Abstract
Phytochemicals are broadly acknowledged for their health-promoting effects owing to the fact of their capacity to counteract free radicals (e.g., superoxide anion radical, hydroxyl radical, hydroperoxyl radical, singlet oxygen, hypochlorite, and nitric oxide) and shield against oxidative stress induced by environmental factors. This study aimed to investigate the relationship between altitude, morphology, soil parameters, in vitro antioxidant potential and phytochemical composition of Phlomis cashmeriana collected from four different locations of Kashmir Himalaya characterized by diverse habitats and elevations. Various factors, such as extraction method, solvent polarity, and habitat conditions, can impact the quantity and efficacy of phytochemicals in plants. The aim of current study was to analyze phytochemical composition and antioxidant activity of P. cashmeriana, an important medicinal plant found in the Kashmir Himalaya region. The antioxidant activity was accessed using several assays and the plant populations were selected based on their diverse habitat features and altitudes. HR-LCMS was conducted for both below-ground and above-ground parts. Some important compounds such as, catechin, vinainsenoside, acutilobin, and kaempferol were reported for the first time from P. cashmeriana. Results showed that methanol was the most efficient solvent for extracting phytochemicals. During the current study, it was also found that the below-ground parts exhibited superior antioxidant activity compared to the above-ground parts. Notably, Site IV demonstrated the highest antioxidant potential; a positive correlation between altitude and antioxidant activity was also found. In conclusion, present research identified specific elite populations having highest antioxidant potential and are well-suited for large-scale cultivation of P. cashmeriana.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Medicinal plants have been utilized as a valuable source for developing various drugs and curing related diseases. Different optimization techniques are used to create safe and effective medicines using phytochemicals as templates. Flavonoids, resins, alkaloids, tannins, terpenoids, steroids, glycosides, fixed oils, phenols, and volatile oils are bioactive compounds present in plants and are stored in different parts of the plant, including roots, leaves, bark, fruits, flowers, and seeds. The therapeutic effects of these plant materials is generally due to the combination of these secondary metabolites. Due to their anti-inflammatory, antibacterial, antifungal, antidiabetic, antioxidant, and radio protective action, these phytochemicals are widely employed for medical purposes (Anand et al., 2022; Kumar et al., 2008; Samadov, 2022).
Plants undergo direct effects from various environmental stresses that are recognized for altering their structural and functional attributes. Factors such as climatic conditions, interactions with other plants, nutrient availability, and genetic composition are known to affect morphology besides secondary phytochemicals. Altitude represents one of the important environmental factors that have been found to have significant impact on plant secondary metabolism. The potential of plant-derived bioactive compounds to inhabit diseases is widely recognized. These chemical entities, referred to as secondary plant metabolites or specialized products, are not indispensable for the plant's growth and development but function as defensive compounds, facilitating interaction with the environment for adaptation, resistance to infections and pests, and also control over flavor, color, and scent. Naturally occurring plant-derived compounds are getting more recognition both in traditional and modern systems of medicines due to their efficiency in treating a variety of disorders.
Oxidative stress has gained popularity in the medical sciences over the past 30 years as it actively influences number of human ailments. Cells generate highly detrimental reactive oxygen species (ROS) through the metabolic process of oxygen metabolism. The rate at which oxidant is eliminated normally counterbalances the pace and magnitude of oxidant formation. An imbalance between poduction and accumulation of ROS causes oxidative stress, these elevated ROS levels have a substantial impact on biological cells’ ability to function, which can cause improper cell activity, aging, or disease (Rodrigo, 2009; Arfin et al., 2021). The scientific community is very interested in investigating antioxidants and their applications in a variety of disciplines, including food engineering, medicine, and pharmacy. Search for naturally occurring antioxidants for use in pharmaceuticals and food industries in substitute of synthetic antioxidants, whose consumption is restricted due to their carcinogenicity, has recently gained a great deal of attention. The global market for antioxidants is increasing every passing day, and it is worth to mention that by year 2027, it is anticipated to be 1.6 billion US$. Numerous medicinal plants still need to be studied for their potential to promote health and scavenge free radicals (Belwal et al., 2019; Bhatt et al., 2017).
P. cashmeriana commonly referred to as Kashmir sage (Darshol) is an important perennial medicinal herb of Kashmir Himalaya belonging to family Lamiaceae. Many species of this family are valued for their therapeutic benefits and economic value due to their naturally occurring volatile oils (Qadir et al., 2022; Sartoratto et al., 2004; Shadia et al., 2007). Several biological and pharmaceutical effects of Phlomis spp. include antimicrobial (Wafa et al., 2016), antiulcerogenic (Amor et al., 2009), antinociceptive (Shang et al., 2011), anti-inflammatory, anti-fibrillation, immunosuppressive (Shang et al., 2016), and antidiabetic (Sarkhail et al., 2007). In Anatolian folk medicine, multiple species of Phlomis are employed as tonics and stimulants, as noted by Aghakhani and Kharazian in 2019. Within the Phlomis genus, various types of glycosides, including diterpenoids, phenylethanoids, phenylpropanoids, iridoids, and flavonoids, have been documented. P. cashmeriana grows frequently in wild or on exposed hillsides and flowers in the summer. P. cashmeriana is native to Afghanistan, Tadzhikistan, West Himalaya, and Pakistan (POWO, 2023). It has multiple stems (40–80 cm), simple as well as branched with woody root stock. The inflorescence has verticillasters, labiate flowers with an upper lip that is plainly falcate, and corolla lobes that are pale purple in color (Fig. 1).
Considering the immense potential of this genus, the current study was initiated with the following objectives in mind: (1) qualitative and quantitative screening of the diverse phytochemicals found in P. cashmeriana; (2) evaluation of in vitro antioxidant potential of different extract of P. cashmeriana; (3) Impact of solvent, altitude, plant part, and various soil parameters on antioxidant potential; and (4) relationship between altitude, morphological attributes, phytochemicals, soil parameters, and the antioxidant potential of P. cashmeriana.
Methodology
Study area
Kashmir Himalaya was chosen for the current study located in India. During the year 2020–2021 extensive filed survey were carried out across the selected study area. Keeping in consideration the availability and accessibility, four populations of P. cashmeriana varying in altitude and habitat conditions were selected to carry out current study (Table 1). The sites selected were Jawahar tunnel (Site IV), Hillar naar (SiteIII), Daksum (SiteII), and KUBG (SiteI) (Fig. 1 and 2).
