Abstract
Bioflocculant exopolysaccharide (EPS) production by 40 cyanobacterial strains during their photoautotrophic growth was investigated. Highest levels of EPS were produced by Nostoc sp. BTA97 and Anabaena sp. BTA990. EPS production was maximum during stationary growth phase, when nitrogenase activity was very low. Maximum EPS production occurred at pH 8.0 in the absence of any combined nitrogen source. The cyanobacterial EPS consisted of soluble protein and polysaccharide that included substantial amounts of neutral sugars and uronic acid. The EPS isolated from Anabaena sp. BTA990 and Nostoc sp. BTA97 demonstrated high flocculation capacity. There was a positive correlation between uronic acid content and flocculation activity. The flocculant bound a cationic dye, Alcian Blue, indicating it to be polyanionic. The 16S rRNA gene sequences for Nostoc sp. BTA97 and Anabaena sp. BTA990 were deposited at NCBI GenBank, and accession numbers were obtained as KJ830951 and KJ830948, respectively. The results of these experiments indicate that strains Anabaena sp. BTA990 and Nostoc sp. BTA97 are good candidates for the commercial production of EPS and might be utilized in industrial applications as an alternative to synthetic and abiotic flocculants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria are good candidates for the utilization of light energy to convert carbon dioxide into useful products. Many strains are relatively easy to grow, and some have the ability to survive desiccation, extremes of temperature, high pH, and/or variable salinity [1]. Cyanobacteria are widely distributed in diverse habitats (e.g., soils, various aquatic environments, symbioses, etc.). Some strains can reduce and assimilate atmospheric dinitrogen as their sole source of nitrogen.
Many strains of cyanobacteria produce substantial quantities of exopolysaccharides (EPS) [2–4]. It has been suggested that EPS produced by cyanobacteria could be useful in various applications due to their water-holding capacity in soils and their ability to remove heavy metals and solid materials from water reservoirs [4–7]. Studies have shown that EPS play a crucial role in biosorption and binding of heavy metals [8–11] and that the biosorption capacity of soluble EPS for heavy metals is greater than that of bound EPS [12]. EPS have been used as an effective absorbent for removing organic pollutants such as dyes and pesticides [13, 14]. The use of cyanobacterial EPS for biotechnological applications requires the identification of culture parameters that maximize the rate of production of desirable polysaccharides under scalable culture conditions.
The purposes of the study described here were to identify strains of cyanobacteria that produce copious quantities of extracellular polymers, determine the influence of nitrate and pH on EPS production, and assess a potential commercial application of cyanobacterial EPS.
Materials and Methods
Cyanobacterial Culture Conditions and Microscopic Observations
For this study, 40 fast-growing cyanobacterial strains were obtained from the freshwater cyanobacterial and microalgal repository of the Institute of Bioresources and Sustainable Development, Imphal, Manipur, India. All of these strains were originally isolated from Indo-Burma biodiversity hotspots of north-eastern India (87° 32′ E–97° 52′ E longitude and 21° 34′ N–29° 50′ N latitude). Cultivation was conducted in 250-ml Erlenmeyer flasks containing 100 ml of BG-11 medium [15]. The nitrate component of the medium was excluded for culturing heterocystous strains except when otherwise indicated but was included for culturing all non-heterocystous strains. Batch cultures were prepared for characterizing EPS production by centrifuging a culture of a desired strain while in exponential growth, then transferring 50 mg of the wet pellet to a flask containing 100 ml of culture medium. Inoculated cultures were grown photoautotrophically at 28 ± 2 °C under a light/dark cycle of 14/10 h at a light intensity of 54–67 μmol photon m−2 s−1. Cultures were left undisturbed during growth except for brief mixing twice daily for sufficient mass transfer of media nutrients to, and secreted metabolites from, the organisms. Aliquots of cultures were negatively stained using Indian ink (HiMedia, India) as described by [16] as prior to their microscopic observation, in order to observe EPS. Microscopic observations utilized an Axio Scope A1 microscope coupled with software AxioVision 4.7.2 (Carl Zeiss, Gottingen, Germany).
