Abstract
Liquid–liquid extraction (LLE) of mixtures of butanol, 1,3-propanediol (PDO), and ethanol was performed using soybean-derived biodiesel as the extractant. The composition of the mixtures simulated the product of the anaerobic fermentation of biodiesel-derived crude glycerol, which has recently been reported for the first time by the authors. Using a biodiesel: with an aqueous phase volume ratio of 1:1, butanol recovery ranged from 45 to 51% at initial butanol concentrations of 150 and 225 mM, respectively. Less than 10% of the ethanol was extracted, and essentially no PDO was extracted. The partition coefficient for butanol in biodiesel was determined to be 0.91 ± 0.097. This partition coefficient is less than that of oleyl alcohol, which is considered the standard for LLE. However, butanol is suitable for blending with biodiesel, which would eliminate the need for separating the butanol after extraction. Additionally, biodiesel is much less costly than oleyl alcohol. If biodiesel-derived glycerol is used as the feedstock for butanol production, and biodiesel is used as the extractant to recover butanol from the fermentation broth, production of a biodiesel/butanol fuel blend could be a fully integrated process within a biodiesel facility. This process could ultimately help reduce the cost of butanol separation and ultimately help improve the overall economics of butanol fermentation using renewable feedstocks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Butanol is one petroleum-based chemical that could be produced using renewable biomass resources. Butanol has been identified as a key biorefinery platform chemical that can be used for the production of new chemical products as well as biobased alternatives to many petroleum chemicals [1]. In addition to conventional uses, butanol can be used as an alternative fuel. Butanol has better physical properties including a higher energy content and lower vapor pressure as compared to ethanol. Butanol also exhibits higher miscibility with gasoline and diesel, making it preferable to ethanol for blending with petroleum fuels [2]. In the early 20th century, the fermentation of sugars by Clostridium acetobutylicum was used extensively for industrial production of a mixture of butanol, acetone, and ethanol (ABE). However, high substrate and fermentation costs combined with low yields eventually resulted in the demise of the fermentation process in favor of petrochemical production routes. In recent years, there has been renewed interest in improving the ABE fermentation. Recent research efforts have included genetic engineering of C. acetobutylicum and another solventogenic strain, Clostridium beijerinckii, to increase butanol production, optimizing fermentation and reactor conditions, and investigating novel separations technologies to recover the solvents [3].
The two most important factors affecting the cost of butanol fermentation processes are feedstock cost and the cost of separating butanol from dilute fermentation broth. While lignocellulosic biomass is a potential low-cost substrate, the pre-treatment processes required to produce fermentable sugars contribute significantly to capital costs and overall production costs. Pretreatment of lignocellulosic biomass also produces a number of compounds that are potent inhibitors of cell growth and solvent formation, which ultimately reduces butanol yield and increases downstream separations costs.
An alternative feedstock for butanol production is glycerol, in particular crude glycerol that is generated during biodiesel production. Biodiesel production yields about 10 wt.% glycerol and the crude glycerol product also contains about 85% glycerol, along with water, methanol, and salts. The bacterium Clostridium pasteurianum has been shown to produce significant amounts of butanol, 1,3-propanediol, and ethanol using both purified and crude glycerol as the sole carbon source [4–6]. A recent paper by the authors reports that C. pasteurianum is capable generating butanol yields up to 30 wt.% using biodiesel-derived crude glycerol as the sole carbon source [6]. This is notably higher than the 15–20 wt.% butanol yields that are typically obtained using C. acetobutylicum or C. beijerinckii. Additionally, glycerol fermentation does not produce acetone and produces much less ethanol, which should simplify the butanol purification process. Finally, if biodiesel-derived crude glycerol is used as the feedstock for butanol production, it could considerably decrease or eliminate the costs associated with feedstock acquisition and pretreatment. Although butanol production via glycerol fermentation using C. pasteurianum offers a number of advantages, the resulting fermentation broth still contains dilute concentrations of butanol. Therefore, if butanol is produced via fermentation, issues regarding separation of a dilute mixture will still need to be addressed.
