Abstract
One of the major limitations of effective nonviral gene carriers is their potential high cytotoxicity. Conjugation of polyethylene glycol (PEG) to polymers is a common approach to decrease toxicity and improve biodistribution. The aim of this study was to evaluate the effect of PEGylation on generation 5 polypropylenimine (PPI) dendrimer by using PEG moieties or alkyl-PEG groups. Polymers were synthesized by grafting of 5 and 10 % primary amines of PPI to NH2–PEG–COOH or Br–(CH2)9–CO–NH–PEG–COOH through Amide bond formation or nucleophilic substitution, respectively. Transfection efficiency and cytotoxicity were analyzed after 4 and 24 h exposure of neuroblastoma cell line (Neuro-2a) with synthesized vectors. Among all of the PEG-PPI derivatives, 5 % PEG-conjugated G5 PPI with alkyl chain (PPI-alkyl-PEG 5 %) resulted in the most efficient gene expression. This vector also significantly decreased the in vitro cytotoxicity and sub-G1 peak in flow cytometry histogram after 24 h incubation. Our results indicate that modification of 5 % primary amines of G5 PPI with PEG using alkyl chain as linker produces a promising vector combining low cytotoxicity, appropriate biodegradability, and high gene transfection efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, gene therapy has been considered as a potential strategy for treatment of different genetic diseases such as severe combined immunodeficiency [1], Alzheimer’s [2], hemophilia [3], cystic fibrosis [4], and Parkinson’s disease [5]. Successful gene therapy depends on development of potent gene delivery vectors to target cells with lower systemic toxicity [6]. So far, a variety of vectors including viral and nonviral vectors has been applied in gene delivery studies. Although viral vectors are considered as efficient gene carriers, most of them suffer from fundamental problems such as toxicity, inflammatory responses, mutagenesis, small capacity, high cost of production, and difficult large-scale pharmaceutical grade production [7, 8]. Hence, there is great attention in the use of nonviral carriers as suitable delivery systems for application in gene delivery area [7]. Nonviral gene carriers fall into two main classes: cationic lipids and cationic polymers [7]. Many different types of cationic polymers including polyethylenimine (PEI), dendrimers, and poly-L-lysine (PLL) have been investigated in gene delivery studies [7]. Poly-(amidoamine) (PAMAM) and poly-(propylenimine) (PPI) are most investigated dendrimers in both drug and gene delivery experiments. Highly branched, three-dimensional structure of dendrimers, well-defined nanostructures, relatively low toxicity, high loading capacity, and a number of available surface groups for conjugation with many different types of functional groups make them attractive carriers for gene and drug delivery [9–11]. Compared to PAMAM dendrimers, PPI are smaller, less polar in internal microenvironment, and more resistant at high temperatures. Different generations of PPI dendrimers are commercially available; however, without suitable modification, they cannot be considered as effective gene carriers [12].
In previous studies, we showed that alkyl grafting onto polycationic vectors such as PEI and PPI enhanced their transfection efficiency while improving their toxicity profiles as well [13–15]. It was suggested by Dehshahri et al. [13] that long hydrophobic chains can provide a hydrophobic environment around the polycation core, which may affect protonation of amine groups as well as acting as a sink for lipids spontaneously released from the endosomal membrane which makes the endosomal membrane fragile. The result of such proposed effects could be a possible facilitated endosomal release. On the other hand, many studies showed that PEGylation of dendrimers increases their solubility, enhances structural stability, improves biocompatibility, prolongs blood circulation half-life, decreases the immunogenicity, reduces intermolecular aggregation, and finally helps to avoid their rapid clearance by reticulo-endothelial system (RES) [11, 12, 16–18]. Polyethylene glycol (PEG) has been widely used in the polymeric gene carriers; however, it was shown that PEGylation could improve or decrease transfection activity of polymers [19, 20].
Therefore, in current study, we investigated the effects of direct PEGylation and indirect PEGylation using alkyl chain linkers on transfection ability and toxicity of generation 5 PPI dendrimer in vitro. G5 PPI has higher gene transfection efficacy but displayed higher cytotoxicity compared to lower generations of dendrimers. It is assumed that modification of PPI primary surface amines with alkyl chains and PEG moieties could be able to enhance the transfection efficiency and reduce cytotoxicity.