Exopolysaccharide Assay and Flocculating Activity Test
Exopolysaccharides from the cell wall of cyanobacteria were separated by putting two steel pins in the culture flasks and kept in a magnetic stirrer for 15 min. The cells were macerated, and cell wall polysaccharides were made to release in the medium. Then, soluble EPS was separated from intact cyanobacteria by centrifugation of cultures at 6,600×g at 15 °C for 20 min (Centrifuge 5430 R, Eppendorf, Germany). The supernatant was concentrated to one fourth of its original volume by drying in a hot air oven (Universal Oven-143, Narang Scientific Works, India) at 60 °C for 10–12 h. The concentrated liquid was precipitated by the gradual addition of three volumes of cold ethanol and was then kept at 4 °C overnight [17]. The precipitate was washed twice by suspension in cold ethanol, followed by centrifugation. The gel-like pellet obtained after the final centrifugation was collected and placed in a dialysis bag (Dialysis Membrane-110, HiMedia, India). The dialysis membrane was cut with respect to the quantity of pellet obtained and tied tightly with a thread at both ends and was dialyzed against five volumes of distilled water overnight at room temperature. The dialysate was then dried at 60 °C to a constant weight.
The dialyzed EPS from two strains, Nostoc sp. BTA97 and Anabaena sp. BTA990, was analyzed chemically. Total neutral sugar content was estimated by Anthrone method [18], using glucose as a standard. Total soluble protein was measured according to [19], using bovine serum albumin as a standard. EPS was hydrolyzed for uronic acid estimation. EPS (1 mg) was dissolved in 1 ml distilled water, 2.4 ml of sulfuric acid was added, and uronic acid content from the hydrolyzed EPS was determined spectrophotometrically using the carbazole method [20], with galacturonic acid as a standard.
Bioflocculant capacity of the EPS extract was determined by a little modification of Alcian Blue binding assay [21]. Alcian Blue 8GX (HiMedia, India) was dissolved at a concentration of 1 mg ml−1 in 0.5 N acetic acid. After culture centrifugation, 0.5 ml of supernatant containing EPS was diluted in 4.25 ml of 0.5 N acetic acid and combined with 0.25 ml of the Alcian Blue dye preparation. After 30-min incubation at room temperature, the solution was centrifuged at 2,900×g for 10 min and the optical density of the supernatant was determined at 610 nm (UV-1800, Shimadzu, Japan). Control assays contained Alcian Blue and acetic acid without any added EPS.
Flocculating activity was calculated as:
where A and B are the absorbance values of sample and control, respectively, at 610 nm.
EPS Production and Nitrogenase Activities of Cyanobacteria
Growth of the organisms and EPS production were studied over the same time period. The quantity of EPS produced in a 100-ml volume of culture was determined during the interval from the time the culture was inoculated until it was harvested. Average biomass was determined as the difference in the biomass at the time of harvest and the time of inoculum. The total weight of EPS was then divided by the average biomass.
Nitrogenase activities were measured at different growth phases by acetylene reduction assays [22]. For each assay, 100 ml of growing cyanobacterial culture was homogenized using a homogenizer (Universal motor, RQ-127A, India), and 5-ml volume was transferred to a serum bottle (GC Crimp Vials, Borosil, India) of 20-ml capacity. Air (1.5 ml = 10 % of the gas volume) was withdrawn from the bottle, and then 1.5 ml of pure acetylene was injected. The culture was incubated at 28 ± 2 °C under cool white fluorescent lamps at an intensity of 54–67 μmol photons m−2 s−1 with gentle brief agitation of the bottles every 30 min. After 90-min incubation, 0.5 ml of 10 % trichloroacetic acid (TCA) was injected into the bottle to arrest enzymatic activity. Using a gas-tight syringe (Hamilton 1001 LTN), 1 ml of gas was withdrawn from the bottle and injected into the gas chromatograph (Chemito Ceres 800 plus, Thermo Scientific, Waltham, MA, USA) fitted with a flame ionization detector Porapak T column (SGE, Australia). The temperature of the injector, detector, and oven was maintained at 150, 200, and 50 °C, respectively. Nitrogen was used as a carrier gas at a flow rate of 1.0 ml min−1. Nitrogenase activity was expressed as nanomole C2H4 per microgram Chl-a per hour. The chromatogram peak area of ethylene was recorded using Chemito-card software version 2.6 (Chrom Card Data System, Thermo Scientific, Waltham, MA, USA). The retention times were calibrated using 96.70 % acetylene (Proton Gases Pvt. Ltd., India) and 99.5 % ethylene (Thermo Fisher Scientific India Pvt. Ltd., India).
Statistical Analysis
Data on the production of EPS under different nitrate concentrations and pH range were compared by one-way analysis of variance (ANOVA), followed by the Tukey’s post hoc test; p < 0.01 was considered statistically significant. Regression correlation analysis and significance of differences were determined to study the correlation between uronic acid content and flocculant activity. Data were analyzed using the SPSS 19 software (IBM, Chicago, USA).
