Abstract
Bile salt hydrolase (BSH, EC 3.5.1.24) is considered as an ideal way with lower cost and less side effects to release the risk of coronary heart disease caused by hypercholesterolemia. As bile salt hydrolase from Lactobacillus plantarum BBE7 could not be efficiently exported by PelB signal peptide of the general secretory (Sec) pathway, three twin-arginine signal peptides from twin-arginine translocation (Tat) pathway were synthesized, fused with bsh gene, inserted into expression vectors pET-20b(+) and pET-22b(+), and transformed into four different Escherichia coli hosts, respectively. Among the 24 recombinant bacteria obtained, E. coli BL21 (DE3) pLysS (pET-20b(+)-dmsA-bsh) showed the highest BSH activity in periplasmic fraction, which was further increased to 1.21 ± 0.03 U/mL by orthogonal experimental design. And, signal peptide dimethyl sulfoxide reductase subunit DmsA (DMSA) had the best activity of exported BSH. More importantly, the presence of BSH in the periplasm had proven to be caused by the export rather than cell leakage. For the first time, we report the periplasmic expression of BSH by signal peptides from the Tat pathway. This will lay a solid foundation for the purification and biochemical characterization of BSH from the supernatant, and strategies adopted here could be used for the periplasmic expression of other proteins in E. coli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In bacteria, a vast majority of the newly synthesized proteins are translocated across the membrane via the general secretory (Sec) system [1]. However, because of the premature folding in the cytoplasm and subsequent degradation by protease, many Sec-targeted proteins cannot be easily released into the medium [2, 3]. Hence, the twin-arginine translocation (Tat) pathway, capable of transporting fully folded or oligomeric proteins across the cytoplasmic membrane, is used as an alternative [4]. In this system, proteins are synthesized as precursors with specialized N-terminal signal peptides bearing a conserved (S/T) RRXFLK motif [2]. The hydrophobic (h-) region of these signal peptides is often less hydrophobic than the corresponding region of signal peptides from Sec pathway. Besides, their C-terminal usually contains positively charged residues compared to neutral residues in this region of Sec signal peptides [4]. Export across the cytoplasmic membrane is initiated by the interaction of the signal peptides with the Tat export machinery, which in Escherichia coli is made up of the membrane protein TatABC [4]. These proteins usually form two complexes, including transport channel complex TatA with the ability of translocating substrates across the membrane and the signal recognition complex TatBC which can specifically recognize the twin-arginine motif of Tat substrates [5].
In bacteria, many proteins have been successfully secreted through the Tat pathway, such as periplasmic ligand-binding proteins, virulence factors, redox enzymes, and enzymes involved in cell envelop biogenesis [4]. Unlike the Sec pathway which only allows the export of unfolded proteins, only proteins that can obtain a folded conformation in the cytoplasm or containing cofactors are competent for translocation via Tat pathway [6]. Furthermore, many hydrolytic enzymes are “non-cofactor” substrates of Tat pathway in E. coli and other microorganisms [7]. Bile salt hydrolase (BSH, EC 3.5.1.24), a hydrolytic and intracellular enzyme, can fold fully in the cytoplasm [8], which makes it a good candidate for translocating via Tat pathway.
Bile salt hydrolase catalyzes the hydrolysis of glycine- or taurine-conjugated bile acids into the free bile acids and a glycine/taurine moiety, which will increase the de novo synthesis of bile acids from cholesterol, thus lowering the serum cholesterol level. This has been demonstrated by the fact that oral administration of BSH-positive probiotics can reduce serum cholesterol levels in humans [9], pigs [10], rats [11], and other mammals. In addition, bacteria with BSH activity are proved to be functional in bile detoxification, which will increase the intestinal persistence and survival of these strains [12]. Consequently, BSH activity has become a desirable trait when selecting probiotics [13] and has been reported in many species such as Lactobacillus, Bifidobacterium, Clostridium, Enterococcus, Bacteroids, Brevibacillus sp., and Brucella obortus [14]. However, few reports show that probiotics have no significant cholesterol-lowering effects on the host, which disputes the hypocholesterolemic claims [15].
