Abstract
Copolymer poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] has been the center of attention in the bio-industrial fields, as it possesses superior mechanical properties compared to poly(3-hydroxybutyrate) [P(3HB)]. The usage of oleic acid and 1-pentanol was exploited as the carbon source for the production of P(3HB-co-3HV) copolymer by using a locally isolated strain Cupriavidus sp. USMAA2-4. In this study, the productivity of polyhydroxyalkanoate (PHA) was improved by varying the frequency of feeding in fed-batch culture. The highest productivity (0.48 g/L/h) that represents 200 % increment was obtained by feeding the carbon source and nitrogen source three times and also by considering the oxygen uptake rate (OUR) and oxygen transfer rate (OTR). A significantly higher P(3HB-co-3HV) concentration of 25.7 g/L and PHA content of 66 wt% were obtained. The 3-hydroxyvalerate (3HV) monomer composition obtained was 24 mol% with the growth of 13.3 g/L. The different frequency of feeding carried out has produced a blend copolymer and has broadened the monomer distribution. In addition, increase in number of granules was also observed as the frequency of feeding increases. In general, the most glaring increment in productivity offer advantage for industrial P(3HB-co-3HV) production, and it is crucial in developing cost-effective processes for commercialization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyhydroxyalkanoates (PHAs) are an example of non-petroleum-based biodegradable plastic that does not cause toxic effect to the host. It is a simple macromolecule produced naturally by many species of microorganisms [1, 2]. The building block of PHAs is 3-hydroxyalkanoic acid (3HA) monomer unit. An example of short-chain-length PHAs (SCL-PHAs) is poly(3-hydroxybutyrate) [P(3HB)] (also known as PHB) which can be found accumulated in granular form in many bacteria [3, 4]. PHA is a potential candidate to replace non-renewable resources, but high production cost always becomes a limiting factor in furthering the usage of PHA. Another example of SCL-PHAs is known as poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] which is less stiff and brittle compared to P(3HB). This copolymer is produced by the incorporation of 3-hydroxyvalerate (3HV) monomer into P(3HB).
It was reported that the mechanical properties of P(3HB) is comparable to polypropylene. However, it has poor elongation at break compared to polypropylene [5]. The melting temperature of this homopolymer is 170 °C which is slightly lower than its degrading temperature [6]. This causes the polymer difficult to be processed and directly limits its applications. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] is the first type of biopolymer that is commercialized under trademark BIOPOLTM. Copolymer P(3HB-co-3HV) has been the center of attention in the bio-industrial fields, as it possesses superior mechanical properties [7]. The properties of the copolymer produced can be tailored by controlling the 3HV monomer composition. This copolymer has wide application compared to P(3HB).
Development of an efficient production technology for copolymer production would be of paramount importance. Industrial fermentation strategies involve producing high cell density product with high volumetric productivity [8]. However, lower productivity of PHA when it comes to larger scale makes the overall production even more uneconomical. To overcome this, it is vital to design and develop proper fermentation strategies in order to improve the fermentation performance. Khanna and Srivastava [9] reported that future studies should be carried out to increase the productivity by using alcohol as the carbon precursor for industrial PHA production. This was most probably because alcohols are less toxic compared to other carbon precursors.
It is well established that high PHA productivity can be achieved by producing culture with high cell density and high PHA concentration or by reducing the cultivation time. In addition, the maximization of the carbon yield of PHA relative to the carbon uptake for cell growth is of primary concern. It was reported by Lee and Choi [10] that large-scale PHA production by using bacterial fermentation is important for the commercialization purposes. Concentration of nutrients that are fed into the bioreactor can be controlled by regulating the feed rate. In fed-batch cultivation, the cells are fed continuously depending on the feeding method [11]. It is well known that fed-batch method yields higher cell densities, thus indirectly reducing the cost of production [10].
In this study, the ability of a locally isolated strain, Cupriavidus sp. USMAA2-4, to produce copolymer P(3HB-co-3HV) by using oleic acid as the main carbon source and alcohol-based 3HV precursor, 1-pentanol, was investigated by using fed-batch strategy. There were no literature available on the production of P(3HB-co-3HV) copolymer by using 1-pentanol and oleic acid with high productivity by using a locally isolated bacterium. Varying the frequency of feeding and consideration of dissolved oxygen has improved the productivity of copolymer. The molecular mass and thermal and mechanical properties of the copolymer produced were also evaluated.
Materials and Methods
Bacterial Strain
Cupriavidus sp. USMAA2-4 (DSM 19379) was isolated from a soil sample collected from Sg. Pinang, Penang, Malaysia.
Synthesis of Copolymer P(3HB-co-3HV) Through Fed-Batch Fermentation
Fed-batch fermentation for copolymer P(3HB-co-3HV) was carried out by using a 3.6-L bioreactor (Infors HT, Switzerland) with 2-L working volume. Approximately 0.06 g/L of inoculum was transferred into a 2 L mineral salt medium (MSM) containing sterilized oleic acid and 1-pentanol at a concentration of 0.56 wt% C. Feeding was carried out based on dissolved oxygen-stat mode (DO-stat mode). MSM was prepared according to Amirul et al. [12]. Approximately 1.1 g/L ammonium sulfate, 6.2 g/L oleic acid, and 1.2 g/L 1-pentanol were fed into the culture when the dissolved oxygen spike was observed. Three types of feeding were carried out: one time feeding, two times feeding, and three times feeding. Each time fixed amount of carbon and nitrogen source was fed. The agitation speed was set at 525 rpm whereas the aeration rate was maintained at 1 vvm based on previous optimization. The pH was maintained at around 7.0. Cell growth was monitored by measuring the optical density (OD) of the culture at 540 nm. Harvested cells were then centrifuged, freeze-dried, and analyzed using gas chromatography (GC). Polymer films were prepared according to Amirul et al. [12].