Strain Identification and Phylogenetic Affiliation
Genomic DNA was extracted by a modified xanthogenate method [23]. PCR amplification of 16S rRNA gene sequences was performed using the universal forward primer 536F and reverse primer 1488R [24]. All PCR reactions were performed in a total volume of 50 μl containing 200 μM dNTPs, 0.3 μM of each primer, 1× Taq buffer, 5 U Taq DNA polymerase, and 2 μl of genomic DNA. Amplification was performed in a thermal cycler (Mastercycler gradient, Eppendorf, Germany). After an initial denaturation at 95 °C for 5 min, the mixture was subjected to 28 cycles including final denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, and final extension at 72 °C for 1 min. The PCR product was detected with standard agarose gel electrophoresis (Elchrom Scientific GEPS 200/2000, Switzerland), and quantification of PCR product was done with BioSpectrometer (Eppendorf, Germany). Sequences of both the directions were aligned using the ClustalW program [25], and partial 16S rRNA gene sequence was compared with other known gene sequences retrieved from the NCBI GenBank. The identification was authenticated through BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi), and neighbor-joining phylogenetic tree was constructed by “Kimura two-parameter” taking 1,000 replicates as bootstrap value by using MEGA 4 software [26].
Results
EPS-Producing Cyanobacterial Strains
The 40 different cyanobacteria used in this study included 21 heterocystous strains, 18 non-heterocystous filamentous strains, and 1 unicellular strain. The strains that produced the most EPS, Nostoc sp. BTA97 and Anabaena sp. BTA990, contained a thick surrounding capsule or slime layer that caused the medium within which they were grown to become highly viscous. Both strains contained heterocysts. Figure 1 illustrates the appearance of cyanobacterial filaments in the absence and presence of Indian ink. The dye was excluded from the filaments and their EPS surroundings but caused some distension of vegetative cells. No EPS was visible in the absence of the Indian ink. The cells appeared slightly larger in the Indian ink-stained cells, and the unstained part around the organisms confirmed the production of mucilaginous boundary of negatively charged EPS by the cyanobacterial strains.
EPS Characterization and Flocculating Activity
All 40 of the examined cyanobacterial strains were screened for EPS production by separating and recovering large molecules from the supernatant of cultures as described in the “Materials and Methods” section. Nostoc sp. BTA97 produced the highest level of exopolysaccharide (1.58 ± 0.06 mg ml−1), followed by Anabaena sp. BTA990 (1.29 ± 0.04 mg ml−1). The total amount of EPS includes the released polysaccharides in the medium and capsular or slime polysaccharides. The total EPS and flocculating activity of ten maximum EPS-producing strains are indicated in Table 1.
Chemical analysis of the EPS extracted from Nostoc sp. BTA97 and Anabaena sp. BTA990 showed the presence of significant levels of neutral sugars, soluble proteins, and uronic acid. The biochemical composition of EPS for the two highest producing strains, Nostoc sp. BTA97 and Anabaena sp. BTA990, is indicated in Table 2.
Kinetics of EPS Production
Results of growth experiments in BG-11 medium revealed that the organisms grew exponentially to a period of 20 days and then entered the stationary phase. The accumulated quantity of EPS increased during growth over time. The maximum amount was obtained from the cultures on day 30, several days after the organisms had entered stationary phase growth. EPS production increased linearly for the first 20 days after inoculation, followed by a more rapid increase as the cultures approached stationary phase. The rate of EPS production with respect to the biomass concentration for Nostoc sp. BTA97 and Anabaena sp. BTA990 is indicated in Fig. 2. The rate of EPS production was maximum during days 25–30 after the date of inoculation. Maximum nitrogenase activity occurred during exponential growth, when EPS production was low, but decreased shortly thereafter in parallel with the increase in EPS production (Table 3).
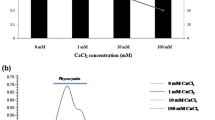
EPS production was examined in cultures grown under various pH values and nitrate concentrations. The highest amount of EPS was produced in the absence of nitrate and at alkaline pH 8.0 (Fig. 3). The rate of EPS production by both Nostoc sp. BTA97 and Anabaena sp. BTA990 gradually increased with the decrease in nitrate concentration and increase in pH which was found to be significant at p < 0.01 significance level.