As it is very difficult to purify BSH from the wide-type Lactobacillus plantarum BBE7 and recombinant bacterium E. coli BL21(DE3) (pET-28a(+)) in which BSH was expressed intracellularly [16], the PelB signal peptide of Sec system was used for the secretion of BSH. However, no proteins and BSH activity were detected by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis and BSH assay, respectively. In this study, we investigated the effects of three different signal peptides from Tat pathway on the export and enzymatic activity of BSH. As bioinformatic analysis suggests that the utilization of the Tat pathway varies greatly among different strains of the same species [4], four different strains of E. coli were used to find the best combination between signal peptides and the hosts. Among the 24 recombinant bacteria constructed, E. coli BL21 (DE3) pLysS harboring vector pET-20b(+)-dmsA-bsh showed the highest periplasmic BSH activity, which was further increased by 68.1 % by optimizing the fermentation conditions. Although most BSHs expressed were inclusion bodies inside the cytoplasm, BSH was successfully liberated into the periplasm. These observations will facilitate the purification and biochemical characterization of BSH from the supernatant, and the periplasmic production of other heterologous proteins in E. coli.
Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions
Table 1 presents bacterial strains, plasmids, and primers used in this study. Sodium glycodeoxycholate (GDCA, G9910) and o-nitrophenyl-β-D-galactopyranoside (ONPG) were obtained from Sigma (St. Louis, MO). The agarose gel DNA purification kit, EZ-10 Spin Column Plasmid Mini-Preps kit, PrimeSTAR HS DNA Polymerase, restriction enzymes, T4 DNA ligase, pMD19-T simple vector, and other molecular biology agents were purchased from Takara Bio Inc. (Kyoto, Japan).
E. coli cells were propagated at 37 °C in Luria-Bertani (LB) medium at 200 rpm in the shaker or on LB broth solidified with 1.5 % (w/v) agar. For gene expression study, E. coli was usually inoculated into Terrific Broth (TB) medium containing 12 g/L peptone, 24 g/L yeast extract, 4 mL/L glycerol, 2.31 g/L KH2PO4, 16.43 g/L K2HPO4, and appropriate amount of antibiotics. When necessary, IPTG was added to induce gene expression. Antibiotic supplements were at the following concentrations: ampicillin, 100 μg/mL; tetracycline, 12.5 μg/mL; kanamycin, 15 μg/mL; and chloramphenicol 34 μg/mL.
Analysis and Synthesis of the Three Tat Signal Peptides
The physicochemical properties of the selected signal peptides were analyzed by the ExPASy tools (http://www.expasy.org/). Transmembrane predictions and expression localization were performed using the TMHMM server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM-2.0/), and PSORTb (version 3.0.2, http://www.psort.org/psortb/index.html), respectively. ProtParam tool, at http://web.expasy.org/protparam/, was used to compute the parameters of the signal peptides, including amino acid composition, isoelectric point, molecular weight, instability index, aliphatic index, and grand average of hydropathicity (GRAVY).
Consequently, the sequences encoding Tat signal peptides trimethylamine N-oxide reductase (TORA), dimethyl sulphoxide reductase subunit DmsA (DMSA), and formate dehydrogenase (FDNG) from E. coli [17] were chosen and optimized based on the codon bias of E. coli. They were then ligated, synthesized, and inserted into plasmid pUC57 by Sangon Biotech Co., Ltd. (Shanghai, China), resulting in a new plasmid pUC-Tat.
Construction of Recombinant Expression Vector
The torA gene fragment encoding the TORA signal peptide was amplified by PCR using plasmid pUC-Tat as a template and primers torA (F) and torA (R) (Table 1). The bsh gene fragment without stop codon was amplified by PCR with primers torA-bsh (F) and bsh (R), using plasmid pET-28a(+)-bsh as a template. Both fragments were purified and adopted as templates in overlapping PCR reaction using primers torA (F) and bsh (R). The resulting PCR fragment containing torA-bsh gene was digested with Nde I and Xho I and ligated to vectors pET-20b(+) and pET-22b(+) after double digestions with Nde I/Xho I, generating two recombinant plasmids pET-20b(+)-torA-bsh and pET-22b(+)-torA-bsh. Other four recombinant vectors, pET-20b(+)-dmsA-bsh, pET-22b(+)-dmsA-bsh, pET-20b(+)-fdnG-bsh, and pET-22b(+)-fdnG-bsh, were constructed in a similar way. Consequently, these six recombinant plasmids were transformed into E. coli JM109 competent cells. After the correctness of insertions was verified by restriction analysis and DNA sequencing, the six correct plasmids were transformed into four different strains of E. coli, respectively, resulting in 24 recombinant bacteria.