Determination of Oxygen Uptake Rate
The dynamic method measures the respiratory activity of microorganisms which are actively growing in the bioreactor. In order to determine the oxygen uptake rate of the microorganism, the gas supply to the bioreactor was turned off. The dissolved oxygen concentration decreased at a rate equal to oxygen consumption by the respiration of microorganism. The value of oxygen uptake rate (OUR) was obtained from the slope of the plot of dissolved oxygen concentration (after stopping air flow) against time. The gas (oxygen) solubility was assumed to be 1.26 mmol/L at 25 °C [13].
Determination of Volumetric Mass Transfer Coefficient (K La) and Oxygen Transfer Rate
Volumetric mass transfer coefficient was measured by elimination of oxygen in the liquid phase by bubbling nitrogen into the liquid until the oxygen concentration drops to zero. Then, the liquid was put into contact again with air in order to measure the variation (increase) of the oxygen concentration with time. The value of K La was obtained from the slope of the plot ln(C-C L) versus time (C represents dissolved oxygen concentration and C L represent dissolved oxygen concentration in equilibrium with mean gaseous oxygen concentration). Oxygen transfer rate was determined by multiplying the K La with the gas solubility. The gas solubility was assumed to be 1.26 mmol/L for oxygen at 25 °C [13].
Characterization of Polymer Films
The mechanical properties (tensile strength, elongation at break, and Young’s modulus) of the copolymers were characterized using tensile machine (GOTECH A1-3000 with U60 software, Taiwan). Tensile test pieces with 4-mm width and 45-mm gauge length (dumbbell shape) were cut from polymer films using steel ASTM regulation punches. The cross-head speed used was 10 mm/min. Load cell of 100 N was used for the analysis. The tensile test was analyzed based on ASTM: D882-91 (ASTM, 1991). Five replicates were prepared for each sample.
The number average molecular weight (M n), the weight average molecular weight (M w), and polydispersity index (M w/M n) data was obtained at 35 °C using a refractive index detector (RID-10A) and Shimadzu High Performance Liquid Chromatography (Kyoto, Japan) with a PLgel mixed C column (Polymer Laboratories, Ltd., UK). Polystyrene standards with a low polydispersity were used to construct a calibration curve. Chloroform was used as eluent at a flow rate of 1.0 mL/min. Concentration of the samples was fixed at 1.0 mg/mL. Molecular weight was calculated by using the Mark Houwink equation ([η] = KMVa). The Mark-Houwink constants for polystyrene in chloroform were taken as K = 0.011 mL/mg and α = 0.73. Similarly, the equivalent constants for P(3HB) in chloroform was K = 0.016 mL/mg and α = 0.76 [14].
The melting temperature (T m) and the enthalpy of fusion (ΔH m) were taken at the peak of the melting endotherm, respectively, whereas the glass transition temperature (T g) was taken at the midpoint of the heat capacity change. Crystallization temperature (T c) and crystallization enthalpy (ΔH c) were also obtained from the differential scanning calorimetry (DSC) curves [15]. The crystallinity of samples was calculated in relation to a hypothetical 100 % crystalline polymer (PET) with a fusion of enthalpy of 140.1 J/g [16].
The randomness of the copolymer P(3HB-co-3HV) was determined using the 13C nuclear magnetic resonance (13C-NMR) analysis. About 25 mg of purified copolymer was dissolved in 1 mL of deuterated chloroform (CDCl3). The 13C-NMR spectra were measured at 100 MHz in a 400 Ultrashield (Bruker, Switzerland). Tetramethylsilane (Me4Si) was used as an internal chemical shift reference, and the chemical shifts were reported relative to Me4Si [17]. Morphology of the isolate and the PHA granules were examined by transmission electron microscopy (Philip CM 12/STEM) as described by Loo and co-workers [18].
Determination of Residual Protein
Samples were centrifuged to obtain the supernatant. Approximately 0.5 mL supernatant and 2.5 mL solution 1 [1 % (1 % CuSO4·5H2O) + 1 % (2 % NaIK tartarate) + 98 % (1 % Na2CO3 in 0.1 M NaOH)] were mixed in a test tube. The test tube was incubated for 10 min at 37 °C. Then, 0.25 mL phenol and 0.25 mL H2O were added into the test tube. The tubes were incubated at room temperature for 30 min. The OD measurement was obtained at 750 nm. The standard was performed by using bovine serum albumin (BSA). The absorbance value of the standard was then plotted against the concentration. This standard curve was then used to determine the protein concentration in the samples. The method was modified from Lowry et al. [19].
Determination of Residual Nitrogen
Samples were centrifuged to obtain the supernatant. Approximately 5 μL of the supernatant and 2.5 mL of Solution A (10 g phenol + 0.0568 g sodium nitroprusside dissolve in 1 L H2O) and 2.5 mL of Solution B (5 g NaOH + 53.6 g disodium phosphate anhydrous + 10 mL sodium hypochlorite dissolve in 1 L H2O) were mixed together in a test tube. The test tube was vortexed for 5 s before incubating it at room temperature for 30 min. The test tube was then placed on ice for 30 min. The test tubes were then left until it reaches room temperature before measuring the OD at 625 nm. The standard was performed by using known concentration of ammonium sulfate. The absorbance value of the standard was then plotted against concentration. The method was modified from Jeong et al. [20] and Solorzano [21].
Determination of Residual Oleic Acid
The samples were centrifuged to obtain the supernatant. Approximately 2 mL of the supernatant was mixed with 2 mL of chloroform in a test tube. The tube was then vortexed for 2 min to mix the solutions. The test tube was then left at room temperature for 1 h for phase separation to occur. The lower layer was transferred into a GC bottle containing anhydrous Na2SO4 to remove the water. About 0.5 mL of this solution was mixed with 2.0 mL of chloroform and 2.0 mL of methanol sulphuric acid in the test tube. Methanolysis was carried out for 30 min. The test tube was left to cool at room temperature. About 1.0 mL of water was added to the test tube and vortexed. The lower layer was then transferred into a GC bottle containing anhydrous Na2SO4. About 0.5 mL of this solution was then transferred into GC vial. Then, 0.5 mL caprylic methyl ester (CME) was added into a GC vial. The standard was performed by using known concentration of oleic acid. The method was modified from Braunegg et al. [22]. The ratio of area (area of oleic acid/area of CME) of the standard was plotted over the concentration. Gas chromatography machine SHIMADZU Gas Chromatograph GC-2010 was used. About 1 μL of the samples was injected using AOC-20i auto-injector.