Correlation Between Uronic Acid Content and Flocculating Activity
Pearson’s correlation value of 0.98** indicates a strong positive correlation between flocculating activity (Alcian Blue binding) and the uronic acid content at p < 0.01 significance level. In the ANOVA analysis, R 2 value of 0.97 and F value of 314.51 also indicate a very high significance. A normal P–P plot showed a positive linear correlation between bioflocculant activity and uronic acid content (Fig. 4).
Strain Identification and Its Phylogeny
The 16S rRNA gene sequences of Nostoc sp. BTA97 and Anabaena sp. BTA990 were found to have closest proximity with Nostoc sp. HK-01 and Anabaena variabilis ATCC 29413 with 100 % query coverage and 99 % homology with maximum score of 1,857 and 1,591 respectively. The 16S rRNA gene sequences were deposited at NCBI GenBank with accession numbers as KJ830951 and KJ830948 for Nostoc sp. BTA97 and Anabaena sp. BTA990, respectively. The clustering in the dendrogram was well supported by bootstrap analysis and partly reflected the morphological similarity of the organisms. The distances in the tree created by 16S rRNA gene sequencing indicated the evolutionary relationships between the studied strains and Nostoc sp. and Anabaena sp. originating from distinct geographical sites (Fig. 5).
Neighbor joining (Kimura) tree based on partial 16S rRNA gene sequence. The tree includes sequences of Nostoc sp. BTA97 and Anabaena sp. BTA990 determined in the present study (bold) with their respective NCBI accession numbers KJ830951 and KJ830948 along with 14 sequences from NCBI GenBank database. Bootstrap values (1,000 replicates) are indicated at the nodes. Synechococcus sp. was used as an out-group. Bar, 0.02 substitutions per site
Discussion
Microscopic observations of negatively stained cells showed the presence of a thick sheath or slime layer surrounding the cyanobacteria examined in this study. In the monograph on Cyanophyta, more than 63.5 % morphotypes of cyanobacteria that showed thin or thick sheath or mucilaginous film or slime were described [27]. The mucilage produced by the organisms prevented the staining of the negative stain, Indian ink, indicating the production of negatively charged EPS.
The presence of non-capsulated morphotypes of cyanobacteria is of crucial importance for the development of biotechnological applications of cyanobacterial since the presence of thick polysaccharidic investments imposes great difficulties for the extraction of bioactive substances and for the molecular biology studies of these strains [28].
The strains utilized in this study produce large amounts of EPS relative to the amounts reported for other cyanobacteria, reaching 1.8 mg ml−1 of culture. Oscillatoria formosa was reported to produce 334.8 μg ml−1 in 24 days [29]. Another filamentous cyanobacterium, Limnothrix redekei PUPCCC 116, produced 304 μg ml−1 of exopolysaccharides [30]. In other reports, strains of Cyanothece, Nostoc calcicola, and Anabaena were reported to produce 1,770, 700, and 55 mg l−1 of EPS, respectively [31–33]. The values observed in the present study can be compared with the highest EPS-producing cyanobacterium or EPS-producing lactic acid bacteria reported in the earlier study [34].
A chemical analysis of the two strains with highest EPS production demonstrated the presence of neutral sugars, soluble proteins, and uronic acid. A diverse range of polysaccharides, with various sugars, uronic acids, and proteins, has been observed previously in cyanobacterial EPS [35]. Analysis of EPS production by the unicellular cyanobacterium, Cyanothece sp. isolated from a rice field of Vietnam, demonstrated a considerable quantity of EPS that contained various sugars and uronic acid [36]. The presence of uronic acid imparts anionic character to EPS, and the capacity of charged groups to bind water molecules can be exploited by the cosmetic industry for product formulations [35]. The presence of uronic acids and sulfate groups confer on cyanobacterial EPS a negative charge that contributes to efficient sequestering of cations, specifically those of heavy metals [37]. In recent years, the heavy metal chelating ability of cyanobacterial EPS has been widely exploited for treatment of wastewater [38].