Cell Fractionation
After recombinant bacteria were cultured overnight at 37 °C in 20 mL LB medium containing appropriate antibiotics, 2 % (v/v) of the seed cultures were inoculated into 20 mL TB medium containing the same antibiotics and cultured at 37 °C until the OD600 (1-cm path length) reach 0.6. The expression of BSH in recombinant bacteria harboring pET-20b(+) was induced with 0.4 mM IPTG for 6 h at 37 °C prior to the next step, while for recombinant bacteria containing pET-22b(+), 1.0 mM IPTG was added. Cultures without induction were used as controls.
Method described in the pET system manual (Novagen) and elsewhere [18] was slightly modified to prepare protein samples from recombinant E. coli cultures. For total cell lysate, 1 mm of cells was collected by a 5-min centrifugation at 10,000 rpm (Mode 5424; Eppendorf). Cell pellets at an OD600 of 2.0 was resuspended in 0.1 mL phosphate-buffered saline (PBS) and sonicated for 3 × 30 s under constant cooling. Ten-milliliter culture was harvested and resuspended in 1 mL 30 mM Tris-HCl, pH 8.0, and 20 % sucrose, and 2 μL 0.5 M EDTA (pH 8.0) was subsequently added. After the cell suspension was incubated at room temperature for 10 min, it was centrifuged at 10,000×g for 10 min at 4 °C. The pellet was resuspended in 1 mL ice-cold 5 mM MgSO4 and incubated on ice for 10 min. The osmotic shocked cells were centrifuged as above, and the supernatant was saved as a periplasmic fraction. After the pellet was sonicated and centrifuged as mentioned above, insoluble and soluble cytoplasmic fractions were prepared. Each fractionated sample was analyzed using SDS-PAGE or BSH activity assay.
BSH Assay and SDS-PAGE Analysis
BSH activity in soluble cytoplasmic and periplasmic fractions was measured by the hydrolysis of GDCA at 37 °C in sodium phosphate buffer (0.1 M, pH 6.0). The amounts of amino acids liberated from conjugated bile salts were determined by the ninhydrin assay [19]. One unit of BSH activity was defined as the amount of enzyme releasing 1 μmol of amino acids from the substrate per minute. Protein concentrations were determined by the Bradford Protein Assay kit (Beyotime, Nantong, China) using bovine serum albumin as a standard.
To confirm the expression and export of BSH enzyme, SDS-PAGE was then conducted using 12 % (w/v) polyacrylamide gels containing 0.1 % (w/v) SDS, according to the manufacturer’s specification (Beyotime, Nantong, China). Total cell lysate, periplasmic fractions, insoluble cytoplasmic fraction, and soluble cytoplasmic fraction were mixed with equal volumes of 2× loading buffer, denatured at 99 °C for 10 min, and 10 μL of them was then loaded for SDS-PAGE analysis.
Optimization of the BSH Activity in Periplasmic Fraction
Nutrient composition and fermentation variables such as temperature, inducer concentration, and other parameters affect the export and production levels of heterologous proteins in E. coli [20]. The effects of induction temperature, inoculation amount, cell concentration (OD600), and IPTG concentration on the export of BSH in E. coli BL21 (DE3) pLysS (pET-20b(+)-dmsA-bsh) were thus investigated using orthogonal experimental design (L9 (34)), which was designed and analyzed by Orthogonality Experiment Assistant II (version 3.1.1, China). The optimal conditions that allowed the best secretion of BSH into the periplasm were adopted for further studies.