Determination of Residual 1-Pentanol
The samples were centrifuged to obtain the supernatant. Approximately 1 mL of the supernatant was mixed with 2 mL of chloroform in a test tube. The tube was then vortexed for 2 min to mix the solutions. The test tube was left at room temperature for 1 h for phase separation to occur. The lower layer was then transferred into GC bottle containing anhydrous Na2SO4 to remove water. About 0.5 mL of this solution was then transferred into GC vial. Then, 0.5 mL CME was added into the same vial. The method was modified from Braunegg et al. [22]. The standard was performed by using known concentration of 1-pentanol. The ratio of area (area of pentanol/ area of CME) of the standard was plotted against the concentration. The gas chromatography (GC) machine used was SHIMADZU Gas Chromatograph GC-2010. About 1 μL of the samples was injected using AOC-20i auto-injector.
Results and Discussion
Production of Copolymer P(3HB-co-3HV) by Cupriavidus sp. USMAA2-4 Through Fed-Batch Strategy
The main goal of any biotechnological process development is to maximize the productivity. This can be obtained by producing a culture with high concentration of PHA in a reaction volume with the shortest time [23]. In addition, the implementation of various production strategies and supplementation of different concentrations of carbon and nitrogen sources can improve the residual cell dry weight (RCDW), PHA content, and 3HV monomer composition which is the targeted product in this study. Currently, repeated batch, continuous, and fed-batch cultures are the main culture strategies being used in order to obtain higher productivity cultures for PHA production. Fed-batch cultures have been widely used for the production of P(3HB) because fed-batch cultures require low investment and production costs [24, 25].
Fed-batch strategy was employed to produce culture with high PHA and cell dry weight (CDW) in this study. The concentration of 1-pentanol (0.04 wt% C), incubation time (54 h), aeration rate (1 vvm), and agitation speed (525 rpm) were fixed based on previous study. The same concentrations of oleic acid (6.2 g/L), 1-pentanol (1.2 g/L), and nitrogen source (1.1 g/L) as initial concentrations were fed based on the dissolved oxygen (DO) spike observed. The controlled parameters in bioreactor were as follows: total volume 3.6 L, working volume 2 L, temperature 28 °C, pH 7, and cell concentration 0.06 g/L.
DO-stat is crucial in increasing the cell density besides minimizing the accumulation of incompletely reduced substrates. Throughout the fermentations that have been carried out, the dissolved oxygen showed a decrease from 100 to 0 % before DO spike occurs. It was maintained until the DO spike occurs which was used as an indicator for depletion of carbon source. At this point, feeding was carried out in order to allow the bacteria to grow and accumulate copolymer. The DO-stat mode was applied since the DO was sensitive with the depletion of carbon source and precursors. Lee and co-workers [26] also applied DO-stat in producing high cell density culture of P(3HB) by using Alcaligenes latus. A few other strategies have been applied for the production of a wide range of 3HV precursors such as alternate feeding [27], sequential feeding [28], and feeding based on pH-stat [29].
Table 1 depicts the production of copolymer where the carbon source and nitrogen source were fed once (after 12 h). The feeding was carried out based on the DO spike observed at 12 h. After 24 h, the DO spike was observed again but feeding was not carried out in order to allow the bacteria to accumulate copolymer since there was still oleic acid present with trace amount of nitrogen. The PHA content increased sharply from 18 ± 6 wt% at 12 h to 53 ± 3 wt% at 24 h (12 h after the feeding was carried out). Under the nitrogen-sufficient condition, cell growth was apparent from the increase of RCDW from 3.4 ± 0.3 to 11.8 ± 0.8 g/L. However, RCDW does not increase under the nitrogen-limited condition, and CDW increased only due to the increase of PHA concentration.
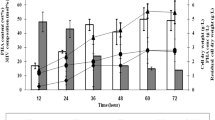
As illustrated in Fig. 1, the P(3HB-co-3HV) synthesis rate reached a maximum value of 0.55 g/L/h after the feeding was carried out and then decreased to 0.30 g/L/h when the PHA content reached a value of 68 wt%. No residual 1-pentanol was present in the bioreactor under the copolymer accumulation conditions (24 h). A cell concentration of 25 g/L was obtained in 24 h, resulting in the highest productivity of 0.55 g/L/h. As predicted, the yield of copolymer production over biomass was found to increase throughout cultivation due to the increase in PHA concentration as the RCDW decrease.
Kinetic analysis of copolymer P(3HB-co-3HV) production in a 3.6-L bioreactor through fed-batch fermentation by feeding one time. Values are mean of triplicate. Dashed line indicates feeding. Sampling for 12 h was carried out before the feeding. Data are mean derived from three independent repeats. Oleic acid at 0.52 wt% C and 1-pentanol at 0.04 wt% C were added for each combination. Nitrogen (1.1 g/L), oleic acid (6.2 g/L), and 1-pentanol (1.2 g/L) were fed after 12 h. It was incubated at 525 rpm at 30 °C with 1 vvm
Based on one time feeding, it was found that after the feeding, the nitrogen and 1-pentanol leftover started to drop. As for the oleic acid, slow utilization of oleic acid was observed until the end of fermentation process. The limitation of carbon source might have contributed to the constant polymer accumulation after 24 h. Therefore, a second set of experiment was carried out by using the same culture condition, but the feeding was carried out two times (12 and 24 h) based on the DO spike observed.