The highest flocculating activity observed in this study utilized EPS isolated from Nostoc sp. BTA97. EPS that could flocculate suspended particles of bentonite are also produced by Phormidium sp., Anabaena circularis, and Oscillatoria sp. [39, 22, 40]. Maximum EPS production occurred in the cultures in stationary phase. Similar results have been seen previously [41–43]. In another study, production of a large amount of EPS was observed during late exponential or stationary phase growth in various cyanobacterial strains [44, 45]. The cyanobacterium Lyngbya stagnina produced a maximum yield of up to 142.4 μg ml−1 of EPS during the stationary phase growth, and the rate of EPS production was maximum during late log phase of growth [46]. EPS production in Cyanothece sp., Nostoc sp., and Oscillatoria sp. has been linked to growth [47, 48]. Stage and age of cultures affect the production of cyanobacterial intracellular polymeric substances [44]. On the other hand, Parikh and Madamwar [49] demonstrated entirely dissociated kinetics of cellular growth and rate of EPS production in Arthrospira platensis. The flocculating activity of MMF1 isolated from the screening medium was 82.9 % [50], which can be compared with our present studied strains. For the industrial applications of p-KG03, as the bioflocculant agent, p-KG03 showed that more than 90 % of the flocculating activity in kaolin suspension occurred at concentrations of 0.5 mg/l with the maximum at 1.0 mg/l [51]. Similarly, the investigated strains showed flocculation of Alcian dye which can make it possible for use in industrial applications. The composition and quantity of EPS are different in different kind of cyanobacteria and may be influenced by environmental conditions such as light intensity, temperature, culture medium, growth stage, or nutrient availability [44, 52]. Both strains examined in detail for this report produced the most EPS in the absence of any nitrogen source in the culture medium. Previous reports indicated that starvation for all sources of nitrogen, including N2, enhanced EPS synthesis in both diazotrophic and non-diazotrophic cyanobacteria [53–55]. Thus, nitrogen starvation appears to be useful for generating high-rate EPS production. EPS production by some cyanobacteria is increased under nitrogen stress, although others, such as Anabaena nidulans [56] and several Cyanothece sp. [57, 2], released larger amounts of polysaccharides under conditions of nitrogen limitation, while others, such as Anabaena cylindrica and Anabaena flos-aquae [58], produced varying amounts of EPS, depending on the nitrogen source. There was decrease in exopolysaccharide production in all nitrate-grown cultures which might indicate that the genes involved in extracellular polysaccharide synthesis may be under nitrogen control. Similar findings were obtained by Herrero et al. [59] where there was complete absence of exopolysaccharide production in nitrate-grown cultures PCC 8113 and PCC 7936. Discrepancies in the literature exist for a variety of reasons, including different ways of measuring EPS, different growth media, different culturing conditions and times of measurement, lack of pH control, and various means of expressing EPS production. Different mechanisms of nitrogen control of the synthesis of exopolysaccharides have been reported for different strains, which suggest that strain-specific optimization will be required to maximize EPS production by cyanobacteria.
pH of the culture medium is important for cell growth and EPS production [60, 31], perhaps relating to its influences on nutrient solubility and uptake, enzymatic activity, cell membrane morphology, by-product formation, and redox reactions [61].
Since the anionic density of the EPS is attributed to carboxyl groups, Alcian Blue, a cationic dye with a high affinity for polyanions was measured [62], to determine the application of bioflocculant. In the present study, cyanobacterial EPS containing a high uronic acid content exhibited high flocculating activity.
There was a direct positive correlation between the flocculant activity and the uronic acid content in both of the strains that were examined extensively. Bioflocculants with a high uronic acid content hold considerable promise as alternatives to synthetic flocculants in various environmentally friendly biotechnological applications. However, high production costs and low yields have limited EPS application as a bioflocculant [63]. The results presented here suggest that higher productivities can be expected with the selection of appropriate cyanobacteria cultured under optimum conditions.
References
Flaibani, A., Olsen, Y., & Painter, T. J. (1989). Polysaccharides in desert reclamation: composition of exocellular proteoglycan complexes produced by filamentous blue green and unicellular green edaphic algae. Carbohydrate Research, 190, 235–248.
De Philippis, R., Margheri, M. C., Materassi, R., & Vincenzini, M. (1998). Potential of unicellular cyanobacteria from saline environments as exopolysaccharide producers. Applied and Environmental Microbiology, 64, 1130–1132.
De Philippis, R., Sili, C., Paperi, R., & Vincenzini, M. (2001). Exopolysaccharide-producing cyanobacteria and their possible exploitation: a review. Journal of Applied Phycology, 13, 293–299.
Ozturk, S., Aslim, B., & Suludere, Z. (2009). Evaluation of chromium (VI) removal behaviour by two isolates of Synechocystis sp. in terms of exopolysaccharide (EPS) production and monomer composition. Bioresource Technology, 100, 5588–5593.
Bender, J., & Phillips, P. (2004). Microbial mats for multiple applications in aquaculture and bioremediation. Bioresource Technology, 94, 229–238.