Materials and Methods
Materials
Generation 5 polypropyleneimine (G5 PPI) was purchased from SyMO-Chem BV (Eindhoven, The Netherlands). Ethidium bromide (EtBr) was obtained from Cinnagen (Tehran, Iran). 10-Bromodecanoic, 1-ethyl-3-[3-dimethyl aminopropyl] carbodiimide hydrochloride (EDC), N-hydroxy benzotriazole (HOBT), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and N-[2-hydroxythyl] piperazine-N′-[2-ethane sulfonic acid] (HEPES) were supplied by Sigma-Aldrich (Munich, Germany). Spectra/Por dialysis membranes were purchased from Spectrum Laboratories (Houston, TX, USA). Bifunctional poly(ethylene glycol) [NH2–PEG–COOH] (weight average MW: 3400) was purchased from NanoCS (New York, USA). Cell culture reagents were purchased from GibcoR Life technologies (Gaithersburg, MD, USA).
Conjugation of PEG onto PPI Surface
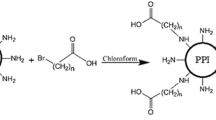
PEGylation of PPI was carried out by grafting of 5 and 10 % primary amines with NH2–PEG–COOH or Br–(CH2)9–CO–NH–PEG–COOH through amidation reaction or nucleophilic substitution, respectively.
Activation of PEG and Alkyl Chain for Amide-Bond Formation
Activated carboxylic groups of PEG or alkyl chain were obtained using EDC and HOBT. PEG (30.35 or 60.71 mg based on 20 mg PPI) or 10-bromodecanoic (2. 21 or 4.41 mg based on mentioned amount of PEG) was dissolved in chloroform. Chloroformic solutions of EDC and HOBT were then added to previous solution and mixed for 45 min.
Conjugation of PEG with Activated Alkylcarboxylate Chain
Bifunctional PEG (30.35 or 60.71 mg) was added to the chloroformic solution of activated alkyl chain obtained in the previous step. After incubation for 24 h at room temperature, the solvent was removed under reduced pressure and the residues were dissolved in double-distilled water (DDW). The final products were dialyzed against water using dialysis membrane (2000 Da cutoff, Spectra/Por membrane) to remove the unreacted materials and then lyophilized.
Modification of PPI with Activated PEG and Alkyl-PEG
PPI (20 mg) was dissolved in chloroform (1 ml). Activated PEG (30.35 or 60.71 mg) or alkyl-PEG (32.43 and 64.87 mg) was then added dropwise to stirred PPI solutions. The substitution degree of PPI surface primary amines was calculated for 5 and 10 %. The reaction was allowed to proceed for 24 h at room temperature, solvent was removed under reduced pressure, and the residue was dissolved in DDW. To remove unreacted agents, the resulting solutions were dialysed against DDW for 3 days using dialysis Spectra/Por membrane ranging between 8 and 12 kDa molecular weight cutoff (Spectrum Laboratories, Houston, USA). After the dialysis, the final product was lyophilized for further use. Amide bond formation was confirmed by Fourier transform infrared spectroscopy (FTIR). The 1H NMR spectra of the G5 PPI with 5 % theoretic conjugation ratio of PEG in D2O was recorded at room temperature using a Bruker Avance-III 300 to analyze the substituted degree of amines.
Preparation of Plasmid DNA
The plasmid pEGFP containing green fluorescent protein reporter gene was transformed into Escherichia coli bacterial strain DH5-α, incubated in selective Luria-Bertani medium, and extracted from the culture pellets using a QIAGEN endotoxin-free Mega Plasmid kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. The concentration and purity of the plasmid were measured as the A260/A280 ratio using an ultraviolet spectrophotometer (Pharmaspec, UV-1700; Spectro, Shimadzu, Japan). Samples with A260/A280 ratios higher than 1.8 were used in transfection experiments.
EtBr Exclusion Assay
DNA condensation ability of PPI and modified PPI were evaluated by EtBr exclusion assay. Fluorescence intensity was determined (exCitation λex 510 nm, and emission λem 590 nm) in a Jasco FP-6200 fluorometer (Tokyo, Japan). Plasmid DNA (5 μg) was complexed with EtBr (400 ng/ml) in 1 ml HBG buffer (20 mM, pH 7.4 containing 5 % glucose), and the recorded fluorescence intensity was taken as the 100 % value. The fluorescence intensity of 400 ng/ml EtBr solution was used as the background value. PPI and PPI derivatives were added to HBG buffer containing plasmid and EtBr at different weight polymer/weight plasmid ratios. Plasmid condensation by PPI or its derivatives was measured in triplicate as decreased fluorescence intensity and results are reported as mean ± SD [21].