Table 2 shows the time profile of the production of copolymer by feeding a second dose of carbon and nitrogen source. The result demonstrated that at the end of cultivation (54 h), the CDW and PHA content obtained were 35.2 ± 0.1 g/L and 57 ± 3 wt%, respectively. The first DO spike was observed at 12 h and the second DO spike was observed at 24 h. After 36 h, the DO increased until the end of fermentation. No further feeding was carried out since there was still remaining oleic acid and nitrogen source left in the medium after 24 h. The second feeding was carried out after 24 h at which the nitrogen in the medium has been exhausted. This resulted in an increase of PHA concentration from 12.8 ± 0.9 g/L at 24 h to 20.8 ± 0.3 g/L at 36 h. This was most probably because the feeding of the carbon source during nitrogen limitation led to an increase in the amount of the copolymer produced. The same trend was reported by Majid et al. [30].
Figure 2 shows the kinetic study of the production of copolymer when two times feeding was carried out. The yield (product/biomass) was highest at the end of cultivation (1.33 g/g) indicating that more PHA accumulates in the cell as less growth was observed. The highest productivity was recorded at 36 h (0.58 g/L/h) at which the CDW was 37.9 ± 0.1 g/L and the PHA concentration was 20.8 ± 0.3 g/L. Ma et al. [31] reported that higher cell densities of PHA contributed to the increase in PHA concentration which results in higher PHA productivity.
Kinetic analysis of copolymer P(3HB-co-3HV) production in a 3.6-L bioreactor through fed-batch fermentation by feeding two times. Values are mean of triplicate. Dashed lines indicate feeding. Sampling was carried out before the feeding. Data are mean derived from three independent repeats. Oleic acid at 0.52 wt% C and 1-pentanol at 0.04 wt% C were added for each combination. Nitrogen (1.1 g/L), oleic acid (6.2 g/L), and 1-pentanol (1.2 g/L) were fed two times (12 and 24 h). It was incubated at 525 rpm at 30 °C with 1 vvm
By comparing one time feeding and two times feeding, it was found that two times feeding has resulted in an increase of productivity from 0.30 g/L/h in one time feeding to 0.37 g/L/h in two times feeding and decrease in yield (product/biomass) from 2.16 g/g at one time feeding to 1.35 g/g in two times feeding. The increase in productivity was due to the increase in PHA concentration with the time.
It was found that after the second feeding, the C/N drops to 11.9. As reported previously, a higher C/N ratio favors the accumulation of copolymer. In order to increase the C/N ratio, a third set of experiment which involves feeding the oleic acid (6.2 g/L), 1-pentanol (1.2 g/L), and nitrogen source (1.1 g/L) three times (12, 24, and 36 h) was carried out. Table 3 shows the profiles of P(3HB-co-3HV) accumulation and the variations in polyester composition during cultivation after three times feeding was carried out. Three DO spikes were observed at 12, 24, and 36 h.
Throughout the feeding period, the residual biomass varied between 3.4 ± 0.3 g/L at 12 h to 16.4 ± 3.2 g/L at 54 h (end of fermentation). The CDW increased nearly ninefold from 12 h (4.1 g/L) to 54 h (37 g/L). Little accumulation (18 wt%) was observed at 12 h, and the copolymer content increased to 56 wt% at the end of fermentation. Figure 3 illustrates the kinetic analysis for the production of copolymer by three times feeding. It was found that after the third feeding, oleic acid and nitrogen source were not utilized. Even after the third feeding, the DO did not drop. This might have contributed to the constant accumulation and growth observed after 36 h. The highest yield (product/biomass) was obtained at the end of fermentation (54 h) which was 1.25 g/g. The highest PHA content was also achieved at this time (56 ± 9 wt%). The productivity was highest at 36 h (0.57 g/L/h) and then decreased to 0.38 g/L/h at the end of fermentation because after 36 h, the CDW and PHA content remain relatively constant; therefore, this resulted in a constant value of PHA concentration.
Kinetic analysis of copolymer P(3HB-co-3HV) production in a 3.6-L bioreactor through fed-batch fermentation by feeding three times. Values are mean of triplicate. Dashed lines indicate feeding. Sampling was carried out before the feeding. Data are mean derived from three independent repeats. Oleic acid at 0.52 wt% C and 1-pentanol at 0.04 wt% C were added for each combination. Nitrogen (1.1 g/L), oleic acid (6.2 g/L), and 1-pentanol (1.2 g/L) were fed three times (12, 24, and 36 h). It was incubated at 525 rpm at 30 °C with 1 vvm
A comparison of two times feeding and three times feeding resulted in increase in CDW and 3HV monomer composition. The CDW increased from 35.2 g/L in two times feeding to 37 g/L in three times feeding whereas the 3HV monomer composition increased from 20 mol% in two times feeding to 24 mol% in three times feeding. PHA content, PHA concentration, and residual biomass remain relatively constant between two times feeding and three times feeding despite additional feeding. As for the productivity, no significant difference was observed. The productivity in two times feeding was 0.37 g/L/h and in three times feeding was 0.38 g/L/h.
Therefore, it can be suggested that the limiting factor after three times feeding was not due to the carbon source or nitrogen source. Choi and Lee [29] reported that during the active P(3HB) production in fed-batch culture, insufficient oxygen resulted in high P(3HB) concentration and high P(3HB) content which directly increase the productivity. Garcia et al. [32] reported that oxygen might be one of the factors that limit the accumulation of PHA. In another study by Quangliano and Miyazaki [33], it was reported that sufficient aeration rate but oxygen-limiting conditions are necessary to achieve high P(3HB) production for A.chrococcum strain. Therefore, by observing the DO which did not drop after three times feeding, it was presumed that limiting the oxygen after the third feeding might have contributed in the increase in growth and accumulation of copolymer. The next set of experiment involved creating a condition of oxygen limitation after the third feeding.