Freire-Nordi, C. S., Vieira, A. A. H., & Nascimento, O. R. (2005). The metal binding capacity of Anabaena spiroides extracellular polysaccharide: an EPR study. Process Biochemistry, 40, 2215–2224.
Ozturk, S., & Aslim, B. (2008). Relationship between chromium (VI) resistance and extracellular polymeric substances (EPS) concentration by some cyanobacterial isolates. Environmental Science and Pollution Research International, 15, 478–480.
Liu, Y., Lam, M. C., & Fang, H. P. (2001). Adsorption of heavy metals by EPS of activated sludge. Water Science and Technology, 43, 59–66.
Guibaud, G., Van Hullebusch, E., Bordas, F., Abzac, P., & Joussein, E. (2009). Sorption of Cd(II) and Pb(II) by exoplymeric substance (EPS) extracted from activated sludges and pure bacterial strains: modeling of the metal/ligand ratio effect and role of the mineral fraction. Bioresource Technology, 100, 2959–2968.
Guibaud, G., Van Hullebusch, E., & Bordas, F. (2006). Lead and cadmium biosorption by extracellular polymeric substances (EPS) extracted from activated sludges: pH-sorption edge tests and mathematical equilibrium modeling. Chemosphere, 64, 1955–1962.
Zhang, D. Y., Wang, J. L., & Pan, X. L. (2006). Cadmium sorption by EPS produced by anaerobic sludge under sulphate-reducing conditions. Journal of Hazardous Materials, 138, 589–593.
Comte, S., Guibaud, G., & Baudu, M. (2006). Biosorption properties of extracellular polymeric substances (EPS) resulting from activated sludge according to their type: soluble or bound. Process Biochemistry, 41, 815–823.
Zumriye, A. (2005). Application of biosorption for the removal of organic pollutants: a review. Process Biochemistry, 40, 997–1026.
Zhang, Z. Q., Xia, S. Q., Wang, X. J., Yang, A. M., Xu, B., Chen, L., Zhu, Z. L., Zhao, J. F., Nicole, J. R., & Didier, L. (2009). A novel biosorbent for dye removal: extracellular polymeric substance (EPS) of Proteus mirabilis TJ-1. Journal of Hazardous Materials, 163, 279–284.
Stanier, R. Y., Kunisawa, R., Mandel, M., & Cohen-Baziere, G. (1971). Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriological Reviews, 35, 171–205.
Plude, J. L., Parker, D. L., Schommer, O. J., Timmerman, R. J., Hagstrom, S. A., Joers, J. M., & Hnasko, R. (1991). Chemical characterization of polysaccharide from the slime layer of the cyanobacterium Microcystis flosaquae C3-40. Applied and Environmental Microbiology, 57, 1696–1700.
Klock, J. H., Weiland, A., Seifert, R., & Michaeli, W. (2007). Extracellular polymeric substances (EPS) from cyanobacterial mats: characterization and isolation method optimization. Marine Biology, 152, 1077–1085.
Spiro, R. G. (1966). Analysis of sugars found in glycoproteins. Methods in Enzymology, 8, 3–26.
Herbert, T. D., Phipps, P. J., & Strange, R. E. (1971). Chemical analysis of microbial cells. In J. R. Morris & D. W. Ribbon (Eds.), Methods of microbiology (pp. 209–234). NY: Academic.
Galambos, J. T. (1967). The reaction of carbazole with carbohydrates. 1. Effect of borate and sulfamate on the carbazole color of sugars. Analytical Biochemistry, 19, 119–132.
Bar-or, Y., & Shilo, M. (1987). Characterization of macromolecular flocculants produced by Phormidium sp. strain J and by Anabaenopsis circularis PCCC 6720. Applied and Environmental Microbiology, 53, 2226–2230.
Hardy, R. F. W., Burns, R. L., & Holsten, R. D. (1973). Applications of the acetylene reduction assay for measurement of nitrogen fixation. Soil Biology and Biochemistry, 5, 47–81.
Avijeet, S. O., Oinam, G., Singh, K. O., & Tiwari, O. N. (2013). Isolation of fresh water cyanobacterial DNA of north east India by modified xanthogenate method. International Journal of Research in BioSciences, 2(2), 75–82.
Nubel, U., Garcia-Pichel, F., & Muyzer, G. (1997). PCR primers to amplify 16S rRNA gene from cyanobacteria. Applied and Environmental Microbiology, 63(8), 3327–3332.
Thompson, J. D., Higgins, D. G., & Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22, 4673–4680.
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596–1599.