Samplings and analysis
The whole plant samples of P. cashmeriana were collected from each population in air tight polyethylene zipper bags. The plant specimens were submitted in the KASH under voucher specimen numbers, 2941, 2942, and 2943. The collected samples were taken to the lab and were portioned into upper ground and lower ground parts. The plant material after proper cleaning was kept at room temperature and shade dried. After shade drying the plant material was grinded. One hundred grams each of upper ground part (leaf, stem, and inflorescence) and below ground (rhizome) was weighed accurately. The weighed samples were packed in extractor of soxhlet apparatus. The plant samples were extracted successively in the soxhlet apparatus (seven cycles for each solvent). Four different solvents were used for the extraction process, with increasing polarity in the following order: petroleum ether, ethyl acetate, methanol, and finally aqueous. Rotatory vaporizer was used to vaporize the solvent and subsequently stored at 4 °C in refrigerator to carry out further phytochemical analysis (Paudel et al., 2018).
Morphological characterization
To examine the phenotypic traits of the species, 20 fully mature flowering plants were randomly collected from each chosen site. These plants were then subjected to a detailed assessment of their morphological characteristics, as outlined in the supplementary table 8 (Qadir et al., 2022).
Quantification of total phenolics (TPC)
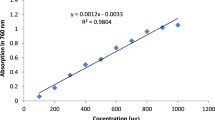
To determine the total phenolic content, 100 mg of the sample’s extract was measured and dissolved in double distilled water (100 mL). Then, 1 mL of this solution was transferred to a test tube and mixed with 0.5 mL of 2N Folin Ciocalteu reagent and 1.5 mL of 20% Na2CO3 solution. The final volume was made 8 mL with addition of double distilled water and thoroughly shaken. Reaction mixture was allowed to stand undisturbed for a duration of 2 h, following which the absorbance was measured at a wavelength of 765 nm. The gallic acid was used as a standard (Y = 9.887x + 0.0129, R2 = 0.9974). The results were expressed as mg of gallic acid equivalents (GAE)/g of plant extract as mean of three replicates (Hagerman et al., 2000).
Quantification of total flavonoids (TFC)
The method is based on the formation of the flavonoids-aluminum complex which has an absorptivity maximum at 415 nm. One hundred microliters of the plant extracts in methanol (10 mg/mL) was mixed with 100 µL of 20% aluminum trichloride in methanol and a drop of acetic acid and then diluted with methanol to 5 mL. The absorption at 415 nm was read after 40 min. Blank samples were prepared from 1 mL of plant extracts and a drop of acetic acid, and then diluted to 5 mL with methanol. All determinations were carried out in triplicates additionally; rutin solution (0.5 mg/mL) in methanol was used as a standard and analyzed using the same conditions in order to make comparisons between the results obtained. The rutin trihydrate was used as standard (Y = 0.0101x + 0.0389, R2 = 0.9922), and the results of TFC were expressed as mg of rutin equivalents (RE)/g of plant extract. Each sample was analyzed in triplicate (Kumaran & karunakaran, 2006).
Phytochemical identification via HR-LCMS
Methanolic extracts of both above and below ground parts were subjected to HR-LCMS analysis. The analysis was carried out at IIT Mumbai, India, having equipment model and make (1260 IN, 6550-QTOF Agilent, USA). For the acquisition technique, a mass range of 50 to 3200 amu was defined, with a mass precision ≤ 1 ppm and a scanning rate of each spectra per second. The investigation was carried out in both positive and negative ESI mode (Rafiq et al., 2022).
Evaluation of the antioxidant activity
DPPH
For DPPH test, method of Braca et al. (2002) was modified in order to test the DPPH radical scavenging capacity of plant extracts. Using 1 mL (0.5 mM) DPPH solution, various concentrations of above and below-ground extract (50–600 g/mL) were diluted. The reaction mixture was vortexed and subsequently kept in dark for 30 min. After incubation, the sample’s absorbance against methanol at 517 nm was assessed spectrophotometrically (Shimadzu 1900i, Kyoto, Japan) (used as a blank). The decrease in absorbance depicted an increase in DPPH’s capacity to scavenge free radicals. The proportion of DPPH inhibition was determined using the following formula:
A0 represented the control’s absorbance (DPPH alone, with no extract), A1 represent the extract absorbance containing DPPH, and A2 the absorbance of the extract excluding DPPH. The 50% suppression of DPPH radicals by an extract was used to express its antioxidant ability (IC50 g/mL of extract). The polynomial regression equation was used to compute the extract’s IC50.
In vitro nitric oxide radical (NO) scavenging assay
Using the Marcocci et al. (1994) approach, NO produced by sodium nitroprusside (SNP) was quantified. Precisely, 5-mL reaction was prepared by mixing 5-mM SNP in phosphate buffer saline having pH 7.3, with varied concentrations of plant extract. This was kept at 26 °C for about 1.5 h under visible polychromatic light (tungsten lamp of 60 W). The absorbance at 546 nm was measured at a 30-min gap by mixing equal amounts, i.e., 1 mL each of incubation mixture and Griess reagent. A standard curve based on sodium nitrite solutions with known concentrations was used to quantify the amount of nitrite produced in presence or absence of the plant extract. Whole procedure was repeated thrice, and data was calculated as the mean of three distinct calculations. % Inhibition was calculated as follows:
where AC and AS denote the absorbance of the control (without the plant extract) and absorbance of the sample (reaction mixture containing plant extracts), respectively.
Reducing power ability
The capacity of the extracts to reduce Fe3+was examined using a modified version of Oyaizu’s, 1986 technique. To carry out the test, equal volumes (0.75 mL) of potassium hexacyanoferrate [K3Fe(CN)6] (at a concentration of 1% w/v) and phosphate buffer (0.2 M, pH 6.6) were taken. The total mixture after placing in a water bath for 20 min at 50 °C. Then, 0.75 mL of trichloroacetic acid (TCA) solution (10%) was added, and centrifuging it for 10 min at 3000 rpm, a solution containing ferric chloride (FeCl3) (at a concentration of 0.1% w/v), 1.5 mL of distilled water, and 0.1 mL of the supernatant was combined and left for 10 min. The reducing power was assessed by measuring the absorbance at 700 nm (Oyaizu’s et al., 1986). Absorbance value directly indicated the reducing potential, higher absorbance was indicative of better reducing power.