Particle Size and Zeta Potential Measurements
Particle size and zeta potential of the polyplexes (vector/pDNA complexes) containing PPI and modified PPI were determined using dynamic light scattering (DLS) and laser Doppler velocimetry (LDV), respectively by Malvern Nano ZS instrument (Malvern Instruments, UK). To perform the measurements, various amounts of PPI and modified PPI were added to a fixed amount of plasmid DNA (5 μg) in DDW. The mixture was then incubated for 30 min at room temperature. Three independent samples (n = 3) were prepared and the results are reported as mean ± SD.
Cell Culture
Neuro-2a murine neuroblastoma cells (ATCC, CCL-131) were cultured in Dulbecco’s modified Eagle culture medium (DMEM-low glucose) supplemented with 10 % fetal bovine serum (FBS), penicillin at 100 U/ml and streptomycin at 100 μg/ml and incubated at 37 °C in a humidified 5 % CO2 atmosphere.
Cytotoxicity Assay
Neuro-2a cells were seeded in 96-well plates at an initial density of 1 × 104 cells per well in 100 μl DMEM complete medium and incubated for 24 h. Polyplexes prepared at different carrier/plasmid w/w (C/P) ratios (2:1, 4:1, and 6:1) were used for cytotoxicity experiments. Twenty microliters of complexes (equivalent of 200 ng plasmid) were added into each well. After 4 h, the media was replaced with fresh medium and further incubated for 18 h. In another experiment, the cells were treated for 24 h. After these incubation times, 20 μl MTT solution per well was added and incubated for 4 h at 37 °C. The medium was then removed and the formazan crystals were dissolved in 100 μl DMSO. The absorbance was read at 570/630 nm on a STAT FAX-2100 microplate reader (Awareness Technology, Palm City, FL, USA) and cell viability (%) relative to control wells containing cell culture medium without polymer was calculated by [A] test/[A] control × 100.
Evaluation of GFP Reporter Gene Expression
For cell transfection, Neuro-2a cells were cultured in 12-well plates at a density of 8 × 104 cells/well and incubated for 24 h. Cells were then treated with prepared polyplexes in serum-free DEMEM containing 3 μg pEGFP at the same C/P ratios [(w/w)] used for the toxicity experiments. After 4 and 24 h exposure time, transfected cells were observed using a fluorescent microscope. The percentage of GFP-positive cells was determined using Partec flow cytometer (Partec, Germany). GFP fluorescence was excited at 488 nm and emission was detected using a 530/40 nm bandpass filter and a 575/25 nm bandpass filter to analyze GFP-positive cells by diagonal gating.
PI Staining
Apoptotic cells were determined by staining method using the propidium iodide (PI) which is described elsewhere [22, 23]. In brief, Neuro-2a cells were seeded at a density of 2 × 104 cells per well in 48-well plates. After 24 h, cells were treated with PPI and modified PPI at C/P ratio 4 and incubated for 4 and 24 h. Floating and adherent cells were then harvested and incubated 2 h at 4 °C in the dark with 150 μl of hypotonic buffer (50 μg/ml PI in 0.1 % sodium citrate, 0.1 % Triton X-100, and 100 μg/ml RNAse A). After centrifugation for 15 min at 5000 rpm, cells were analyzed with flow cytometer (Paratec, Germany) to detect subG1 Peak (apoptotic cells) which show reduced DNA stainability following staining with a PI as DNA specific fluorochromes.