Testing of Cell Leakage by β-Galactosidase Assay
After E. coli BL21 (DE3) pLysS (pET-20b(+)-dmsA-bsh) was cultured overnight at 37 °C in 30 mL LB medium containing appropriate antibiotics, 2 % (v/v) of the seed cultures were inoculated into a 3-L fermentor containing 1.5 L TB medium and antibiotics and cultured at 37 °C until the OD600 reach 6.0–7.0. Subsequently, the temperature was shifted to 20 °C, and 0.4 mM IPTG was added to induce gene expression. Since then, the culture was collected every 12 h to determine the optical density at 600 nm (OD600), BSH activity, and β-galactosidase activity.
β-Galactosidase activity was measured by previously described methods [21] with slight modification. Enzyme samples were incubated with 10 mM ONPG in 50 mM sodium phosphate buffer (pH 7.5) at 37 °C for 10 min, after which an equal volume of 1.0 M Na2CO3 was added to terminate the reaction. The absorbance at 420 nm (1-cm path length) was measured with the UV-VIS spectrophotometer (model UVmini-D40, Suzhou, China) to determine the amount of o-nitrophenol released. One unit of activity was defined as the amount of enzyme that liberated 1 μmol of o-nitrophenol per minute.
Results
Effects of Signal Peptide Replacement on the Intracellular Expression of BSH
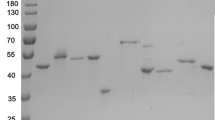
According to the results of SDS-PAGE analysis and BSH assay (data not shown), BSH could not be expressed both intracellularly and extracellularly by signal peptide PelB from Sec system in E. coli; three signal peptides of the Tat pathway were thus chosen to direct BSH to the periplasm. Figure 1 presents the schematic diagram of the construction of recombinant plasmids using signal peptides from Tat pathway. As can be seen from Fig. 2, BSH was successfully expressed at different levels inside the cells by all of the recombinant bacteria. However, as compared with the theoretical molecular mass (MM) of BSH (about 37.0 kDa), BSH expressed intracellularly had an apparent MM of approximately 42.0 kDa in Fig. 2a, c, e, g. This was possibly due to the fact that the Tat signal peptides of intracellular BSH have not been cut by signal peptidases, and it was further demonstrated by the N-terminal sequencing of the purified BSH from E. coli BL21 (DE3) pLysS (pET-20b(+)-dmsA-bsh) with a MM of 37.0 kDa [19]. Table 2 shows that the intracellular activity of BSH from different recombinant bacteria varied considerably. Without induction by IPTG, E. coli BL21 (DE3) pLysS (pET-20b(+)-dmsA-bsh) showed the highest intracellular BSH activity of 1.00 ± 0.05 U/mL, while no intracellular BSH activity could be detected in some bacteria with IPTG induction, such as E. coli BL21 (DE3) pLysS (pET-20b(+)-torA-bsh) and E. coli BL21 (DE3) pLysS (pET-20b(+)-fgnG-bsh). Both results of SDS-PAGE analysis and BSH assay indicated that these three signal peptides, the four strains of E. coli, and the concentration of IPTG had significant effects on the intracellular expression of BSH.