In order to determine the exact amount of oxygen being supplied, the OUR, oxygen transfer rate (OTR), and K La need to be determined. Due to the low solubility of oxygen, these three parameters need to be determined to ensure an adequate delivery of oxygen [34]. Table 4 shows the data for OUR, OTR, and K La under various agitation and aeration. Based on the table, the OTR and K La decrease from 53.58 mmol/L/h at 525 rpm, 1 vvm, to 13.57 mmol/L/h at 200 rpm, 0.5 vvm, whereas the K La decreases from 44.76 h−1 at 525 rpm, 1 vvm, to 11.34 h−1 at 200 rpm, 0.5 vvm. The same trend was reported by Zafar et al. [35] that K La value increased with increase in agitation and aeration rates.
The OUR by the microorganisms remains relatively constant (7.3 to 7.5 mmol/L/h) regardless of agitation and aeration. The highest K La was recorded when the agitation and aeration rate was the highest. This indicates that more oxygen was transferred to the culture medium. The oxygen transfer rate of microbial cell strongly affects growth and product formation [13]. Oxygen is known to play a crucial role as terminal electron acceptor during ATP production. Since oxygen plays a role as the terminal electron acceptor during ATP production, more ATP will be produced when more oxygen is supplied. This resulted in the acceleration of the bacterial metabolic process [36]. Therefore, the agitation and aeration rate at which the OTR was almost near to OUR was chosen (200 rpm, 0.5 vvm). Agitation rate with 200 rpm and 0.5 vvm has the lowest K La. Low value of K La represents the reduction in the dissolved oxygen of the culture medium. Another set of experiment was carried out with the optimized agitation and aeration rate.
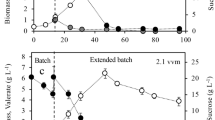
Table 5 shows the production of copolymer after three times feeding was carried out. After the third feeding, the agitation and aeration rate was reduced. The dissolved oxygen concentration was maintained at a low level until the end of fermentation to allow the accumulation of copolymer. Based on Table 5, an almost comparable result was obtained between three times feeding and the last run based on OUR and OTR up to 36 h. The PHA concentration and PHA content increase after 36 h proving the efficiency of controlling the dissolved oxygen level in the fermentation medium. The CDW and PHA content recorded were 37.0 ± 1.0 g/L and 52 ± 2 wt%, respectively, at 36 h. At the end of fermentation (54 h), the CDW and PHA content increase to 39.0 ± 0.1 g/L and 66 ± 2 wt%, respectively. The 3HV molar fraction and 3HV concentration also increased to 24 mol% and 6.16 g/L.
A lower K La value indicates lower DO concentration in the medium. Therefore, in the last run carried out with a low K La value, a higher copolymer content was obtained as compared to that with a high K La value. Choi et al. [37] stated that PHA content is usually enhanced at a lower K La value because more carbon flux will be directed to the PHA biosynthetic pathway when oxygen is limited. Figure 4 shows the kinetic analysis for the last run. It can be seen that the productivity of the copolymer was the highest at 36 h (0.54 g/L/h) and the value decreases after that period to 0.48 g/L/h. The yield of the copolymer increased almost linearly from 12 to 54 h. Table 6 shows the comparison between the optimized condition obtained from bioreactor (before and after various feeding). Overall, the method of feeding has significantly increased the productivity of copolymer. By comparing the three times feeding run with the last run, it clearly shows that the productivity of the copolymer increased 26 % from 0.38 g/L/h in three times feeding to 0.48 g/L/h in the last run. The yield of the copolymer increased 54 % from 1.25 g/g in three times feeding run to 1.93 g/g in the last run. It can be suggested that limiting the oxygen after the third feeding allows the transition of culture from growth phase to accumulation phase. Besides, consumption of excess oleic acid and nitrogen source that was still present after the third feeding was initiated by creating a condition of oxygen limitation. The maximum amount of 3HV obtained was 24 mol% after the third feeding.
Kinetic analysis of copolymer P(3HB-co-3HV) production in a 3.6-L bioreactor through fed-batch fermentation by feeding three times and by considering the OUR and OTR. Sampling was carried out before the feeding. Values are mean of triplicate. Agitation was reduced to 200 rpm and aeration to 0.5 vvm after 36 h. Dashed lines indicate feeding. Data are mean derived from three independent repeats
Characterization of Copolymer P(3HB-co-3HV) Produced by Cupriavidus sp. USMAA2-4 Through Fed-Batch Strategy
Table 7 shows the molecular weight and the randomness of the copolymer produced. The copolymers were extracted and purified before analyzing their properties. Based on the table, it was observed that the number average molecular weight (M n) values of the copolymer obtained ranged from 23 to 344 kDa with polydispersity index of 2.5 to 7.8. Based on the table, it can be inferred that the polydispersity of the copolymer increases (2.5 to 7.8) as the number of feeding increases. The increase in polydispersity suggests that the incorporation of 3HV monomer in chain segment has widened its monomer distribution. More repeated chain segments containing 3HB and 3HV monomer units were produced instead of random assembly of this monomer on the chain segment. The same trend was reported by Madden and Anderson [27] that the polydispersity produced by alternate feeding was slightly higher than the random copolymer.
Weight averaged molecular weight (M w) decreased as the mol% of 3HV monomer increased (number of feeding increases). The M w of the copolymer produced ranged between 233 and 849 kDa. It can be suggested that the copolymer with higher composition of 3HV has a lower molecular weight. Feeding was carried out when there is depletion of carbon and nitrogen source. It was assumed that each time the carbon source and nitrogen source were fed, the formation of copolymer chain might stop. This contributes to more cut in the chain formation which resulted in the shortening of polymer chain. Nyman [38] reported that periods of substrate accessibility might affect the M w of the copolymer produced. This study shows that it is possible to regulate M n and PDI of the copolymer by manipulating the frequency of feeding. The ability to control the molecular weight of the copolymer is an added advantage as it offers the prospects of exploring a wide range of biopolymer applications.