Erythrocyte Leakage Assay
Erythrocyte hemolysis assay was tested as previously described [24]. Human erythrocytes were isolated from fresh, citrate-treated blood and washed four times with phosphate buffered saline (PBS) by centrifuging at 800×g for 10 min at 4 °C. The procedures were performed in accordance with the Institutional Ethical Committee and Research Advisory Committee of Mashhad University of Medical Sciences based on the national guidelines from Ministry of Health and Medicinal Education of Iran, adopted from the 86/609/EEC Directives of European Community. The erythrocyte pellet was diluted in 900 μl 150 mM NaCl. The efficient and low toxic vectors in transfection and MTT experiments were chosen for the hemolysis assay. The vectors were serially diluted in 150 μl HEPES buffered containing 150 mM NaCl using a V-bottom 96-well plate. The concentration of PPI and PPI derivatives varied from 2.5 to 80 μg/ml. As positive control, 1 % Triton X-100 was added to the erythrocyte suspension for 100 % hemolysis. The plates were incubated at 37 °C for 30 min under constant shaking. After removal of the unlysed erythrocytes by centrifugation at 2200 rpm for 10 min, 70 μl supernatant was analyzed for hemoglobin release at 405 nm using a microplate reader. Experiments were performed in triplicate. The results were represented as % hemolysis (compared to Triton X-100 1 %) against carrier concentration (μg/ml).
Statistical Analysis
The statistical analysis of data was determined by Student’s t test using SPSS 16.0 software. P values of ≤0.05 were considered significant. Results are reported as the mean ± SD of triplicates.
Results
Synthesis of PEGylated and PEGylated-Alkylated PPI
The procedure used for modification of G5 PPI is presented in Scheme 1. To synthesize the modified PPI, 5 and 10 % of PPI surface amines were substituted by PEG and alkyl-PEG chains. The synthesized vectors were abbreviated as PPI-PEG n% and PPI-Alkyl-PEG n%, in which n% represents the PEG or alkylated PEG grafting percentage as 5 and 10 %. The conjugation of the PEG and alkyl-PEG moieties to PPI which do not contain Amide groups was confirmed using FTIR spectra. The carbonyl absorbance peak in the range 1650 cm−1 confirmed the presence of Amide bonds in PPI derivatives (Fig. 1).
The 1H NMR was displayed by G5 PPI with 5 % theoretic conjugation ratio of PEG (Fig. 2). The proton chemical (δ) are assigned to δ 3.86 (dd, O-CH2-CO) and δ 1.59-1.71(m, PPI CH2, aliphatic). Regarding the integration of the relative peaks, the percentage of grafting of PEG onto PPI was calculated to be approximately 4.5 %.
EtBr Exclusion Assay
DNA condensation ability of PPI and modified PPI was evaluated by EtBr exclusion assay. By adding 2.5 μl aliquots of PPI and PPI derivatives to a solution containing EtBr bound to plasmid DNA, some intercalated EtBr gradually will be released from the DNA resulting in a recordable decrease in fluorescence intensity. Our results indicated that PEGylated PPI (PPI-PEG) and alkyl-PEG modified PPI (PPI-alkyl-PEG) could efficiently condense pDNA at C/P ratio 1 and 1.5, respectively (Fig. 3). Therefore, compared to PEGylated PPI, PPI-Alkyl-PEG series resulted in slightly less compact polyplexes (complete condensation at C/P ratio 1.5).
Particle Size and Surface Charge Analysis
The ability of the vectors to condense pDNA into nano-sized particles is an important property which affects cell uptake efficiency. All polyplexes were able to condense pDNA into nanoparticles with average sizes about 150–400 nm (Fig. 4a). Our results showed that alkyl-PEG grafting into PPI backbone resulted in smaller complexes than unmodified PPI, and this reduction was statistically significant in both G5 PPI-Alkyl-PEG 5 and 10 %. On the other hand, substitution of PPI amines with PEG or alkyl-PEG chains partially increases zeta potentials of the vectors (Fig. 4b). Overall, electrical charge was between 5 and 16 mV.
Cytotoxicity Assay
The cytotoxicity of PPI and modified PPI polyplexes was evaluated by MTT assay in Neuro-2a cells, where cell viability is measured by the capability of the live cells to reduce MTT to its colored derivative. Cell viability was tested for all polyplexes at 4 and 24 h exposure times. In comparison with unmodified PPI, modified PPI were less toxic (Fig. 5). This trend was more apparent after 24 h incubation with vectors. Furthermore, results showed a C/P ratio-dependent enhancement in cytotoxicity at all modified and unmodified vectors, with relative viability reduction down to less than 5 % at C/P ratio 6 in unmodified PPI. On the other hand, compared to PPI-PEG vectors, PPI-alkyl-PEG vectors were less toxic (Fig. 5).