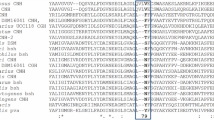
Schematic presentation of the Tat-BSH fusion proteins. The bsh gene sequence is inserted downstream of a Shine-Dalgarno (SD) sequence. The signal peptides of Tat pathway (SP (Tat)) are fused directly to the BSH domain. The twin-arginine motif of “SRRXXXX” is underlined, and arrows indicate the recognition sites for type I signal peptidases
SDS-PAGE analysis of the intracellular and extracellular expression of BSH in different recombinant bacteria harboring different Tat signal peptides. (a, c, e, g) Proteins in total cell lysates; (b, d, f, h) proteins in periplamic fractions; (a, b), recombinant E. coli BL21 (DE3); (c, d), recombinant E. coli BL21 (DE3) pLysS; (e, f), recombinant E. coli Rosetta (DE3); (g, h), recombinant E. coli Rosetta-gami (DE3); (i) recombinant E. coli BL21 (DE3) pLysS. Left four lanes, proteins in the insoluble cytoplasmic fraction; right four lanes, proteins in the soluble cytoplasmic fraction. M, protein molecular weight standard; NI, not induced by IPTG; I, induced by 0.4 mM IPTG
Effects of Different Signal Peptides on the Export of BSH
Both the SDS-PAGE analysis (Fig. 2b, d, f, h) and periplasmic BSH assay (Table 2) demonstrated that BSH enzymes were successfully exported to the periplasm by all of the recombinant bacteria. However, SDS-PAGE analysis of proteins in the insoluble and soluble fractions from E. coli BL21 (DE3) pLysS (pET-20b(+)-dmsA-bsh) indicated that most proteins were inclusion bodies inside the cells, with only a few released into the periplasm (Fig. 2i). The periplasmic BSH activity of all the recombinant bacteria differed greatly (Table 2), which demonstrated that the secretion efficiency of the three signal peptides was greatly affected by their physiochemical properties. Besides, signal peptide DMSA showed the highest secretion efficiency among all of the recombinant bacteria. Without induction, E. coli BL21 (DE3) pLysS (pET-20b(+)-dmsA-bsh) showed the highest periplasmic BSH activity of 0.72 ± 0.05 U/mL, but periplasmic BSH activity could not be detected in several bacteria induced by IPTG, including E. coli BL21 (DE3) (pET-20b(+)-fdnG-bsh) and E. coli Rosseta (DE3) (pET-20b(+)-torA-bsh). From the above, E. coli BL21 (DE3) pLysS (pET-20b(+)-dmsA-bsh), with the highest BSH activity both inside the cells and in the periplasm, was chosen for further studies.
Optimization of Periplasmic BSH Activity by Orthogonal Experimental Design
In order to increase the periplasmic BSH activity of E. coli BL21 (DE3) pLysS (pET-20b(+)-dmsA-bsh), the induction conditions were optimized by the orthogonal experimental design (L9 (34)). As shown in Table 3, the induction temperature, IPTG concentration, cell optical density at 600 nm (OD600), and inoculation amount dramatically influenced the periplasmic translocation of BSH. Their effects on the export of BSH followed a descending order as follows: induction temperature, IPTG concentration, cell concentration (OD600), and inoculation amount. When the induced temperature, the concentration of IPTG, OD600, and inoculation amount were 20 °C, 0.4 mM, 0.6, and 1 % (v/v), respectively, the BSH activity in the periplasm reached its maximum (1.21 ± 0.03 U/mL).
Testing of Leakage from Recombinant Bacteria by β-Galactosidase Activity Measurement
As can be seen from the results of protein and BSH assays, 4.1 % of BSH was excreted into the periplasm by E. coli BL21 (DE3) pLysS (pET-20b(+)-dmsA-bsh), with a periplasmic BSH activity of 1.13 ± 0.04 U/mg. Since cell leakage could also lead to the translocation of the recombinant proteins, the level of cell leakage was determined by measuring the activity of β-galactosidase, a cytosolic marker enzyme, in the supernatant. β-Galactosidase activity was measured in medium samples obtained from cells of E. coli BL21 (DE3) pLysS (pET-20b(+)-dmsA-bsh) in a 3-L fermentor. As shown in Fig. 3, leakage of β-galactosidase did take place in the supernatant of recombinant cells. However, after the recombinant bacterium was induced for 5 h, BSH activity decreased with the descending of OD600 instead of increasing with the ascending of β-galactosidase activity. Besides, according to the results of N-terminal sequencing, the purified extracellular BSH (specific activity was 274.23 U/mg) whose signal peptides had been cut by signal peptidases has a MM of 37 kDa [19]. Post-translational processing from inactive precursors, characterized by the N-terminal methionine excision (NME), can also generate the exported BSH and a nucleophile with a free α-amino acid (Cys at the N-terminal) [22]. All of these demonstrated that the presence of BSH in the periplasm was caused by translocation through Tat pathway rather than the leakage of the cells.