In order to determine the relationship between the polydispersity index and the granule formation, the cells of Cupriavidus sp. USMAA2-4 under different feeding were observed under transmission electron microscope. Figure 5 shows the transmission electron micrograph image for the copolymer produced at different feeding times. Under electron microscope, cells occurred singly and gradually swollen during polymer accumulation. The granules were smaller in size when no feeding was carried out. The granules do not occupy the cells fully. When one time feeding was carried out, one to two granules were observed. About three granules were observed when two times feeding was carried out, and the number of granules increases to five granules after three times feeding. The last run with high PHA content and high CDW produced a granule which was fully occupied with the copolymer. This was most probably due to the crowding and fusion of granules. It can be postulated that as the number of feeding increases, the formation of short-chain polymer occurred which contributed to the increase in granule formation. This result was strengthened further with the polydispersity value obtained whereby the value increases as the number of feeding increases.
Randomness of different copolymers was determined by using the 13C-NMR carbon carbonyl resonance spectrum. Table 7 shows the dyad sequence distribution of the 3HB and 3HV units in the copolymers as determined from the peak areas of the carbonyl resonance in the 13C-NMR spectra. The dyad sequence distribution of the 3HB and 3HV units in the copolymers can be summarized as follows:
The parameter D was defined to measure the degree of variation from a random sequence of monomer units. This can be calculated from the diad fractions using the equation below:
The copolymer is a statistically random copolymer if the D value is close to 1. The D values less than 1 represent alternating copolymer while the copolymers with D value larger than 1 represent block or blend copolymers [39].
Based on the table, the D value for all the copolymers produced were in the range of 3.2 to 11.9, suggesting that these copolymers are blends or blocky copolymers having a block copolymer with either a mixture of rich HV and HB homopolymers or a mixture of HV and HB-rich random copolymers [39]. These NMR diad fractions correlate well with the monomer composition whereby as the number of feeding increases the 3HV molar fraction shows increment. Excess of 3HB inside the microbial cell led to a higher level of block homopolymer arrangement of the monomer units, and this is consistent with the values of D parameter calculated. Madden and Anderson [27] reported that alternate substrate feeding of glucose and propionic acid in their studies resulted in a significant increase in VV diad fractions. Ivanova et al. [40] also reported that the total B/V ratio in the polymer can be completely reversed by changing the feeding strategy. This proves that the chemical composition and microstructure of P(3HB-co-3HV) can be manipulated through the feeding procedure.
Table 8 shows the mechanical and thermal analysis of the P(3HB) and P(3HB-co-3HV) polymers. One of the factors that contribute to tensile strength of polymer is molecular weight. The increase in molecular weight caused an increase in tensile strength. The tensile strength was in the range of 14 to 25 MPa. However, as the number of feeding increases, the tensile strength decreases. This result correlates with the molecular weight obtained whereby the molecular weight decreases as the number of feeding increases.
A reduction in Young’s modulus from 245 to 187 MPa was also seen as the number of feeding increases (as the mol% of 3HV increases). The lower Young’s modulus represents high flexibility of the copolymer produced. Therefore, feeding of the carbon and nitrogen source resulted in a more flexible copolymer. The copolymer produced in last run exhibits the lowest Young’s modulus of 107 MPa. The lower Young’s modulus obtained in this study could be associated with the low molecular weight obtained as reported by Luo and co-workers [41]. Elongation at break increases as the number of feeding increases (as the mol% 3HV increases) whereby 3HV fraction from 7 to 23 mol% showed an increase in elongation at break from 111 to 193 %. This was most probably because the rigidness of the 3HB polymer backbone was reduced by the incorporation of 3HV monomers.
Thermal stabilities of the copolymer were studied using differential scanning calorimetry (DSC). Scandola et al. [42] reported that melting temperature (T m) and glass transition temperature (T g) are the two important physical features that determine their properties commercially. The incorporation of 3HV monomer was found to lower the T g. T g decreased from –6.9 to −9.3 °C as the 3HV monomer increased from 7 to 23 mol%. The decrease of glass transition temperature will lead to an increase in elastomeric properties of the copolymer [43]. This was most probably because the incorporation of 3HV monomer to P(3HB-co-3HV) copolymer increased the mobility in the amorphous state. This offers a larger volume of molecular movement [44]. Regulation of the copolymer composition can change their T g to suit certain applications since T g can affect the polymer morphology [45]. It could also be seen that the Young’s modulus and tensile strength of the copolymer decreased with the decrease of T g indicating that the polymers become soft and flexible as the number of feeding increases.
The T m decreases as the 3HV monomer composition increases. The T m of P(3HB) in this study was 170 °C and the enthalpy of melting (ΔH m) was 79 J/g indicating that the polymer crystallizes in the P(3HB) lattice. The lowered melting temperature allowed the molding of the polymer besides reducing the thermal degradation of the polymer for better processing. ΔH m is the indicator of crystallinity. Copolymer with lower ΔH m value indicates lower crystallinity degree. The enthalpy of the copolymer decreased from 64 to 28 J/g as the 3HV monomer increased from 7 to 23 mol% reflecting the depression in crystallinity for the copolymer sample. That was because the 3HV monomers was incorporated within the P(3HB) lattice where they act as defects in the lattice causing an increase in dimension and decrease in melting temperature [46].
The calculated crystallinity percentages are shown in Table 8. It was found that the crystallinity decreases from 46 to 20 % as the 3HV monomer composition increases from 7 to 23 mol% (number of feeding increases). The same trend was reported by Galego et al. [47] whereby the crystallinity decreases from 58.7 to 54.7 % as the 3HV monomer composition increases from 8 to 24 mol%. It was reported by Kunioka et al. [46] that the rate of crystallization depends on crystal growth and rates of nucleation. At lower 3HV monomer composition, the P(3HB-co-3HV) copolymer crystallized in the P(3HB) lattice.