Transfection Efficiency
To determine the transfection efficiency of prepared complexes, Neuro-2a cells were exposed to pEGFP plasmid, complexed with PPI derivatives at C/P ratios of 2, 4, and 6. The transfection results indicate that grafting of G5 PPI with 5 and 10 % alkyl-PEG chains enhanced its transfection ability (Fig. 6). On the other hand, among modified PPI, PPI-alkyl-PEG 5 % showed the highest transfection in both 4 and 24 h exposure times. Therefore, substitution of 5 % primary amines of G5 PPI with alkyl-PEG chains could result in the most efficient vector in the modified series for in vitro gene transfection.
PEGylation Alleviates the Apoptotic Effects of G5 PPI
The effects of modified and unmodified PPI on induction of apoptosis were examined 4 and 24 h post-transfection using PI staining. Our results indicated that in both 4 and 24 h treatments, G5 PPI modified with 5 and 10 % alkyl-PEG chains (PPI-alkyl-PEG 5 % and PPI-alkyl-PEG 10 %) exhibit the lowest toxicities on the Neuro-2a cells, whilst unmodified PPI showed 62 and 82 % cell apoptosis after 4 and 24 h of polyplex (C/P ratio 6) treatments, respectively (Fig. 7).
Erythrocyte Leakage Assay
Cationic polymers may induce hemolysis and leakage of the membrane of human red blood cells (RBCs). Therefore, the effects of current constructed carriers were assayed on the cell viability and induction of cell hemolysis. We evaluated the membrane-destabilizing activity of PPI and PPI derivatives on human RBCs with varying concentrations at pH 7.5 (close to physiologic pH). The polymeric vectors showed a concentration-dependent hemolytic effect on RBCs; however, none of them exhibited a significant hemolytic activity (Fig. 8).
Discussion
Poly-(propyleneimine) (PPI) has been investigated as efficient dendrimer in drug and gene delivery because of its highly branched structure and a defined number of surface primary amines which can be modified and functionalized with different moieties that provide both selectivity and reactivity [25, 26]. However, this structure suffers from the fact that it has cytotoxic effects due to its polycationic nature as well as possessing low transfection efficacy which limits its efficiency in the biomedical applications [25, 26]. The positively charged surface groups of dendrimers are potentially available to destruct cell membrane and consequently cause cell lysis. The dendrimers lytic effect in the bloodstream is very dangerous and could limit the in vivo application [27].
To address these issues, several modifications have been made on PPI dendrimers to improve transfection efficiency and reduce their toxicity, including grafting with oligoethyleneimine [28], guanidyl-groups, arginine moieties [29], and coupling with targeting ligands such as transferrin [30], galactose, folate, and dextran [31]. We previously showed that alkyl grafting onto PPI enhanced their transfection efficiency while improving their toxicity [14].
In several studies, modification of cationic polymers such as dendrimers with PEG chains was used to enhance the gene transfer ability, decrease toxicity, and enhance hemocompatibility [27, 32]. Furthermore, grafting with PEG chains have been used to increase steric stability of polyplexes and prevent nonspecific interactions with surrounding molecules, resulted in reduced toxicity [18].
In this study, we investigated the effects of PEGylation on transfection and toxicity of G5 PPI which itself has high toxicity and low transfection efficiency. Polymers were synthesized by grafting of 5 and 10 % primary amines of PPI to NH2–PEG–COOH or Br–(CH2)9–CO–NH–PEG–COOH through Amide bond formation or nucleophilic substitution, respectively.
The ability of pDNA packaging is a main step in design of any gene delivery carrier. For this purpose, EtBr exclusion assay was carried out for the evaluation of PEGylation on the dendrimer–DNA interactions. Our results indicated that PEGylated PPI and alkyl-PEGylated PPI could efficiently condense pDNA, respectively, at higher C/P ratio compared to unmodified PPI. Thus, substitution of PPI amines with hydrophobic alkyl-PEG chains shifted the maximum condensation C/P ratio by 0.5 to 1.5 units higher. But, the reduction was not so significant to interfere with the formation of polyplexes at suitable C/P ratios.