Hydroxyl radical scavenging (OH) activity
In order to evaluate hydroxyl scavenging activity method given by Klein, the mixture contained the following ingredients was prepared: 1.0 mL of varied extract concentrations (2–10 mg/mL), iron-EDTA solution 1 mL, EDTA (0.018%) 0.5 mL, DMSO 1 mL, and 0.22% ascorbic acid 0.5 mL. After heating at 80–90 °C for 15 min using a well-sealed water bath within the tubes, the reaction was halted by introducing 1.0 mL of ice-cold TCA (17.5%) to the previously mentioned reaction mixture. Following this, 3.0 mL of the Nash reagent, which includes 75.0 g of ammonium acetate, 3 mL of glacial acetic acid, 2.0 mL of acetyl acetone, and 1 L of double-distilled water, was added. The color development was then allowed to proceed for 15 min at 27 °C while measuring the absorbance at 412 nm. Standards used were ascorbic acid and gallic acid. By comparing the test with the standard, the percentage of inhibition was calculated using following formula.
where AC stands for the absorbance of the control (without the plant extract) and AS for the absorbance of the sample (reaction mixture containing plant extracts) (Klein et al., 1981).
Soil sampling and analysis
During August 2020, both plant and soil samples were collected simultaneously. At each site, three soil samples were collected from depths of 0–30 cm. These three samples were combined into one sample per site after manually removing any roots and stones present. The soil samples were then dried in air for 10 days; properly dried soil samples were grinded using mortar and pestle and then passed through a 0.5-mm metallic sieve. The powdered soil samples were stored at room temperature for later physicochemical analysis, which was performed at the Soil Testing Laboratory in Lal mandi Srinagar, Jammu and Kashmir, India. The pH of each soil sample was measured in a 1:2 soil water suspension using a digital pH meter. Various parameters such as organic carbon, available nitrogen, phosphorus, and potassium content were analyzed in each layer using standard methodology described by Bray and Kurtz (1945), Subbiah and Asija (1956), Walkley and Black (1934), and Morwin and Peach (1951). To convert the organic carbon percentage into organic matter percentage, a factor of 1.724 was used.
Statistical analysis
The findings presented are an average of three analyses, and a linear regression model was used to derive equations for standards, gallic acid, and quercetin. These equations were then applied to calculate the total flavonoid and polyphenol contents based on linear relationships. To compare the significance of differences in polyphenol and flavonoid levels among the extracts, the Tukey’s test was conducted with a significance level of p ≤ 0.05. Different concentrations varying from 50 to 600 µL were used. During preliminary investigation, concentration at 600 µL was seen to exhibit the best activity in all the four assays. Thus, in order to carry further analysis, only 600 µL concentration was used for experimentation. The data was analyzed using three-way ANOVA. To evaluate the antioxidant activity of all the extracts, the IC50 values were calculated using a suitable regression equation (linear or non-linear) based on the percentage activity at various concentrations. The significance of the results was assessed using the F-statistic with a significance level of p ≤ 0.05. The statistical software SPSS 23 (SPSS Inc., Chicago, IL, USA) was employed to analyze variance (ANOVA), and Tukey’s test was used for multiple comparisons of the antioxidant tests. Origin Pro 2021 was used for pairwise comparison and other correlation plots.
Results
Qualitative and Quantitative Analysis of Phytochemical: while examining various extracts from different parts of P. cashmeriana, it was revealed that there is a notable to substantial presence of various bioactive compounds, as summarized in the Supplementary Table 1. The total phenolics and flavonoids varied significantly from both parts i–e above and below ground of P. cashmeriana across all studied sites (Supplementary Table 2). Methanolic fractions of Site IV exhibited higher phenolic (below ground 159.4 ± 9.17; above ground 107.93 ± 1.66 mg GAE/g) and flavonoid content (below ground 188.07 ± 2.04; above ground 112.60 ± 4.33mg rutin/g, respectively) compared to other populations. Between the different extracts TPC and TFC of P. cashmeriana, a significant deviation was observed, and a particular trend followed was petroleum ether > ethyl acetate extracts > aqueous > methanolic extract. Concentration of phenolic compounds was found maximum (159.4 ± 9.17 mg GAE/g) in methanolic extracts obtained from below ground part followed by 52.73 ± 1.70 mg GAE/g and 46.27 ± 2.29 mg GAE/g in ethyl acetate and aqueous extract, respectively. The methanolic extract exhibited the highest flavonoid content in the rhizome, measuring at 188.07 ± 2.04 mg rutin/g extract. This was followed by the ethyl acetate extract, which contained 76.18 ± 2.07 mg rutin/g extract (Fig. 3).
HR-LCMS
Said approach was utilized in analyzing and identifying phytochemicals in methanolic extract of P. cashmeriana from below and aboveground parts (Tables 2 and 3). The major compounds found in these extracts were acutilobin, lentiginosine, secoxylognin, doronine, catechin, cassine, anemonin, nelumboside, columbin, sophoronone, glaucarublin, kuwanon z, Isoscopoletin vinainsenoside, acutilobin, kaempferol, sophoranone, and acutilobin. Methanol-based extracts from both below ground and above ground parts displayed 15 significant peaks in ESI + and 20 in ESI − modes using HR-LCMS, as shown in Fig. 4.
Antioxidant assays
All four in vitro antioxidant assays revealed significant variation (p > 0.05) between the solvents across all the study sites (Fig. 5). Moreover, absorbance and antioxidant percent inhibition and significant variation observed between below and above ground parts were observed as depicted in paired comparison plot (Fig. 6). Furthermore, variation in site, solvent and plant parts, and their interaction significantly affected antioxidant activity of P. cashmeriana (Table 4). Among all the four assays, DPPH exhibited maximum activity (79.25 ± 2.82) in methanolic extract of below ground part from Site IV followed by OH radical antioxidant assay (77.09 ± 2.68). While correlating the flavonoid, phenolic content, and antioxidant activity (Fig. 7 and Supplementary Table 7), all parameters have a significant relationship (p ≤ 0.001).