Conclusion
The development of fermentation strategies through fed-batch has shown promising results in terms of productivity whereby the highest productivity obtained was 0.48 g/L/h. Undoubtedly, consideration of OUR and OTR in this strain seems to be a preeminent strategy in improving the P(3HB-co-3HV) production. By comparing with the batch fermentation, fed-batch fermentation has resulted in 200 % increment in productivity. The different frequency of feeding carried out has produced a blend copolymer and has broadened the monomer distribution. In addition, an increase in the number of granules was also observed as the frequency of feeding increased. The copolymer produced exhibited a wide range of material properties depending on the feeding frequency and the composition of the copolymer. Therefore, these strategies will facilitate the process developments and can be applied in industrial production of copolymer P(3HB-co-3HV) by using oleic acid and 1-pentanol as the substrate.
References
Chien, C. C., Chen, C. C., Choi, M. H., Kung, S. S., & Wei, Y. H. (2007). Production of poly [beta]-hydroxybutyrate (PHB) by Vibrio spp. isolated from marine environment. Journal of Biotechnology, 132, 259–263.
Suriyamongkol, P., Weselake, R., Narine, S., Moloney, M., & Shah, S. (2007). Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants – A review. Biotechnology Advances, 25, 148–175.
Akiyama, M., Taima, Y., & Doi, Y. (1992). Production of poly(3-hydroxyalkanoates) by a bacterium of the genus Alcaligenes utilizing long-chain fatty acids. Applied Microbiology and Biotechnology, 37, 698–701.
Ayub, N. D., Pettinari, M. J., Méndez, B. S., & López, N. I. (2007). The polyhydroxyalkanoate genes of a stress resistant Antarctic Pseudomonas are situated within a genomic island. Plasmid, 58, 240–248.
Iwata, T., Tsunoda, K., Aoyagi, Y., Kusaka, S., Yonezawa, N., & Doi, Y. (2003). Mechanical properties of uniaxially cold-drawn films of poly ([R]-3-hydroxybutyrate). Polymer Degradation and Stability, 79, 217–224.
Sudesh, K., Abe, H., & Doi, Y. (2000). Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Progress in Polymer Science, 25, 1503–1555.
Kim, D. Y., Park, D. S., Kwon, S. B., Chung, M. G., Bae, K. S., Park, H. Y., & Rhee, Y. H. (2009). Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co polyesters with a high molar fraction of 3-hydroxyvalerate by an insect symbiotic Burkholderia sp. IS-01. The Journal of Microbiology, 47, 651–656.
Cerrone, F., Duane, G., Casey, E., Davis, R., Belton, I., Kenny, S. T., Guzik, M. W., Woods, T., Babu, R. P., & O’Connor, K. (2014). Fed batch strategies using butyrate for high cell density cultivation of Pseudomonas putida and its use as a biocatalyst. Applied Microbiology and Biotechnology, 98(22), 9217–9228.
Khanna, S., & Srivastava, A. K. (2005). Recent advances in microbial polyhydroxyalkanoates. Process Biochemistry, 40, 607–619.
Lee, S. Y., & Choi, J. I. (1998). Effects of fermentation performance by Alcaligenes latus. Polymer Degradation and Stability, 59, 387–393.
Chen, G. Q., Zhang, G., Park, S. J., & Lee, S. Y. (2001). Industrial scale production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Applied Microbiology and Biotechnology, 57, 50–55.
Amirul, A. A., Yahya, A. R. M., Sudesh, K., Azizan, M. N. M., & Majid, M. I. A. (2009). Isolation of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) producer from Malaysian environment using γ-butyrolactone as carbon source. World Journal Of Microbiology and Biotechnology, 25, 1199–1206.
Ochoa, F. G., & Gomez, E. (2009). Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnology Advances, 27, 153–176.
Majid, M. I. A. (1988). PhD thesis, University of Bath.
Chee, J.-W., Amirul, A. A., Majid, M. I. A., & Mansor, S. M. (2008). Factors influencing the release of Mitragyna speciosa crude extracts from biodegradable P(3HB-co-4HB). International Journal of Pharmaceutics, 361, 1–6.
Mancini, S. D., & Zanin, M. (1999). Recyclability of Pet from virgin resin. Materials Research, 2, 33–38.
Amirul, A. A., Syairah, S. N., Yahya, A. R. M., Azizan, M. N. M., & Majid, M. I. A. (2008). Synthesis of biodegradable polyesters by Gram negative bacterium isolated from Malaysian environment. World Journal of Microbiology and Biotechnology, 24, 1327–1332.
Loo, C. Y., Lee, W. H., Tsuge, T., Doi, Y., & Sudesh, K. (2005). Biosynthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from palm oil products in a Wautersia eutropha mutant. Biotechnology Letters, 27, 1405–1410.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the folin phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
Jeong, H., Park, J., & Kim, H. (2013). Determination of NH4 + in environmental water with interfering substances using the modified Nessler method. Journal of Chemistry, Article ID 359217.
Solorzano, L. (1969). Determination of ammonia in natural waters by the phenolhypochlorite method. Limnology and Oceonography, 14(5), 799–801.
Braunegg, G., Sonnleitner, B., & Lafferty, R. M. (1978). A rapid gas chromatography method for determination of the poly-β-hydroxybutyric acid in microbial biomass. European Journal of Applied Microbiology and Biotechnology, 6, 29–37.
Lenczak, J. L., Schmidell, W., & Aragao, G. M. F. (2013). High cell density strategies for polyhydroxyalkanoate production: a review. Journal of Industrial Microbiology and Biotechnology, 40(3-4), 275–286.
Yamane, T., Chen, X., & Ueda, S. (1996). Growth-associated production of poly(3-hydroxyvalerate) from n-pentanol by a methylotrophic bacterium, Paracoccus denitrificans. Applied and Environmental Microbiology, 62, 380–384.
Shang, L., Jiang, M., & Chang, H. N. (2003). Poly(3-hydroxybutyrate) synthesis in fed batch culture of Ralstonia eutropha with phosphate limitation under different glucose concentrations. Biotechnology Letters, 25, 1415–1419.