Size and surface charge of nanoparticles (NPs) are another main factors which affects the stability, cellular uptake, and gene transfer efficacy of polyplexes [33]. Although cellular uptake of particles cannot be predicted by measuring the particle size itself, particles in the submicron size range have shown to use uptake pathways such as caveolae-mediated endocytosis or clathrin-mediated endocytosis for entering the cells [21]. Based on particle size and zeta potential measurements, nanoparticles (NPs) in this study are assumed to have a suitable size range and sufficient positive charges to bind to cell surfaces. Nevertheless, grafting of PPI amines with 5 and 10 % alkyl-PEG resulted in smaller complexes than unmodified PPI. These results suggest that hydrophobic interactions as well as the flexibility of the polymer might lead to a reduced size in alkyl-PEG grafted constructs, similar to the observations by Alshamsan et al. [34].
Transfection ability and cytotoxicity were assayed after 4 and 24 h exposure of neuroblastoma cell line (Neuro-2a) with synthesized vectors. In this study, we introduced two types of PEG chains into G5 PPI backbone; PEG moieties or alkyl-PEG groups with 5 and 10 % of grafting. The aim of using alkyl chains as linker was to prevent unwanted reactions between functional groups and add a partial hydrophobicity to PPI scaffold. Previous studies showed that a balanced hydrophobic–hydrophilic property is needed to achieve high transfection and low toxicity through regulation of interactions of cationic polymers with proteins, cells, and other biocomponents [35, 36]. For example, several studies indicated that conjugation of hydrophobic moieties to polycations has led to increased gene transfection ability in a range of constructs such as PEI, PAMAM, PLL, dendrimers, dendrons, and other nonviral structures [13, 15, 36, 37]. Our transfection studies showed that among all of the PEG-PPI dendrimers, PEG-alkyl-PPI 5 % resulted in the most efficient gene expression. This vector also significantly decreased the in vitro cytotoxicity and sub-G1 peak in flow cytometry histogram after 24 h incubation.
Similar results have been recently reported by Yuan et al. with introducing PEG onto PAMAM dendrimer using bis-aryl hydrazine (BAH) which possesses protonable pyridine and amines. This new derivative of PAMAM could maintain or increase the buffering capacity of the unmodified dendrimer and result in enhanced transfection [11].
It seems that the higher transfection efficacy of PEG-alkyl-PPI 5 % could be due to the appropriate balance of both hydrophobic and hydrophilic segments. Alkyl chains may affect the interactions of carrier with negatively charged cell membranes as well as interaction with endosome membrane for improving their endosome escape ability. Thus, hydrophobic linkages could improve transfection activity of PEGylated PPI. It is also observed that gene expression obtained from the 24 h transfection series was prominently higher than the ones obtained from 4 h transfection series. The probable reason for such observation could be that the slightly more hydrophilic nanoparticles need more time to reach their peak of cellular uptake and efficiency.
Conclusion
In this study, we described the effects of direct PEGylation and indirect PEGylation using alkyl chain linkers on gene delivery activity and toxicity of PPI dendrimers in vitro. Our transfection results showed that among all of the PEG-PPI dendrimers, PPI with 5 % alkyl-PEG grafting, i.e., PEG-alkyl-PPI 5 % resulted in the most efficient gene expression. This vector also significantly decreased the in vitro cytotoxicity and sub-G1 peak in flow cytometry histogram after 24 h incubation.
References
Hacein-Bey-Abina, S., Hauer, J., Lim, A., Picard, C., Wang, G. P., Berry, C. C., Martinache, C., Rieux-Laucat, F., Latour, S., & Belohradsky, B. H. (2010). Efficacy of gene therapy for X-linked severe combined immunodeficiency. New England Journal of Medicine, 363, 355–364.
Tuszynski, M. H., Thal, L., Pay, M., & Salmon, D. P. (2005). A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nature Medicine, 11, 551–555.
Mannucci, P. M., & Tuddenham, E. G. (2001). The hemophilias—from royal genes to gene therapy. New England Journal of Medicine, 344, 1773–1779.
Lee, T., Matthews, D., & Blair, G. (2005). Novel molecular approaches to cystic fibrosis gene therapy. The Biochemical Journal, 387, 1–15.
Yamada, M., Mizuno, Y., & Mochizuki, H. (2005). Parkin gene therapy for α-synucleinopathy: a rat model of Parkinson’s disease. Human Gene Therapy, 16, 262–270.
Li, S., & Huang, L. (2000). Nonviral gene therapy: promises and challenges. Gene Therapy, 7, 31–34.