As the boiling point of butanol is higher than water, conventional distillation requires removal of very large amounts of water, and the energy costs to remove the water make the process economically unfavorable. Various other separations technologies have been investigated as options for butanol purification. These options include gas stripping, liquid–liquid extraction (LLE), and pervaporation [3]. Of these options, LLE is particularly attractive, as when performed in situ, butanol can be removed as it is produced. Since butanol is known to be toxic to cell growth and product formation, in situ removal can increase productivity. Potential solvents for in situ butanol extraction must be non-toxic to the organism, have a high partitioning coefficient for butanol, be immiscible and not form emulsions with the fermentation broth, and be inexpensive and readily available [3]. A variety of solvents have been evaluated for their potential to extract dilute concentrations of alcohols such as butanol and ethanol from dilute solutions, including vegetable oils, fatty esters, paraffinic hydrocarbons, and primary alcohols [7]. Oleyl alcohol is typically recognized as the best solvent for butanol extraction, as it has a high affinity for butanol and is not toxic to the cells at short exposure times (24–48 h) [3]. Unfortunately, the cost of oleyl alcohol combined with the cost of recovering butanol from the extractant make this process economically unfavorable.
An alternative extractant to recover butanol from dilute fermentation broth is biodiesel. Research evaluating different types of biodiesel has shown it to be an effective solvent for many compounds, and biodiesel is also biodegradable and non-toxic to humans and the environment, making it a “green” solvent. If biodiesel-derived crude glycerol were used as the feedstock, a butanol fermentation process could be integrated into a biodiesel production facility, and biodiesel could be used as the extractant at little cost. This closed-loop process would result in either a biodiesel-butanol biofuel blend or, following a secondary distillation step, a pure butanol product.
The overall goal of this research is to evaluate the effectiveness of biodiesel as a solvent for butanol extraction. Several aspects set this work apart from previous efforts: (1) this research specifically evaluated the LLE of butanol–PDO–ethanol mixtures that would be generated from glycerol fermentation, and (2) this research evaluated biodiesel produced from soybean oil, which is the primary feedstock for biodiesel production in the US. Previous published efforts evaluating LLE focused on acetone, butanol, ethanol (ABE) mixtures, and only evaluated solvents such as oleyl alcohol and biodiesel derived from palm oil and sunflower oil. These efforts also used biodiesel from an industrial biodiesel facility producing over 25 million gallons per year, while previous efforts used biodiesel produced in a laboratory setting. Specific objectives include determining the extent to which soybean derived biodiesel can extract butanol at concentrations that would likely be produced during glycerol fermentation, determining the selectivity for butanol, and determining the value of the partition coefficient, K, of biodiesel for butanol.
Experimental Procedures
Oleyl alcohol (85%, Fisher Scientific) and soybean-derived biodiesel (Green River Biodiesel, Moundville, AL) were used as extractants. Aqueous mixtures of butanol (Sigma Aldrich), 1,3-propanediol (Fisher Scientific), and ethanol (Sigma Aldrich) were prepared in the laboratory at concentrations reported from previous studies of glycerol fermentation using C. pasteurianum [5]. Experiments were divided into “high” and “low” concentrations. The “high” concentration mixtures contained approximately 225 mM butanol, 59 mM PDO, and 31 mM ethanol (17, 4.5, and 1.5 g/L, respectively). This concentration of butanol is close to the maximum that has been reported in batch fermentation. The “low” concentration mixtures contained 150 mM butanol, 25 mM PDO, and 20 mM ethanol (11, 2, and 1 g/L, respectively). Biodiesel:aqueous phase volume ratios of 1:1, 1:2, and 2:1 were tested. Oleyl alcohol extractions were performed using mixtures of 225 mM butanol, 59 mM PDO, and 31 mM ethanol at alcohol:aqueous phase volume ratios of 1:1, 2:1 and 1:2.