Desikachary, T. V. (1959). Cyanophyta. New Delhi: Indian Council of Agricultural Research.
Otero, A., & Vincenzini, M. (2003). Extracellular polysaccharide synthesis by Nostoc strains as affected by N source and light intensity. Journal of Biotechnology, 102, 143–152.
Jindal, N., Singh, D. P., & Khattar, J. S. (2011). Kinetics and physico-chemical characterization of exopolysaccharides produced by the cyanobacterium Oscillatoria formosa. World Journal of Microbiology and Biotechnology, 27, 2139–2146.
Khattar, J. S., Singh, D. P., Jindal, N., Kaur, N., Singh, Y., Rahi, P., & Gulati, A. (2010). Isolation and characterization of exopolysaccharides produced by the cyanobacterium Limnothrix redekei PUPCCC 116. Applied Biochemistry and Biotechnology, 162, 1327–1338.
Nicolaus, B., Panico, A., Lama, L., Romano, I., Manca, M. C., De Giulio, A., & Gambacorta, A. (1999). Chemical composition and production of exopolysaccharides from representative members of heterocystous and non-heterocystous cyanobacteria. Phytochemistry, 52, 63–647.
Singh, S., & Das, S. (2011). Screening, production, optimization and characterization of cyanobacterial polysaccharide. World Journal of Microbiology and Biotechnology, 27, 1971–1980.
Mota, R., Guimaraes, R., Buttel, Z., Rossi, F., Colica, G., Silva, C. J., Santos, C., Gales, L., Zille, A., De Philippis, R., Pereira, S. B., & Tamaqnini, P. (2013). Production and characterization of extracellular carbohydrate polymer from Cyanothece sp. CCY 0110. Carbohydrate Polymers, 92, 408–1415.
Raus Madieldo, P., & De Los Reyes Gavilan, C. G. (2005). Invited review: methods for the screening, isolation and characterization of exopolysaccharides produced by lactic acid bacteria. Journal of Dairy Science, 88, 843–856.
Sutherland, I. W. (1994). Structure-function relationships in microbial exopolysaccharides. Biotechnology Advances, 12, 393–448.
Ohki, K., Nguyen, Q. T., Yoshikawa, S., Kanesaki, Y., Okajima, M., Kaneko, T., & Tran, H. T. (2014). Exopolysaccharide production by a unicellular freshwater cyanobacterium Cyanothece sp. isolated in a rice field of Vietnam. Journal of Applied Phycology, 26(1), 265–272.
De Philippis, R., Colica, G., & Micheletti, E. (2011). Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: molecular basis and practical applicability of the biosorption process. Applied Microbiology and Biotechnology, 92, 697–708.
Fattom, A., & Shilo, M. (1984). Phormidium J-1 bioflocculant: production and activity. Archives of Microbiology, 139, 421–426.
Bender, J., Rodriguezeaton, S., Ekanemesang, U. M., & Phillips, P. (1994). Characterization of metal-binding bioflocculants produced by the cyanobacterial component of mixed microbial mats. Applied and Environmental Microbiology, 60, 2311–2315.
Kroen, W. K., & Rayburn, W. R. (1984). Influence of growth status and nutrients on extracellular polysaccharides synthesis by the soil alga Chlamydomonas mexicana (Chlorophyceae). Journal of Phycology, 20, 253–257.
Moreno, J., Vargas, M. A., Madiedo, J. M., & Munoz, J. (2000). Chemical and rheological properties of an extracellular polysaccharide produced by the cyanobacterium Anabaena sp. ATCC 333047. Biotechnology and Bioengineering, 67, 283–290.
West, T. P. (2000). Exopolysaccharide production by entrapped cells of the fungus Aureobasidium pullulans ATCC 201253. Journal of Basic Microbiology, 40, 397–401.
West, T. P. (2011). Effect of carbon source on polysaccharide production by alginate-entrapped Aureobasidium pullulans ATCC 42023 cells. Journal of Basic Microbiology, 51, 673–677.
Kumar, C. G., Joo, H., Kavali, R., & Choi, J. (2004). Characterization of an extracellular biopolymer flocculant from a haloalkalophilic Bacillus isolate. World Journal of Microbiology and Biotechnology, 20, 833–836.
De Philippis, R., & Vincenzini, M. (1998). Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiology Reviews, 22, 151–175.
Kumar, A. S., Mody, K., & Jha, B. (2007). Bacterial exopolysaccharides: a perception. Journal of Basic Microbiology, 47, 103–117.