A–D The plot presents a comparison of different antioxidant assays (D reducing power, A DPPH radical scavenging, B nitric oxide radical scavenging, and C hydroxyl radical scavenging) across study sites. The plot illustrates the variation in absorbance and % inhibition, and significant differences between study sites are indicated by asterisks (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001) while non-significant differences are denoted as “ns”
Paired comparison plot showing partwise variation (1, aboveground; 2, belowground parts) in absorbance and % inhibition of antioxidant assays. A Reducing power activity, B DPPH radical scavenging activity, C nitric oxide radical scavenging activity, and D hydroxyl radical scavenging activity. The significance level in antioxidant potential between belowground and above ground parts denoted as stars (***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05, ns, non-significant)
Correlation plot showing relationship between altitude, morphology, soil characteristics, and different antioxidant assays. ALT-altitude, P-phosphorous, K-potassium, S-sulfur, N-nitrogen, OC- organic carbon(%), OM-organic matter(%), Total ph.-total phenolics, Total flv-total flavonoids, PH-plant height, RL-rhizome length, RB-rhizome breadth, NOL-number of leaves, BLL-basal leaf length, BLB-basal leaf breadth, BPL-basal petiole length, APL-apical petiole length, ALL-apical leaf length, ALB-apical leaf breadth, DPPH-DPPH radical scavenging activity, NO-nitric oxide radical scavenging assay, OH-hydroxyl radical scavenging activity, RPA-reducing power ability, FLAV-flavonoids mg Rutin/g, PHE-phenolics mg GAE/g
DPPH radical scavenging activity
The quantitative assessment ability of various P. cashmeriana extracts to neutralize DPPH radicals was conducted. Upon addition of DPPH to plant extracts, swift diminish in optical density took place at 517 nm, signifying the extract’s strong scavenging capability. The extract displayed noteworthy antioxidant activity, which increased with higher doses in a manner comparable to ascorbic acid, serving as the control standard antioxidant. By plotting the concentration against activity, the extract’s IC50 value, which represents the concentration needed for a 50% reduction in DPPH radicals, was determined to be 207.144 µg/mL. In comparison, ascorbic acid required 158.162 µg/mL in methanolic extracts from the below-ground part of Site IV population, followed by Site III with 302.6 µg/mL (Supplementary Table 3).
Nitric oxide radical (NO) scavenging assay
Noticeable variations were noted in the NO scavenging activity of P. cashmeriana extracts and their fractions. Extracts possessed concentration-dependent potential in-order to scavenge NO radicals. Methanolic extract from below-ground part from Site IV (47.71 ± 0.27), followed by the ethyl acetate extract (38.61 ± 0.14) from the same location was found to possess most robust power in terms of scavenging. The IC50 values for different extracts varied between 714.21 and 3409.93 µg/mL, whereas ascorbic acid exhibited an IC50 value of 332.38 µg/mL as presented in (Supplementary Table 4).
Reducing power ability
Extracts shows dependence on concentration and solvent utilized. Higher absorbance indicates greater capacity for reducing activity in the samples. When compared to other extracts, the methanolic extracts from below-ground part demonstrated the most robust reducing power capability, having the highest value 0.973 ± 0.001, followed by ethyl acetate (698 ± 0.001), aqueous (612 ± 0.001), and petroleum ether (PE) (532 ± 0.001) extracts. Rhizomes reducing ability was found to be relatively lower. Concerning different collection sites, Site 4 exhibited the highest antioxidant potential, while Site 1 displayed the lowest (Supplementary Table 5).
Hydroxyl radicals (OH)
The methanolic extract exhibited dependence on concentration for inhibition of hydroxyl radical-induced deoxyribose degradation. The highest inhibition percentage, reaching 71.42 ± 0.58%, was noted on concentration of 600 µg/mL from the below-ground extract of Site IV, followed closely by Site III with a percentage of 70.84 ± 0.58. In the case of above-ground extracts, Site 2 recorded the highest inhibition at 64.81 ± 0.39, followed by Site 1 and Site IV at 61.71 ± 0.38 and 58.20 ± 0.19, respectively, while the lowest activity was observed at Site 1, with a percentage of 56.45 ± 0.78. The IC50 values for different extracts (ethyl acetate, methanol, petroleum ether, and aqueous) of below-ground parts were 62.16 µg/mL, 70.60 µg/mL, 126.93 µg/mL, and 562.78 µg/mL, respectively (refer to Supplementary Table 6). Site 1 exhibited the highest reduction in OH radicals and had the lowest value for IC50, followed by Sites II, III, and IV.
Soil analysis
Soil properties like soil mineral components varied significantly among study sites. Detailed results obtained from soil samples of different study sites. The percentage electric conductivity was recorded maximum (0.344 ± 0.017%) for study Site IV and minimum (0.185 ± 0.036%) for Site I. pH was recorded maximum (8.05 ± 0.29) for Site I and minimum (5.927 ± 0.56) for Site IV. Phosphorous, potassium, and sulfur were recorded maximum (102.734 ± 5.17, 334.466 ± 8.838, and 27.21 ± 2.84) for Site I and minimum (51.767 ± 3.53, 204.5 ± 2.51, and 18.785 ± 2.03 ppm) for Site IV. Nitrogen, organic carbon, and organic matter was found maximum (537.58 ± 7.11, 8.022 ± 0.22, 13.829 ± 0.35 ppm) for Site I and minimum (392.737 ± 3.45, 6.780 ± 0.06, and 11.70 ± 0.11 ppm) for Site 4 (Table 5).
Morphology
Morphological traits of P. cashmeriana were studied across the study sites and significant variations in phenotypic traits were recorded across populations at different altitudes (Supplementary Table 8). Site I had the plants with maximum height (59.41 ± 12.43 cm), while Site IV had the shortest (41.5 ± 5.99 cm) plants. Root length and breadth of 28.6 ± 5.98 and 1.80 ± 0.29 cm respectively were highest at Site I, and the lowest at Site IV at 13.03 ± 2.99 and 1.49 ± 0.24 cm, respectively. Number of leaves varied from 22.31 ± 11.41 per plant (Site I) to 19.89 ± 4.81 (Site IV), while mean apical and basal leaf length per plant ranged from 10.99 ± 0.89 and 16.11 ± 3.05 cm (Site I) to 5.99 ± 0.45 and 8.98 ± 1.97 cm (Site IV), correspondingly. Finally, leaf width with respect to apical and basal ranged from 3.11 ± 0.39 and 5.66 ± 0.87 cm per plant (Site I) to 1.82 ± 0.37 and 2.41 ± 0.52 cm (Site IV), respectively. Flower number varied from 19.02 ± 2.72 (Site I) to 15.3 ± 3.11 (Site IV), respectively. Petiole length of apical and basal leaves ranged from 1.49 ± 0.15 cm (Site I) and 16.11 ± 3.03 cm and 1.82 ± 0.37 cm and 6.58 ± 1.42 cm (Site IV), respectively.