Discussion
Based on the results of epidemiological and clinical studies, coronary heart disease, one of the leading causes of disability and death all over the world, has a positive correlation with the high serum cholesterol levels [23]. High level of serum cholesterol is also a risk factor for the development of atherosclerosis. Although drug therapy can be used to cure these diseases, the undesirable side effects limit its usage [23]. However, the hypocholesteremic effect of BSH-producing probiotics has been discovered in many mammals [9–11]. This provides a more natural way for lowering serum cholesterol levels in humans and has become one of focused research in recent years. Hence, large-scale production of secreted and functional BSH has become important to investigate the cholesterol-lowering effect and the functional mechanism of BSH in clinical trials.
Nevertheless, the successful secretion of proteins is a multistage effort that needs an optimal balance between all stages of the translocation pathway. Signal peptide is one of the most important factors that have a great impact on the production of a secreted protein [24]. Besides, the physiochemical properties of a signal peptide play a significant role in its secretion efficiency. As reported by Choo and Ranganathan [25], the median net charge for signal peptides of Gram-negative bacteria was +2, and Gram-negative signal peptides had median pI values of 10.0. Until now, many computational tools have been used to predict the physiochemical characteristics of the signal peptides, such as isoelectric point, molecular weight, instability, aliphatic index, and GRAVY.
In the present study, three signal peptides (TORA, DMSA, and FDNG) of the Tat pathway were characterized, synthesized, and used for the export of BSH in E. coli. In contrast to PelB signal peptide from Sec pathway, these three signal peptides allowed both the intracellular and periplasmic expression of BSH in E. coli, although the periplasmic yield of BSH was very low. Besides, signal peptide DMSA showed relatively higher secretion efficiency than other two signal peptides. Without induction by IPTG, E. coli BL21 (DE3) pLysS (pET-20b(+)-dmsA-bsh) showed the highest periplasmic BSH activity. These could be attributed to the fact that DMSA had the highest GRAVY score, stability, and aliphatic index as compared to other two signal peptides based on the computational analyses (Table 4).
The formation of inclusion bodies and low secretion efficiency of BSH may be due to any (or all) of the following reasons: (a) When Tat proteins are expressed by multi-copy plasmids, they are prone to aggregate and Tat export will saturate [4]; (b) incompatibility between the signal peptide and the target protein which could not form the proper structure needed for translocation and for processing by signal peptidase I [24, 26]; (c) utilization of the Tat pathway differs widely among various strains of the same species [4]; and (d) interactions among the overall charge balance, hydrophobicity profile, and the n-, h-, and c-regions of the signal peptide can affect the secretion efficiency [27].
Therefore, in order to further boost the export of BSH, it is necessary to test many other signal peptides of Tat pathway to find a good combination between the signal peptide and target protein [28, 29]. Increasing the positive charge of the n-region and/or hydrophobicity of the signal peptide will be another effective method for enhancing the secretion efficiency [27, 30, 31]. Gram-positive bacteria can also be used as host organisms for the extracellular expression of BSH, because they do not contain an extra outer membrane and target protein can be easily directed into the medium [3]. Furthermore, the inclusion bodies formed inside the cells can be reduced by several strategies, for example, chaperone co-expression, fusion protein strategies, and modulating the cytoplasmic redox environment [32].
In summary, this is the first study reporting the successful periplasmic translocation of BSH using signal peptides of Tat pathway in E. coli, although most of the proteins expressed were inclusion bodies inside the cells. The physicochemical properties of the signal peptides influenced their secretion efficiency. More importantly, it was confirmed that extracellular BSH was a result of translocation through Tat pathway rather than of cell leakage. The modification of signal peptide sequences will be adopted to further enhance the secretory expression of BSH in E. coli. These observations will facilitate the easy recovery and characterization of BSH from the supernatant as well as the periplasmic production of other heterologous proteins in E. coli.
References
Driessen, A. J. M., & Nouwen, N. (2008). Protein translocation across the bacterial cytoplasmic membrane. Annu Rev Biochem, 77, 643–667.
Fisher, A. C., Kim, J. Y., Perez‐Rodriguez, R., Tullman‐Ercek, D., Fish, W. R., Henderson, L. A., & DeLisa, M. P. (2008). Exploration of twin-arginine translocation for expression and purification of correctly folded proteins in Escherichia coli. Microb Biotechnol, 1, 403–415.