Select specifications of the biodiesel were provided by Green River Biodiesel and are as follows: Water content, 78 mg/kg; kinematic viscosity at 40 °C, 4.003 mm2/s; sulfur, 6 mg/kg; ester content 99.6% wt/wt; free glycerine, <0.01% wt/wt; monoglyceride, 0.47% wt/wt; diglyceride, 0.15% wt/wt; triglyceride, 0.14% wt/wt; bound glycerine, 0.14% wt/wt; total glycerine, 0.14% wt/wt; methanol, 0.06% wt/wt; polyunsaturated methyl ester 0.1% wt/wt; linolenic acid methyl ester, 7.1% wt/wt; group I metals (Na + K), 0.1 mg/kg; group II metals (Ca + Mg), <0.1 mg/kg; phosphorus, <0.1 mg/kg; acid value, 0.53 mg KOH/g.
Extractions were performed at room temperature in 15-mL centrifuge tubes. The total volume of liquid was 10 mL (for 1:1 experiments) or 12 mL (for 1:2 and 2:1 experiments). All experiments were agitated by hand for several minutes to facilitate liquid–liquid contact. Experiments using biodiesel as the extractant were centrifuged at 700g for 5 min and the biodiesel was decanted. Experiments using oleyl alcohol as the extractant could not be centrifuged, as this formed an emulsion. After shaking, these experiments were allowed to settle for 5 min before decanting the oleyl alcohol. All experiments were performed in triplicate.
The concentration of butanol, PDO, and ethanol in the aqueous phase was measured before and after extracting using high pressure liquid chromatography. The HPLC system was equipped with a Bio-Rad (Hercules, CA) Aminex HPX-87H column and a refractive index detector. The mobile phase was 5 mM H2SO4, pumped at a flow rate of 0.4 mL/min.
The experimental error associated with solution preparation and HPLC analysis was estimated as one combined term for each of the components. The error was estimated by averaging the initial concentration of each of the components as determined by HPLC analysis. For the 18 initial butanol samples that were analyzed, the concentration as determined by HPLC analysis varied by an average of 4.1%. The initial concentration of the PDO samples varied by an average of 5.0%, and the initial concentration of the ethanol varied by 6.9%. Therefore, the concentrations of butanol, PDO, and ethanol, were assumed correct within 4.1, 5.0, and 6.9%, respectively.
The concentration of butanol, PDO, and ethanol in the extractant was calculated by performing mass balance calculations using the results of HPLC analysis. Any solute not detected in the aqueous phase was assumed to have partitioned into the biodiesel phase. The partition coefficient, K, which is defined as y/x, is calculated by dividing the concentration of the component in the extractant phase (y, in g/L) by the concentration of the component in the aqueous phase following extraction (x, in g/L) [8].
Results and Discussion
Soybean-derived biodiesel proved effective at extracting butanol from aqueous mixtures simulating product concentrations that would be produced during glycerol fermentation by Clostridium pasteurianum. Figure 1 shows the initial and final concentrations of butanol, 1,3-propanediol (PDO), and ethanol at biodiesel:aqueous phase volume ratios of 1:2, 1:1, and 2:1. As shown in the figures, significant amounts of butanol were extracted into the biodiesel, while only limited amounts of ethanol, and essentially no PDO were extracted. As would be expected, increasing the amount of solvent increased the amount of butanol extracted. Similar results were observed with ethanol, but only to a limited extent. Biodiesel exhibited little to no affinity for PDO, independent of PDO concentration or the ratio of aqueous phase to solvent.
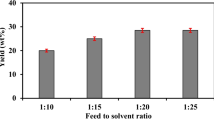
Figure 2 shows the percent of butanol, PDO, and ethanol extracted at each of the initial butanol concentrations and aqueous phase:biodiesel ratios. Only 31% of the butanol was extracted using a biodiesel:aqueous phase volume ratio of 1:2. At a ratio of 1:1, 45% of the butanol was extracted at an initial butanol concentration of 150 mM, while 51% of the butanol was extracted at an initial butanol concentration of 225 mM. A statistical t test at the 95% confidence interval determined that the butanol recovery at these two initial butanol concentrations is not significantly different. Over 60% of the butanol was extracted at a biodiesel:aqueous phase volume ratio of 2:1.