Discussion
The findings demonstrated that solvent polarity and subsequent extraction of phytochemicals have an impact on variations in TPC and TFC of various extracts. The antioxidant activity of plant extracts can be related with acutilobin, lentiginosine, secoxylognin, doronine, catechin, cassine, anemonin, nelumboside, columbin, sophoronone, glaucarublin, vinainsenoside, acutilobin, kaempferol, sophoranone, and acutilobin identified by LC–MS. This study offered the first account of an HR-LCMS/MS-based phytochemical analysis of P. cashmeriana. The role of Isoscopoletin, catechin, Kawanon Z, as an antioxidants, has been elucidated by several workers (Ahmad & Ghosh, 2022; Bai et al., 2019; luo et al., 2022). In order to increase antioxidant activity, these molecules may interact with other phytochemicals (Okur et al., 2021; Sharma & Cannoo, 2016). Effect of extraction solvents/techniques on polyphenolic contents and antioxidant potential of the aerial parts of Nepeta leucophylla and the analysis of their phytoconstituents using RP-HPLC–DAD and GC–MS. RSC advances, 6(81), 78,151–78,160.). It is well known that due to different polarity of extraction solvents, varied amounts of polyphenol and flavonoid components from plants are obtained (Złotek et al., 2016; Dhanani et al., 2017). To dissolve phytochemicals with similar polarities, a variety of extraction solvents were utilized starting from low to high polarity. As a general phenomenon higher the polarity of solvents higher will be the quantity of phytochemicals; therefore, low-polarity solvent such as P.E often leads to minimal extraction of TPC and TFC. Contrarily, ethyl acetate and methanol extracts both displayed high TPC and TFC, likely as a result of the large number of chemicals in these extracts that include polyphenol and flavonoid groups. The current study is supported by earlier documented research on the extraction of various flavonoid and polyphenol in different plant species owing to the fact of different solvents (Boeing et al., 2014, Chand et al., 2016; Saeed et al., 2012). For several kinds of plants in the area, there is a clear intraspecific variation in the antioxidant activity and phytochemical content among plant samples. These variations are typically linked to one of the following factors: (i) plants genetic makeup, (ii) stage of plant development (Rawat et al., 2014), (iii) impact of light intensity, (iv) growing altitude of the plant (Alonso-Amelot et al., 2007), and (v) local microclimate (Oloumi & Hassibi, 2011). The phytochemical heterogeneity found in our study may also be attribute to varied habitat and climatic conditions besides genetic background of each collected plant samples from four different habitats having altitudinal range of 1593–2582 m asl. The differences in phenolic mass fraction between different blueberry cultivars were determined to be caused by the growing season (Dragovi-Uzelac et al., 2010), while altitude of plant affects the tannin, glycyrrhizin flavonoid, and alkaloid content of licorice (Glycyrrhiza glabra). Binns et al., (2002) documented that in Echinacea angustifolia, phytochemical diversity shows strong positive correlation with altitude. Comparable reports are also available on various other species, such as Centella asiatica (Devkota et al., 2010) and Eucommia ulmodies (Tong et al., 2008). Moreover, Azadirachta indica’s phytochemicals and antioxidant activity were examined in relation to the initial temperature treatment (Vats, 2016). The diversity of habitat and microclimatic circumstances may be responsible for the variance in content of total phenolics and antioxidant activity across different sites (Lutharia and Mukhopadhyay, 2006). According to reports, phenolic compounds build up more with rising altitude as a form of defense against UV-B radiation from sunshine, which is why there is a maximum concentration at higher altitudes (> 2200 m asl) (Chaves et al., 1997). In plants, the accumulation of secondary metabolites is significantly influenced by altitude, because of the higher UV exposure, stress conditions, and lower temperature (zidorn et al., 2005; Spitaler et al., 2008; Giupponi et al., 2020), the findings of this investigation demonstrated that some physico-chemical characteristics of soil were significantly impacted by changes in altitude, understanding how changes in soil properties affect plant phytochemistry and, in turn, the plants’ ability to produce antioxidants.
Throughout the gradient in altitude, there was a reduction in the total N and OM content. High precipitation and low temperatures may slow the decomposition of soil organic matter. It is likely that variations in decomposition and nitrogen turnover rates contributed to variations in soil nitrogen storage. One of the fundamental source of OM in forest is litter and its quantity/quality relies on the plant species which is dominant (Magray et al., 2022). The primary element of the forest floor, OM, promotes microbiological diversity and maintains a steady supply of nutrients over the long term. The majority of soil C comes from OM and controls the soil’s characteristics (Woomer et al., 1999). The availability of N and K exhibited a strong positive association with the amount of OC and OM. Since, quantity of these vital nutrients is inversely correlated with humus concentration lends weight to this (Gupta & Sharma, 2008), OC and accessible P content had a negative relationship. Concentration of N in the forest soil is determined by OM content of soil (Magray et al., 2022). The assessed forest sites’ N (%) content revealed minimal variance between them. At elevation, the pH did not change significantly. Site IV had a mildly acidic environment. Moreover, Engda et al., (2008) observed that tree roots continuously absorb basic cations, which may be another factor contributing to the acidic nature of forest soils. Generally high-altitude soils have high organic matter content and favorable pH (Fitzsimons & Michael, 2017). However, this study revealed that the sites at higher altitudes were partially to fully rocky habitats. Environments that are mostly rocky are generally less fertile compared to other habitats. Which could explain the low levels of OC and OM at these altitudes. There is more biomass of herbaceous plants at higher altitudes than at lower altitudes. Wild animals that graze on herbaceous biomass at higher altitudes may cause compaction and reduce the volume and network of soil pores. According to a survey, intensive grazing shrink’s soil pores and compacts the soil (Yüksek & Yüksek, 2021). The current study aids in understanding the interrelations among various soil parameters and antioxidant activities across different habitats and altitudes. The level of nutrients in the soil has a crucial impact on the number of secondary metabolites, specifically flavonoids and phenolics, as shown in Fig. 6. Our study has uncovered a negative correlation between nitrogen and TPC and TFC indicating that an increase in soil nutrients, particularly nitrogen, leads to a decrease in secondary metabolites. The carbon/nutrient balance (CNB) hypothesis (Bryant et al., 1983) supports this relationship, suggesting that nitrogen fertilization leads to increased growth but decreased production of carbon-based secondary metabolites like terpenoids and phenolics, and potentially increased concentrations of nitrogen-based compounds like alkaloids (Fig. 7). According to CNB hypothesis presence of light, nitrogen and carbon have significant impact on the formation of carbon- and nitrogen-based allelochemicals, which subsequently has impact on the growth rate and production of secondary metabolites. Changes in the plant’s carbon-to-nutrient ratio impact the quantity of secondary metabolites produced. When there is a deficiency of nutrients, the plant synthesizes non-nitrogenous metabolites (via shikimic acid pathway). Previous research has also indicated the same results (Duarte et al., 2012). During current study, it was found that the antioxidant potential of P. cashmeriana had a negative relationship with morphological traits (height of plant, root/leaf dimensions, and parameters of soil). Morphological as well as concentration of phytochemicals showed significant variation relaying on the elevation of plants from the study site as revealed from the study; this variation in morphology is due to the diverse phenotypic expressions resulting from different habitats (Javid et al., 2023a, b; Suyal et al., 2019). Similar variations have also been observed in other Himalayan medicinal plants. The direct relation in morphological and soil parameters is due to the fact that plants tend to grow better in soils having good amount of nutrients than in poor ones (Inagaki et al., 2011). With nitrogen availability being a key limiting factor for plant growth (Kant et al., 2011). However, in nutrient-deficient soils, plant growth is restricted, as seen in Site IV. Negative correlation shown by antioxidant potential and morphology is due to the fact that higher altitudes often create more stress for plants, resulting in an enhanced content of phytochemicals which ultimately leads to higher activity of antioxidants but a reduction in overall plant growth (Cirak et al., 2017; Javid et al., 2023a, b).