Meissner, D., Vollstedt, A., van Dijl, J. M., & Freudl, R. (2007). Comparative analysis of twin-arginine (Tat)-dependent protein secretion of a heterologous model protein (GFP) in three different Gram-positive bacteria. Appl Environ Microbiol, 76, 633–642.
Tullman-Ercek, D., DeLisa, M. P., Kawarasaki, Y., Iranpour, P., Ribnicky, B., Palmer, T., & Georgiou, G. (2007). Export pathway selectivity of Escherichia coli twin arginine translocation signal peptides. J Biol Chem, 282, 8309–8316.
Gohlke, U., Pullan, L., McDevitt, C. A., Porcelli, I., De Leeuw, E., Palmer, T., Saibil, H. R., & Berks, B. C. (2005). The TatA component of the twin-arginine protein transport system forms channel complexes of variable diameter. Proc Natl Acad Sci U S A, 102, 10482–10486.
Zhu, F.-M., Ji, S.-Y., Zhang, W.-W., Li, W., Cao, B.-Y., & Yang, M.-M. (2008). Development and application of a novel signal peptide probe vector with PGA as reporter in Bacillus subtilis WB700: twenty-four tat pathway signal peptides from Bacillus subtilis were monitored. Mol Biotechnol, 39, 225–230.
Yang, C., Freudl, R., Qiao, C., & Mulchandani, A. (2010). Cotranslocation of methyl parathion hydrolase to the periplasm and of organophosphorus hydrolase to the cell surface of Escherichia coli by the Tat pathway and ice nucleation protein display system. Appl Environ Microbiol, 76, 434–440.
Kim, G. B., & Lee, B. H. (2005). Biochemical and molecular insights into bile salt hydrolase in the gastrointestinal microflora-a review. Asian-Aust J Anim Sci, 18, 1505–1512.
Jones, M., Martoni, C., Parent, M., & Prakash, S. (2012). Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br J Nutr, 107, 1505–1513.
Park, Y. H., Kim, J. G., Shin, Y. W., Kim, H. S., Kim, Y. J., Chun, T., Kim, S. H., & Whang, K. Y. (2008). Effects of Lactobacillus acidophilus 43121 and a mixture of Lactobacillus casei and Bifidobacterium longum on the serum cholesterol level and fecal sterol excretion in hypercholesterolemia-induced pigs. Biosci Biotechnol Biochem, 72, 595–600.
Guo, C. F., & Li, J. Y. (2014). A combination of Tween 80 with CaCl2 enhances the hypocholesterolemic activity of bile salt hydrolase-active Lactobacillus casei F0422 in rats fed a cholesterol-rich diet. J Funct Foods, 9, 131–140.
Ruiz, L., Margolles, A., & Sanchez, B. (2013). Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front Microbiol, 4, 396.
Jiang, J., Hang, X., Zhang, M., Liu, X., Li, D., & Yang, H. (2010). Diversity of bile salt hydrolase activities in different Lactobacilli toward human bile salts. Ann Microbiol, 60, 81–88.
Begley, M., Hill, C., & Gahan, C. G. (2006). Bile salt hydrolase activity in probiotics. Appl Environ Microbiol, 72, 1729–1738.
Hatakka, K., Mutanen, M., Holma, R., Saxelin, M., & Korpela, R. (2008). Lactobacillus rhamnosus LC705 together with Propionibacterium freudenreichii ssp shermanii JS administered in capsules is ineffective in lowering serum lipids. J Am Coll Nutr, 27, 441–447.
Dong, Z., Zhang, J., Lee, B., Li, H., Du, G., & Chen, J. (2012). A bile salt hydrolase gene of Lactobacillus plantarum BBE7 with high cholesterol-removing activity. Eur Food Res Technol, 235, 419–427.
Robinson, C., Matos, C. F., Beck, D., Ren, C., Lawrence, J., Vasisht, N., & Mendel, S. (2011). Transport and proofreading of proteins by the twin-arginine translocation (Tat) system in bacteria. Biochim Biophys Acta, 1808, 876–884.
Balachandran, P., Hollingshead, S. K., Paton, J. C., & Briles, D. E. (2001). The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J Bacteriol, 183, 3108–3116.