A maximum of 11% ethanol was extracted at an initial ethanol concentration of 31 mM and a biodiesel:aqueous phase volume ratio of 2:1. Overall, the amount of ethanol extracted ranged from 1.6 to 7.1% at the lower ethanol concentration and 7.5–11% at the higher ethanol concentration. The most PDO that was extracted was 3.5%. However, the standard deviation of the PDO results is larger than the average values, indicating that it cannot be concluded with any certainty that PDO was actually extracted.
In several experiments, the concentration of PDO in the aqueous phase was higher following the extraction process. However, the percent difference was within the percentage error as described in Experimental Procedures. Since it was not possible for any more solute to be added to the aqueous phase during extraction, in any experiment in which this was observed, it was assumed that no extraction occurred, and the concentration of PDO or ethanol in the biodiesel phase following extraction was assumed to be zero.
Using the concentration of butanol, PDO, and ethanol in the aqueous phase following extraction, the concentration of each component in the biodiesel phase was calculated. The ratio of the concentration of solute in the extractant to the concentration of solute in the aqueous phase is the partition coefficient, K. Table 1 shows the average K value for each of the three solutes when biodiesel is used as the extractant. As would be expected in dilute extraction, there was no statistical difference in K values calculated for the solutions with lower concentrations of solute versus those with higher concentrations. However, extraction theory states that the equilibrium relationship between the solute and the two phases is not necessarily linear, and the K value may not be constant for a given range of concentrations, particularly at higher concentrations. Similarly, changing the biodiesel:aqueous phase volume ratio did not affect the K values. However, as expected, larger amounts of extractant did result in larger amounts of butanol being extracted (i.e., higher percent recovery).
As stated in the “Experimental Procedures”, experiments were also performed under identical conditions using oleyl alcohol as the extractant rather than biodiesel. Oleyl alcohol is widely considered the standard for dilute solvent extraction. Table 1 also shows the average K values determined from the extraction of mixtures of butanol, ethanol, and PDO using oleyl alcohol at ratios of 1:1, 2:1, and 1:2 with respect to the aqueous phase. Oleyl alcohol has a much higher affinity for butanol, as indicated by the K value. Oleyl alcohol also extracted 58, 75, and 84%, of the butanol at alcohol:aqueous phase volume ratios of 1:2, 1:1, and 2:1, respectively. However, biodiesel was able to extract up to 71% of the butanol in a two-stage extraction process (data not shown), indicating that, despite the lower K value, high mass recovery is possible using biodiesel as the extractant in multi-stages.
Table 1 also shows the K values for the extraction of mixtures of butanol and ethanol (with acetone and trace amounts of acetate and butyrate) using methylated crude palm oil (CPO) and oleyl alcohol [9]. The partition coefficient for butanol using CPO reported by Ishizaki et al. [9] is the same as that observed in this work using biodiesel. However, the partition coefficient for butanol using oleyl alcohol in this work is notably lower than that reported previously. The partition coefficient could have been affected by the different composition of the aqueous phase, as Ishizaki et al. [9] evaluated mixtures of butanol, ethanol, acetone, acetate, and butyrate, while this work evaluated mixtures of butanol, ethanol, and PDO. Ishizaki et al. [9] did not provide any standard deviation values for their reported K values. Ishizaki et al. [9] also reported that about 47% of the butanol was extracted by the CPO at ratios of 1:1. Crabbe et al. [10] performed extractions of butanol, acetone, ethanol, acetate, and butyrate using CPO and also reported 40–50% total recovery of butanol in the extractant but did not report any partition coefficient. Grobben et al. [11] determined the partition coefficients for butanol and ethanol using biodiesel produced from sunflower oil as the extractant. These K values are also given in Table 1 (standard deviations were not provided). The values are higher, particularly with respect to ethanol, but the extractions were performed at pH 4.5, which is the final pH of fermentation broth following solvent production.