Luten, J., van Nostrum, C. F., De Smedt, S. C., & Hennink, W. E. (2008). Biodegradable polymers as nonviral carriers for plasmid DNA delivery. Journal of Controlled Release, 126, 97–110.
Mintzer, M. A., & Simanek, E. E. (2008). Nonviral vectors for gene delivery. Chemical Reviews, 109, 259–302.
Choi, Y. J., Kang, S. J., Kim, Y. J., Lim, Y.-B., & Chung, H. W. (2010). Comparative studies on the genotoxicity and cytotoxicity of polymeric gene carriers polyethyleneimine (PEI) and polyamidoamine (PAMAM) dendrimer in Jurkat T-cells. Drug and Chemical Toxicology, 33, 357–366.
Šebestík, J., Reiniš, M., Ježek, J., Dendrimers in gene delivery. In Biomedical Applications of Peptide-, Glyco-and Glycopeptide Dendrimers, and Analogous Dendrimeric Structures, Springer: 2012; pp 141–147.
Yuan, Q., Yeudall, W. A., & Yang, H. (2010). PEGylated polyamidoamine dendrimers with bis-aryl hydrazone linkages for enhanced gene delivery. Biomacromolecules, 11, 1940–1947.
Taratula, O., Garbuzenko, O. B., Kirkpatrick, P., Pandya, I., Savla, R., Pozharov, V. P., & Minko, T. (2009). Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. Journal of Controlled Release, 140, 284–293.
Dehshahri, A., Oskuee, R. K., Shier, W. T., Hatefi, A., & Ramezani, M. (2009). Gene transfer efficiency of high primary amine content, hydrophobic, alkyl-oligoamine derivatives of polyethylenimine. Biomaterials, 30, 4187–4194.
Hashemi, M. S. F., H. Amel, F.S. Parhiz, H. Ramezani, M. (2013). Gene transfer enhancement by alkylcarboxylation of poly(propylenimine). Nanomedicine Journal, 1, 55-62.
Oskuee, R. K., Dehshahri, A., Shier, W. T., & Ramezani, M. (2009). Alkylcarboxylate grafting to polyethylenimine: a simple approach to producing a DNA nanocarrier with low toxicity. The Journal of Gene Medicine, 11, 921–932.
Dutta, T., Jain, N. K., McMillan, N. A., & Parekh, H. S. (2010). RETRACTED: dendrimer nanocarriers as versatile vectors in gene delivery. Nanomedicine: Nanotechnology, Biology and Medicine, 6, 25–34.
Guillaudeu, S. J., Fox, M. E., Haidar, Y. M., Dy, E. E., Szoka, F. C., & Fréchet, J. M. (2008). PEGylated dendrimers with core functionality for biological applications. Bioconjugate Chemistry, 19, 461–469.
Luo, D., Haverstick, K., Belcheva, N., Han, E., & Saltzman, W. M. (2002). Poly (ethylene glycol)-conjugated PAMAM dendrimer for biocompatible, high-efficiency DNA delivery. Macromolecules, 35, 3456–3462.
Fitzsimmons, R., & Uludağ, H. (2012). Specific effects of PEGylation on gene delivery efficacy of polyethylenimine: interplay between PEG substitution and N/P ratio. Acta Biomaterialia, 8, 3941–3955.
Qi, R., Gao, Y., Tang, Y., He, R.-R., Liu, T.-L., He, Y., Sun, S., Li, B.-Y., Li, Y.-B., & Liu, G. (2009). PEG-conjugated PAMAM dendrimers mediate efficient intramuscular gene expression. The AAPS Journal, 11, 395–405.
Parhiz, H., Hashemi, M., Hatefi, A., Shier, W. T., Farzad, S. A., & Ramezani, M. (2013). Molecular weight-dependent genetic information transfer with disulfide-linked polyethylenimine-based nonviral vectors. Journal of Biomaterials Applications, 28, 112–124.
Mousavi, S. H., Tavakkol-Afshari, J., Brook, A., & Jafari-Anarkooli, I. (2009). Role of caspases and Bax protein in saffron-induced apoptosis in MCF-7 cells. Food and Chemical toxicology : an International Journal Published for the British Industrial Biological Research Association, 47, 1909–1913.
