P. Dhyani, Director of GBPIHED, for the facilities and encouragement. Colleagues of Biodiversity Conservation and Management and Ecophysiology and biotechnological application group are thanks for the cooperation and help during the study. Financial support is from GBPIHED in house project no. 10, and Science and Engineering Research Board, Department of Science and Technology (DST No. SB/YS/LS-162/262), New Delhi, is acknowledged for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jugran, A.K., Bahukhandi, A., Dhyani, P. et al. Impact of Altitudes and Habitats on Valerenic Acid, Total Phenolics, Flavonoids, Tannins, and Antioxidant Activity of Valeriana jatamansi . Appl Biochem Biotechnol 179, 911–926 (2016). https://doi.org/10.1007/s12010-016-2039-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2039-2