In conclusion, this work demonstrated that soybean-derived biodiesel is an effective extractant for butanol from fermentation mixtures containing butanol, ethanol, and PDO. The average partition coefficient for butanol in soybean-derived biodiesel is 0.91. Single-stage extraction using a biodiesel:aqueous phase volume ratio of 1:1 can remove up to 50% of the butanol when the initial concentration is comparable to that produced during anaerobic fermentation, while two stages could remove up to 71% of the butanol. This work also showed that biodiesel is highly selective for butanol and removes very little ethanol and essentially no PDO. Therefore, if biodiesel were used as an extractant, subsequent separation could produce a very pure butanol product. However, since butanol is a suitable fuel for blending with biodiesel, recovery of the butanol may not be necessary. Although butanol is a promising alternative fuel, particularly for blending, it should be mentioned that there are currently no ASTM standards for butanol–biodiesel blends. Since biodiesel has also been shown to be non-toxic to species of clostridia [9], in situ extraction could be applied during fermentation to remove the butanol as it is being produced. In situ extraction would mitigate the issue of butanol toxicity and increase butanol productivity by minimizing product inhibition. If biodiesel-derived glycerol is used as the feedstock for butanol production via fermentation, and biodiesel is used as the extractant to recover butanol from the fermentation broth, production of a biodiesel/butanol fuel blend could be a fully integrated process within a biodiesel facility. Even though the partition coefficient for butanol is lower for biodiesel than other solvents such as oleyl alcohol, this potential for full integration certainly makes biodiesel a more favorable extractant. Additionally, biodiesel is significantly less expensive than oleyl alcohol (about one-half of the cost). However, additional improvements to improving feedstock utilization and increasing butanol yield concentration need to be made in order to improve the overall economics of the butanol fermentation process.
References
Paster M, Pellegrino JL, Carole TM (2003) Industrial bioproducts: today and tomorrow. Report prepared by Energetics, Inc., for the United States Department of Energy, Office of Energy Efficiency and Renewable Energy, Office of the Biomass Program, Washington, D.C.
Qureshi N, Blaschek HP (2001) ABE production from corn: a recent economic evaluation. J Ind Microbiol Biotechnol 27:292–297
Ezeji TC, Qureshi N, Blaschek HP (2004) Butanol fermentation research: upstream and downstream manipulations. Chem Rec 4:305–314
Dabrock B, Bahl H, Gottschalk G (1992) Parameters affecting solvent production by Clostridium pasteurianum. Appl Environ Microbiol 58(4):1233–1239
Biebl H (2001) Fermentation of glycerol by Clostridium pasteurianum: batch and continuous culture studies. J Ind Microbiol Biotechnol 27:18–26
Taconi K, Venkataramanan K, Johnson D (2009) Growth and solvent production by Clostridium pasteurianum ATCC® 6013™ utilizing biodiesel derived crude glycerol as the sole carbon source. Environ Prog Sustain Energy 28(1):100–110
Offeman RD, Stephenson SK, Robertson GH, Orts WJ (2006) Solvent extraction of ethanol from aqueous solutions using biobased oils, alcohols, and esters. J Am Oil Chem Soc 83(2):153–156
Harrison RG, Todd P, Rudge SR, Petrides DP (2003) Bioseparations science and engineering. Oxford University Press, New York
Ishizaki A, Michiwaki S, Crabbe E, Kobayashi G, Sonomoto K, Yoshino S (1999) Extractive acetone–butanol–ethanol fermentation using methylated crude palm oil as extractant in batch culture of Clostridium saccharoperbutylacetonicum N1–4 (ATCC 13564). J Biosci Bioeng 87:352–356
Crabbe E, Nolasco-Hipolito C, Kobayashi G, Sonomoto K, Ishizaki A (2001) Biodiesel production from crude palm oil and evaluation of butanol extraction and fuel properties. Process Biochem 37:65–71
Grobben NG, Eggink G, Cuperus FP, Huizing HJ (1993) Production of acetone, butanol, and ethanol (ABE) from potato wastes: fermentation with integrated membrane extraction. Appl Microbiol Biotechnol 39:494–498
Acknowledgments
This research was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2007-35504-18253.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Adhami, L., Griggs, B., Himebrook, P. et al. Liquid–Liquid Extraction of Butanol from Dilute Aqueous Solutions Using Soybean-Derived Biodiesel. J Am Oil Chem Soc 86, 1123–1128 (2009). https://doi.org/10.1007/s11746-009-1447-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-009-1447-7