Zhang, X. D., Gillespie, S. K., & Hersey, P. (2004). Staurosporine induces apoptosis of melanoma by both caspase-dependent and -independent apoptotic pathways. Molecular Cancer Therapeutics, 3, 187–197.
Russ, V., Günther, M., Halama, A., Ogris, M., & Wagner, E. (2008). Oligoethylenimine-grafted polypropylenimine dendrimers as degradable and biocompatible synthetic vectors for gene delivery. Journal of Controlled Release, 132, 131–140.
Dutta, T., Garg, M., & Jain, N. K. (2008). Poly (propyleneimine) dendrimer and dendrosome mediated genetic immunization against hepatitis B. Vaccine, 26, 3389–3394.
Murugan, E., Geetha Rani, D., & Yogaraj, V. (2014). Drug delivery investigations of quaternised poly (propylene imine) dendrimer using nimesulide as a model drug. Colloids and Surfaces B: Biointerfaces, 114, 121–129.
Wang, W., Xiong, W., Zhu, Y., Xu, H., & Yang, X. (2010). Protective effect of PEGylation against poly(amidoamine) dendrimer-induced hemolysis of human red blood cells. Journal of biomedical materials research. Part B, Applied Biomaterials, 93, 59–64.
Russ, V., Gunther, M., Halama, A., Ogris, M., & Wagner, E. (2008). Oligoethylenimine-grafted polypropylenimine dendrimers as degradable and biocompatible synthetic vectors for gene delivery. Journal of Controlled release : Official Journal of the Controlled Release Society, 132, 131–140.
Kim, T.-I., Baek, J.-U., Zhe Bai, C., & Park, J.-S. (2007). Arginine-conjugated polypropylenimine dendrimer as a non-toxic and efficient gene delivery carrier. Biomaterials, 28, 2061–2067.
Koppu, S., Oh, Y. J., Edrada-Ebel, R., Blatchford, D. R., Tetley, L., Tate, R. J., & Dufès, C. (2010). Tumor regression after systemic administration of a novel tumor-targeted gene delivery system carrying a therapeutic plasmid DNA. Journal of Controlled Release, 143, 215–221.
Kesharwani, P., Tekade, R. K., Gajbhiye, V., Jain, K., & Jain, N. K. (2011). Cancer targeting potential of some ligand-anchored poly (propylene imine) dendrimers: a comparison. Nanomedicine: Nanotechnology, Biology and Medicine, 7, 295–304.
Duncan, R., & Izzo, L. (2005). Dendrimer biocompatibility and toxicity. Advanced Drug Delivery Reviews, 57, 2215–2237.
Shahidi-Hamedani, N., Shier, W. T., Moghadam Ariaee, F., Abnous, K., & Ramezani, M. (2013). Targeted gene delivery with noncovalent electrostatic conjugates of sgc-8c aptamer and polyethylenimine. The Journal of Gene Medicine, 15, 261–269.
Alshamsan, A., Haddadi, A., Incani, V., Samuel, J., Lavasanifar, A., & Uludag, H. (2008). Formulation and delivery of siRNA by oleic acid and stearic acid modified polyethylenimine. Molecular Pharmaceutics, 6, 121–133.
Kurisawa, M., Yokoyama, M., & Okano, T. (2000). Transfection efficiency increases by incorporating hydrophobic monomer units into polymeric gene carriers. Journal of Controlled Release, 68, 1–8.
Liu, Z., Zhang, Z., Zhou, C., & Jiao, Y. (2010). Hydrophobic modifications of cationic polymers for gene delivery. Progress in Polymer Science, 35, 1144–1162.
Santos, J. L., Oliveira, H., Pandita, D., Rodrigues, J., Pêgo, A. P., Granja, P. L., & Tomás, H. (2010). Functionalization of poly (amidoamine) dendrimers with hydrophobic chains for improved gene delivery in mesenchymal stem cells. Journal of Controlled Release, 144, 55–64.
Acknowledgments
This study was funded by Mashhad University of Medical Sciences, Mashhad, Iran.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Maryam Hashemi and Sara Ayatollahi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hashemi, M., Ayatollahi, S., Parhiz, H. et al. PEGylation of Polypropylenimine Dendrimer with Alkylcarboxylate Chain Linkage to Improve DNA Delivery and Cytotoxicity. Appl Biochem Biotechnol 177, 1–17 (2015). https://doi.org/10.1007/s12010-015-1723-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1723-y