Dong, Z., Zhang, J., Lee, B. H., Li, H., Du, G., & Chen, J. (2013). Secretory expression and characterization of a bile salt hydrolase from Lactobacillus plantarum in Escherichia coli. J Mol Catal B Enzym, 93, 57–64.
Jana, S., & Deb, J. (2005). Strategies for efficient production of heterologous proteins in Escherichia coli. Appl Microbiol Biotechnol, 67, 289–298.
Wang, S. D., Guo, G. S., Li, L., Cao, L. C., Tong, L., Ren, G. H., & Liu, Y. H. (2014). Identification and characterization of an unusual glycosyltransferase-like enzyme with beta-galactosidase activity from a soil metagenomic library. Enzym Microb Technol, 57, 26–35.
Lodola, A., Branduardi, D., De Vivo, M., Capoferri, L., Mor, M., Piomelli, D., & Cavalli, A. (2012). A catalytic mechanism for cysteine N-terminal nucleophile hydrolases, as revealed by free energy simulations. PLoS ONE, 7, e32397.
Sridevi, N., Vishwe, P., & Prabhune, A. (2009). Hypocholesteremic effect of bile salt hydrolase from Lactobacillus buchneri ATCC 4005. Food Res Int, 42, 516–520.
Baradaran, A., Sieo, C. C., Foo, H. L., Illias, R. M., Yusoff, K., & Rahim, R. A. (2013). Cloning and in silico characterization of two signal peptides from Pediococcus pentosaceus and their function for the secretion of heterologous protein in Lactococcus lactis. Biotechnol Lett, 35, 233–238.
Choo, K. H., & Ranganathan, S. (2008). Flanking signal and mature peptide residues influence signal peptide cleavage. BMC Bioinforma, 9(Suppl 12), S15.
Richter, S., & Bruser, T. (2005). Targeting of unfolded PhoA to the TAT translocon of Escherichia coli. J Biol Chem, 280, 42723–42730.
Ng, D. T., & Sarkar, C. A. (2013). Engineering signal peptides for enhanced protein secretion from Lactococcus lactis. Appl Environ Microbiol, 79, 347–356.
Kjærulff, S., & Jensen, M. R. (2005). Comparison of different signal peptides for secretion of heterologous proteins in fission yeast. Biochem Biophys Res Commun, 336, 974–982.
Knappskog, S., Ravneberg, H., Gjerdrum, C., Tröβe, C., Stern, B., & Pryme, I. F. (2007). The level of synthesis and secretion of Gaussia princeps luciferase in transfected CHO cells is heavily dependent on the choice of signal peptide. J Biotechnol, 128, 705–715.
Zhang, L., Leng, Q., & Mixson, A. J. (2005). Alteration in the IL-2 signal peptide affects secretion of proteins in vitro and in vivo. J Gene Med, 7, 354–365.
Zanen, G., Houben, E. N., Meima, R., Tjalsma, H., Jongbloed, J. D., Westers, H., Oudega, B., Luirink, J., van Dijl, J. M., & Quax, W. J. (2005). Signal peptide hydrophobicity is critical for early stages in protein export by Bacillus subtilis. FEBS J, 272, 4617–4630.
Basu, A., Li, X., & Leong, S. S. (2011). Refolding of proteins from inclusion bodies: rational design and recipes. Appl Microbiol Biotechnol, 92, 241–251.
Acknowledgments
This project was financially supported by Synergetic Innovation Center of Food Safety and Nutrition, the National Natural Science Foundation of China (No. 31100064), the National High Technology Research and Development Program of China (863 Program, No. 2011AA100905), Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1135), the Major State Basic Research Development Program of China (973 Program, 2012CB720802, 2012CB720806), and the Natural Science Foundation of Jiangsu Province (No. BK2012553).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dong, Z., Zhang, J., Du, G. et al. Periplasmic Export of Bile Salt Hydrolase in Escherichia coli by the Twin-Arginine Signal Peptides. Appl Biochem Biotechnol 177, 458–471 (2015). https://doi.org/10.1007/s12010-015-1755-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1755-3