Conclusion
The study emphasizes the screening of diverse plant populations to identify rich sources of phytochemicals and antioxidants. In our investigation, below-ground parts of P. cashmeriana exhibited the highest phytochemical content followed by the above-ground parts, suggesting potential benefits from the entire plant. Various extracts of P. cashmeriana may serve as therapeutic agents against oxidative and non-oxidative damage caused by reactive oxygen and nitrogen species. Altitude significantly influenced antioxidant activity, indicating enhanced potential in higher altitude cultivation. Changes in altitude also affected morphological features and soil properties, underscoring the importance of understanding their impact on plant phytochemistry. Future research aims to identify elite populations with specific habitat conditions and altitude for mass propagation.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aghakhani Kaaji, F., & Kharazian, N. (2019). Flavonoid diversity and morphological variations among seven Phlomis species in Zagros Iran. Iranian Journal of Science and Technology, Transactions A: Science, 43, 415–431.
Ahmad, S. R., & Ghosh, P. (2022). A systematic investigation on flavonoids, catechin, β-sitosterol and lignin glycosides from Saracaasoca (ashoka) having anti-cancer & antioxidant properties with no side effect. Journal of the Indian Chemical Society, 99(1), 100293.
Alonso-Amelot, M. E., Oliveros-Bastidas, A., & Calcagno-Pisarelli, M. (2007). Phenolics and condensed tannins of high altitude Pteridium arachnoideum in relation to sunlight exposure, elevation, and rain regime. Biochemical Systematics and Ecology, 35, 1–7.
Amor, I. L. B., Boubaker, J., Sgaier, M. B., Skandrani, I., Bhouri, W., Neffati, A., & Chekir-Ghedira, L. (2009). Phytochemistry and biological activities of Phlomis species. Journal of ethnopharmacology, 125(2), 183–202.
Anand, U., Tudu, C. K., Nandy, S., Sunita, K., Tripathi, V., Loake, G. J., ... & Proćków, J. (2022). Ethnodermatological use of medicinal plants in India: From ayurvedic formulations to clinical perspectives–A review. Journal of ethnopharmacology, 284, 114744.
Arfin, S., Jha, N. K., Jha, S. K., Kesari, K. K., Ruokolainen, J., Roychoudhury, S., ... & Kumar, D. (2021). Oxidative stress in cancer cell metabolism. Antioxidants, 10(5), 642.
Bai, X., Pan, R., Li, M., Li, X., & Zhang, H. (2019). HPLC profile of longan (cv. Shixia) pericarp-sourced phenolics and their antioxidant and cytotoxic effects. Molecules, 24(3), 619.
Belwal, T., Pandey, A., Bhatt, I. D., Rawal, R. S., & Luo, Z. (2019). Trends of polyphenolics and anthocyanins accumulation along ripening stages of wild edible fruits of Indian Himalayan region. Scientific Reports, 9(1), 5894.
Bhatt, I. D., Rawat, S., Badhani, A., & Rawal, R. S. (2017). Nutraceutical potential of selected wild edible fruits of the Indian Himalayan region. Food Chemistry, 215, 84–91.
Binns, S. E., Arnason, J. T., & Baum, B. R. (2002). Phytochemical variation within populations of Echinacea angustifolia (Asteraceae). Biochemical Systematics and Ecology, 30, 837–854.
Boeing, J. S., Barizão, É. O., e Silva, B. C., Montanher, P. F., de CinqueAlmeida, V., & Visentainer, J. V. (2014). Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chemistry Central Journal, 8, 48.
Braca, A., Sortino, C., Politi, M., Morelli, I., & Mendez, J. (2002). Antioxidant activity of flavonoids from Licania licaniaeflora. Journal of Ethnopharmacology, 79(3), 379–381.
Bray, R. H., & Kurtz, L. T. (1945). Determination of total, organic and available forms of phosphorus in soils. Soil Science, 59, 39–45.
Bryant, J. P., Chapin, F. S., III., & Klein, D. R. (1983). Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos, 40, 357–368.
Chand, M. B., Paudel, M. R., & Pant, B. (2016). The antioxidant activity of selected wild orchids of Nepal. Journal of Coastal Life Medicine, 4, 731–736.
Cirak, C., Radusiene, J., Jakstas, V., Ivanauskas, L., Seyis, F., & Yayla, F. (2017). Altitudinal changes in secondary metabolite contents of Hypericum androsaemum and Hypericum polyphyllum. Biochemical Systematics and Ecology, 70(2), 108–115.
Devkota, A., Dall Acqua, S., Jha, P. K., & Innocenti, G. (2010). Variation in the active constituent contents in Centella asiatica grown in different habitats in Nepal. Botnica Orientalis- Journal of Plant Science, 7, 43–47.
Dhanani, T., Shah, S., Gajbhiye, N. A., & Kumar, S. (2017). Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arabian Journal of Chemistry King Saud University, 10, S1193–S1199.
Dragovi-Uzelac, V., Savi, Z., Brala, A., Levaj, B., Kovaceveic, D. B., & Bisko, A. (2010). Evaluation of phenolic content and antioxidant capacity of blueberry cultivars (Vaccinium corymbosum L.) grown in the northwest Croatia. Food Technology and Biotechenology, 48, 214–221.