Jindal, N., Singh, D. P., & Khattar, J. S. (2013). Optimization, characterization, and flow properties of exopolysaccharides produced by the cyanobacterium Lyngbya stagnina. Journal of Basic Microbiology, 53, 902–912.
Shah, V., Garg, N., & Madamwar, D. (1999). Exopolysaccharide production by a marine cyanobacterium Cyanothece sp.: application in dye removal by its gelation phenomenon. Applied Biochemistry and Biotechnology, 82, 81–90.
Parikh, A., & Madamwar, D. (2006). Partial characterization of extracellular polysaccharides from cyanobacteria. Bioresource Technology, 97, 1822–1827.
Trabelsi, L., Msakni, N., Ouada, H. B., Bacha, H., & Roudesli, S. (2009). Partial characterisation of extracellular polysaccharides produced by cyanobacterium Arthrospira platensis. Biotechnology and Bioprocess Engineering, 14, 1407–1415.
Zhang, Z., Bo, L., Xia, S., Wang, X., & Yang, A. (2007). Production and application of a novel bioflocculant by multiple-microorganism consortia using brewery wastewater as carbon source. Journal of Environmental Sciences, 19(6), 667–673.
Yim, J. H., Kim, S. J., Ahn, S. H., & Lee, H. K. (2007). Characterization of a novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodinium impudicum KG03. Bioresource Technology, 98, 361–367.
Pereira, S., Zille, A., Micheletti, E., Moradas-Ferreira, P., De Philippis, R., & Tamagnini, P. (2009). Complexity of cyanobacterial exopolysaccharides: composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiology Reviews, 33, 917–941.
Phlips, E. J., Zeman, C., & Hansen, P. (1989). Growth, photosynthesis, nitrogen fixation and carbohydrate production by a unicellular cyanobacterium Synechococcus sp. (cyanophyta). Journal of Applied Phycology, 1, 137–145.
Fresnedo, O., & Serra, J. L. (1992). Effect of nitrogen starvation on the biochemistry of Phormidium laminosum (Cyanophyceae). Journal of Phycology, 28, 786–793.
Sangar, V. K., & Dugan, P. R. (1972). Polysaccharide produced by Anacystis nidulans: its ecological implication. Applied Microbiology, 24, 732–734.
De Philippis, R., Margheri, M. C., Pelosi, E., & Ventura, S. (1993). Exopolysaccharide production by a unicellular cyanobacterium isolated from a hypersaline habitat. Journal of Applied Phycology, 5, 387–394.
Tischer, R. G., & Davis, E. B. (1971). The effect of various nitrogen sources upon the production of extracellular polysaccharide by the blue-green alga Anabaena flosaquae A-37. Journal of Experimental Botany, 22, 546–551.
Herrero, A., Muro-Pastor, A., & Flores, E. (2001). Nitrogen control in cyanobacteria. Journal of Bacteriology, 183, 411–425.
Li, P., Harding, S. E., & Liu, Z. (2001). Cyanobacterial exoplysaccharides: their nature and potential biotechnological applications. Biotechnology and Genetic Engineering, 18, 375–404.
Bajaj, I. B., Lele, S. S., & Singhal, R. S. (2009). A statistical approach to optimisation of fermentive production of poly (gamma glutamic acid) from Bacillus licheniformis NCIM 2324. Bioresource Technology, 100, 826–832.
Ramus, J. (1977). Alcian-blue: a quantitative aqueous assay for algal acid and sulfated polysaccharides. Journal of Phycology, 13, 345–348.
Smith, R. W., & Miettinen, M. (2006). Microorganisms in flotation and flocculation: future technology or laboratory curiosity? Minerals Engineering, 19, 548–553.
Acknowledgments
The authors are thankful to the Director, IBSD, Imphal, Manipur, India for providing laboratory facilities and Dr. Yogesh S. Shouche, Scientist, MCC-NCCS, Pune for scientific input in the present manuscript and the Department of Biotechnology, Government of India for financial assistance (vide grant no. BT/218/NE/TBP/2011).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1117 kb)
Rights and permissions
About this article
Cite this article
Tiwari, O.N., Khangembam, R., Shamjetshabam, M. et al. Characterization and Optimization of Bioflocculant Exopolysaccharide Production by Cyanobacteria Nostoc sp. BTA97 and Anabaena sp. BTA990 in Culture Conditions. Appl Biochem Biotechnol 176, 1950–1963 (2015). https://doi.org/10.1007/s12010-015-1691-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1691-2