Lee, S. Y., Choi, J. I., & Wong, H. H. (1999). Recent advances in polyhydroxyalkanoate production by bacterial fermentation: mini-review. International Journal of Biological Macromolecules, 25, 31–36.
Madden, L. A., & Anderson, A. J. (1998). Synthesis and characterization of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxybutyrate) polymer mixtures production in high-density fed –batch. Macromolecules, 31, 5660–5667.
Shang, L., Yim, S. C., Park, H. G., & Chang, H. N. (2004). Sequential feeding of glucose and valerate in a fed-batch culture of Ralstonia eutropha for production of poly(hydroxybutyrate-co-hydroxyvalerate) with high 3-hydroxyvalerate fraction. Biotechnology Progress, 20, 140–144.
Choi, J. I., & Lee, S. Y. (1999). High-level production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by fed-batch culture of recombinant. Escherichia coli Applied Environmental and Microbiology, 65, 4363–4368.
Majid, M. I. A., Akmal, D. H., Few, L. L., Agustien, A., Toh, M. S., Samian, M. R., Najimudin, N., & Azizan, M. N. (1999). Production of poly(3-hydroxybutyrate) and its copolymer poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Erwinia sp. USMI-20. International Journal of Biological Macromolecules, 25, 95–104.
Ma, C.K., Chua, H., Yu, P.H.U., & Hong, K. (2000). Optimal production of polyhydroxyalkanoates in activated sludge biomass. Applied Biochemistry and Biotechnology, 84-86, 981-989.
Garcia, I. L., Lopez, J. A., Dorado, M. P., Kopsahelis, N., Alexandri, M., Papanikolaou, S., Villar, M. A., & Koutinas, A. A. (2013). Evaluation of by-products from the biodiesel industry as fermentation feedstock for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Cupriavidus necator. Bioresource Technology, 130, 16–22.
Quangliano, J. C., & Miyazaki, S. S. (1997). Effect of aeration and carbon/nitrogen ratio on the molecular mass of the biodegradable polymer poly-ß-hydroxybutyrate obtained from Azotobacter chroococcum 6B. Applied Microbiology and Biotechnology, 48, 662–664.
Calik, P., Yilgor, P., Ayhan, P., & Demir, A. S. (2004). Oxygen transfer effects on recombinant benzaldehyde lyase production. Chemical Engineering Science, 59, 5075–5083.
Zafar, M., Kumar, S., & Dhiman, A. K. (2012). Modeling and optimization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production from cane molasses by Azohydromonas lata MTCC 2311 in a stirred-tank reactor: effect of agitation and aeration regimes. Journal of Industrial Microbiology and Biotechnology, 39, 987–1001.
Third, K. A., Newland, M., & Ruwisch, R. C. (2003). The effect of dissolved oxygen on PHB accumulation in activated sludge cultures. Biotechnology and Bioengineering, 82, 238–250.
Choi, J. C., Shin, H. D., & Lee, Y. H. (2002). Pilot scale production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by fed batch culture of recombinant. Eschericia Coli Enzyme and Microbial Technology, 32, 178–185.
Nyman, A. K. (2010). Master thesis.
Kamiya, N., Yamamoto, Y., Inoue, Y., & Chujo, R. (1989). Microstructure of bacterially synthesized poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Macromolecules, 22, 1676–1682.
Ivanova, G., Serafim, L. S., Lemos, P. C., Ramos, A. M., Reis, M. A. M., & Cabrita, E. J. (2009). Influence of feeding strategies of mixed microbial cultures on the chemical composition and microstructure of copolyesters P(3HB-co-3HV) analyzed by NMR and statistical analysis. Magnetic Resonance in Chemistry, 47, 497–504.
Luo, S., Grubb, D. T., & Netravali, A. N. (2002). The effect of molecular weight on the lamellar structure, thermal and mechanical properties of poly(hydroxybutyrate-co- hydroxyvalerates). Polymer, 43, 4159–4166.
Scandola, M., Ceccoralli, G., & Doi, Y. (1990). Viscoelastic relaxations and thermal properties of bacterial poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). International Journal of Biological Macromolecules, 12, 112–117.
Doi, Y. (1990). Microbial polyesters. Ch 3. New York: VCH Publishers.
You, J. W., Chiu, H. J., Shu, W. J., & Don, T. M. (2003). Influence of hydroxyvalerate content on the crystallization kinetics of poly(hydroxybutyrate-co-hydroxyvalerate). Journal of Polymer Research, 10, 47–54.
Zhang, H. F., Ma, L., Wang, Z. H., & Chen, G. Q. (2009). Biosynthesis and characterization of 3-hydroxyalkanoate terpolyesters with adjustable properties by Aeromonas hydrophila. Biotechnology and Bioengineering, 104(3), 582–589.
Kunioka, M., Tamaki, A., & Doi, Y. (1989). Crystalline and thermal properties of bacterial copolyesters: Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules, 22, 694–697.
Galego, N., Rozsa, C., Sanchez, R., Fung, J., Vazquez, A., & Tomas, J. S. (2000). Characterization and application of poly(ß-hydroxyalkanoates) family as composite Biomaterial. Polymer Testing, 19, 485–49.
Acknowledgments
The authors acknowledge the USM Science Fellowship awarded to Shantini [RU(1001/441/CIPS/AUPE001)] that has resulted in this article.
Compliance with Ethical Standards
All the co-authors have seen and agreed with the contents of the manuscript, and there is no financial interest to report. We certify that the submission is the original work by us and is not under review in any other publication. We also would like to justify that we do not have any conflict of interest to declare and this study does not involve the usage of animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shantini, K., Yahya, A.R.M. & Amirul, A.A. Influence of Feeding and Controlled Dissolved Oxygen Level on the Production of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Copolymer by Cupriavidus sp. USMAA2-4 and Its Characterization. Appl Biochem Biotechnol 176, 1315–1334 (2015). https://doi.org/10.1007/s12010-015-1648-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1648-5