Duarte, A. R., Santos, S. C., Seraphin, J. C., & Ferri, P. H. (2012). Influence of spatial, edaphic and genetic factors on phenols and essential oils of Myrciaria cauliflora fruits. Journal of the Brazilian Chemical Society, 23, 737–746.
Engda, T. A., Kassahun, H. T., Collick, A. S., Adissu, T., Ashagrie, B. B., Tessema, Z. K.,& Steenhus, T. S. (2008). Soil properties and fertility status dynamics of North Western Ethiopia as influenced by land use changes: case of Dibanke watershed.
Fitzsimons, J. A., & Michael, D. R. (2017). Rocky outcrops: A hard road in the conservation of critical habitats. Biological Conservation, 211(2), 36–44.
Giupponi, L., Leoni, V., Pavlovic, R., & Giorgi, A. (2020). Influence of altitude on phytochemical composition of hemp inflorescence: A metabolomic approach. Molecules, 25(6), 1381.
Gupta, M. K., & Sharma, S. D. (2008). Effect of tree plantation on soil properties, profile morphology and productivity index I poplar in Uttarakhand. Annals of Forestry, 16(2), 209–224.
Hagerman, A., Harvey-Mueller, I., & Makkar, H. P. S. (2000). Quantification of tannins in tree foliage – A laboratory manual (pp. 4–7). Vienna (Austria): FAO/IAEA.
Inagaki, M., Kamo, K., Miyamoto, K., Titin, J., Jamalung, L., Lapongan, J., & Miura, S. (2011). Nitrogen and phosphorus retranslocation and N: P ratios of litterfall in three tropical plantations: Luxurious N and efficient P use by Acacia mangium. Plant and Soil, 341(1–2), 295–307.
Javid, H., Qadir, R. U., Magray, J. A., Wani, B. A., Nawchoo, I. A., & Gulzar, S. (2023a). Growth dynamics and resource allocation of Bistorta amplexicaulis (D. Don) Greene: An alteration across different habitats and altitudes. Proceedings of the Pakistan Academy of Sciences: B. Life and Environmental Sciences, 60(1), 57–70.
Javid, H., Ul Qadir, R., Magray, J. A., Wani, B. A., Nawchoo, I. A., & Gulzar, S. (2023b). Variability in morphology, phytochemicals and antioxidant activity in Bistorta amplexicaulis (D. Don) Greene populations under variable habitats and altitudes. Natural Product Research, 16, 1–8.
Kant, S., Bi, Y. M., & Rothstein, S. J. (2011). Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. Journal of Experimental Botany, 62(4), 1499–1509.
Klein, S. M., Cohen, G., & Cederbaum, A. I. (1981). Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical-generating systems. Biochemistry, 20(21), 6006–6012.
Kumar, A., Ilavarasan, R., Jayachandran, T., Deecaraman, M., Aravindan, P., Padmanabhan, N., & Krishan, M. R. V. (2008). Anti diabetic activity of Syzygiumcumini seed and its isolate compounds against streptozotocin induced diabetic rats. Journal of Medicinal Plants Research, 2(9), 246–249.
Kumaran, A., & Karunakaran, R. J. (2006). Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chemistry, 97(1), 109–114.
Luo, S. Y., Zhu, J. Y., Zou, M. F., Yin, S., & Tang, G. H. (2022). Mulberry Diels–Alder-type adducts: Isolation, structure, bioactivity, and synthesis. Natural Products and Bioprospecting, 12(1), 31.
Luthria, D. L., Mukhopadhyay, S., & Krizek, D. T. (2006). Content of total phenolics and phenolic acids in tomato (Lycopersicon esculentum Mill.) fruits as influenced by cultivar and solar UV radiation. Journal of food composition and analysis, 19(8), 771–777.
Magray, J. A., Wani, B. A., Islam, T., Ganie, A. H., & Nawchoo, I. A. (2022). Phyto-ecological analysis of Phytolacca acinosa Roxb. assemblages in Kashmir Himalaya, India. Frontiers in Forests and Global Change, 5, 976902.
Marcocci, L., Maguire, J. J., Droylefaix, M. T., & Packer, L. (1994). The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochemical and Biophysical Research Communications, 201(2), 748–755.
Morwin, H. D., & Peach, P. M. (1951). Exchangeability of soil potassium in and silt and clay fractions as influenced by the nature of complementary exchangeable cations Pro Nat Acad Sci Uni Stat Amer 15:125–128 of Indian Himalayan region. Scientifc Reports, 9(1), 1–11.
Okur, M. E., Karadağ, A. E., Özhan, Y., Sipahi, H., Ayla, Ş, Daylan, B., Kültür, Ş, Demirci, B., & Demirci, F. (2021). Anti-inflammatory, analgesic and in vivo-in vitro wound healing potential of the Phlomis rigida Labill extract. Journal of Ethnopharmacology, 266(1), 113408.
Oloumi, H., & Hassibi, N. (2011). Study the correlation between some climate parameters and the content of phenolic compounds in roots of Glycyrrhiza glabra. Journal of Medicinal Plant Research, 5, 6011–6016.
Oyaizu, M. (1986). Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. The Japanese Journal of Nutrition and Dietetics, 44(6), 307–315.
Paudel, M. R., Chand, M. B., Pant, B., & Pant, B. (2018). Antioxidant and cytotoxic activities of Dendrobium moniliforme extracts and the detection of related compounds by GC-MS. BMC Complementary and Alternative Medicine, 18(1), 1–9.
POWO (2023). “Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet; http://www.plantsoftheworldonline.org/Retrieved 10 March 2023.”
Qadir, R. U., Javid, H., Shapoo, G. A., Wani, B. A., Magray, J. A., Nawchoo, I. A., & Gulzar, S. (2022). Phenotypic variability and resource allocation in Kashmir Sage (Phlomis cashmeriana Royle ex Benth.) in relation to different habitats and altitudes. Proceedings of the Pakistan Academy of Sciences: B. Life and Environmental Sciences, 59(2), 35–47.
Rafiq, S., Wagay, N. A., Elansary, H. O., Malik, M. A., Bhat, I. A., Kaloo, Z. A., Hadi, A., Alataway, A., Dewidar, A. Z., El-Sabrout, A. M., et al. (2022). Phytochemical screening, antioxidant and antifungal activities of Aconitum chasmanthum Stapf ex Holmes wild rhizome extracts. Antioxidants, 11(6), 1052.
Rawat, S., Andola, A., Giri, L., Dhyani, P., Jugran, A., Bhatt, I. D., & Rawal, R. S. (2014). Assessment of nutritional and antioxidant potential of selected vitality strengthening medicinal plants. International Journal of Food Properties, 17, 703–712.
Rodrigo, R. (2009). oxidative stress and antioxidants: Their role in human diseases (pp. 9–10). Nova: New York, NY, USA.
Saeed, N., Khan, M. R., & Shabbir, M. (2012). Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilisleptophylla L. BMC Complementary and Alternative Medicine, 12, 221.
Samadov, B. S. (2022). The chemical composition of the medicinal plant Momordica Charantia L used in folk medicine. Thematics Journal of Chemistry, 6(1).
Sarkhail, P., Rahmanipour, S., Fadyevatan, S., Mohammadirad, A., Dehghan, G., Amin, G., & Abdollahi, M. (2007). Antidiabetic effect of Phlomis anisodonta: Effects on hepatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Pharmacological Research, 56(3), 261–266.
Sartoratto, A., Machado, A. L. M., Delarmelina, C., Figueira, G. M., Duarte, M. C. T., & Rehder, V. L. G. (2004). Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Brazilian Journal of Microbiology, 35, 275–280.
Shadia, E., El-Aziz, A., Omer, E. A., & Sabra, A. S. (2007). Chemical composition of Ocimum americanum essential oil and its biological effects against, Agrotisipsilon, (Lepidoptera: Noctuidae). Research Journal of Agriculture and Biological Sciences, 3, 740–747.
Shang, X., Wang, J., Li, M., Miao, X., Pan, H., Yang, Y., & Wang, Y. (2011). Antinociceptive and anti-inflammatory activities of Phlomis umbrosa Turcz extract. Fitoterapia, 82(4), 716–721.
Shang, X., Chao, Y., Zhang, Y., Lu, C., Xu, C., & Niu, W. (2016). Immunomodulatory and antioxidant effects of polysaccharides from Gynostemma pentaphyllum Makino in immunosuppressed mice. Molecules, 21(8), 1085.
Sharma, A., & Cannoo, D. S. (2016). Effect of extraction solvents/techniques on polyphenolic contents and antioxidant potential of the aerial parts of Nepeta leucophylla and the analysis of their phytoconstituents using RP-HPLC-DAD and GC-MS. RSC Advances, 6(81), 78151–78160.
Spitaler, R., Winkler, A., Lins, I., Yanar, S., Stuppner, H., & Zidorn, C. (2008). Altitudinal variation of phenolic contents in flowering heads of Arnica montana cv. ARBO: A 3-year comparison. Journal of Chemical Ecology, 34, 369–375.
Subbiah, B. V., & Asija, C. L. (1956). A rapid procedure for the estimation of available nitrogen in soils. Current Science, 25, 256–260.
Suyal, R., Rawat, S., Rawal, R. S., & Bhatt, I. D. (2019). Variability in morphology, phytochemicals, and antioxidants in Polygonatum verticillatum (L.) All populations under different altitudes and habitat conditions in Western Himalaya, India. Environmental monitoring and assessment, 191(S3), 1–18.
Tong, L., Wang, Y., Xiong, J., Cui, Y., Zhou, Y., & Yi, L. (2008). Selection and fingerprints of the control substances for plant drug Eucommiaulmodies Oliver by HPLC and LC–MS. Talanta, 76, 80–84.
Vats, S. (2016). Effect of initial temperature treatment on phytochemicals and antioxidant activity of Azadirachta indica A. Juss. Applied Biochemistry and Biotechnology, 1–9.
Wafa, N., Sofiane, G., & Mouhamed, K. (2016). The antioxidant and antimicrobial activities of flavonoids and tannins extracted from Phlomis bovei De Noé. European Journal of Experimental Biology, 6(3), 55–61.
Walkley, A., & Black, I. A. (1934). An examination of the Degti jareff method for determining soil organic matter, and a proposed modification of the chromic soil titration method. Soil Science, 38, 29–38.
Woomer, P. L., Martin, A., Albrecht, A., Reseck, D. V. S., & Scharpenseel, H. W. (1999). The importance and management of soil organic matter in the tropics. In P. L. Woomer & M. J. Swift (Eds.), The biological management of tropical soil fertility (pp. 47–80). Wiley.
Yüksek, T., & Yüksek, F. (2021). Effects of altitude, aspect, and soil depth on carbon stocks and properties of soils in a tea plantation in the humid Black Sea region. Land Degradation & Development, 32(15), 4267–4276.
Zidorn, C., Schubert, B., & Stuppner, H. (2005). Altitudinal differences in the contents of phenolics in flowering heads of three members of the tribe Lactuceae (Asteraceae) occurring as introduced species in New Zealand. Biochemical Systematics and Ecology, 33(9), 855–872.
Złotek, U., Mikulska, S., Nagajek, M., & Świeca, M. (2016). The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi Journal of Biological Sciences, 23, 628–33.
Acknowledgements
We express our gratitude to the Department of Botany at the University of Kashmir for granting us access to the essential research facilities. Additionally, we extend our thanks to our colleagues for their valuable support in both the field and laboratory work.
Funding
This work received funding from MANF2018–19-JAM-97230 in favor of HJ.
Author information
Authors and Affiliations
Contributions
Roof Ul Qadir, Hanan Javid and Irshad Ahmad Bhat perceived the research idea. Irshad A Nawchoo and Shaban Gulzar supervised the research work. Bilal Ahmad Wani, Roof Ul Qadir, and Junaid Ahmad Magray collected field and herbarium data. Hanan Javid, Junaid Ahmad Magray conducted the data analysis; validation and visualization were carried out by Roof Ul Qadir, Irshad Ahmad Bhat and Bilal Ahmad Wani. The original draft was written by Roof Ul Qadir and Hanan Javid with a detailed review, editing and inputs from Irshad Ahmad Nawchoo and Shabana Gulzar. All the authors reviewed and approved the final draft for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qadir, R.U., Bhat, I.A., Javid, H. et al. Exploring morphological variability, in vitro antioxidant potential, and HR-LCMS phytochemical profiling of Phlomis cashmeriana Royle ex Benth. across different habitats of Kashmir Himalaya. Environ Monit Assess 196, 241 (2024). https://doi.org/10.1007/s10661-024-12338-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-024-12338-2