Abstract
In this study, we investigated the bioremediation of petrochemical wastewater containing BTEX compounds by immobilized Comamonas sp. JB cells. Three kinds of magnetic nanoparticles were evaluated as immobilization supports for strain JB. After comparison with Fe3O4 and a-Fe2O3 nanoparticles, r-Fe2O3 nanoparticle was selected as the optimal immobilization support. The highest biodegradation activity of r-Fe2O3-magnetically immobilized cells was obtained when the concentration of r-Fe2O3 nanoparticle was 120 mg L−1. Additionally, the recycling experiments demonstrated that the degradation activity of r-Fe2O3-magnetically immobilized cells was still high and led to less toxicity than untreated wastewater during the eight recycles. qPCR suggested the concentration of strain JB in r-Fe2O3-magnetically immobilized cells was evidently increased after eight cycles of degradation experiments. These results supported developing efficient biocatalysts using r-Fe2O3-magnetically immobilized cells and provided a promising technique for improving biocatalysts used in the bioremediation of not only petrochemical wastewater but also other hazardous wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
BTEX compounds (benzene, toluene, ethylbenzene, and o-, m-, and p-xylenes) are an important family of aromatic hydrocarbons that are components of petroleum, and its products such as gasoline and diesel fuel are widely used in industrial syntheses [1–3]. Meantime, because of the application of petroleum and its products in industrial processes, BTEX compounds are frequently found as the major organic pollutants in petrochemical, coking, and other industrial wastewater [4]. As it is well known, BTEX compounds possess toxic to humans and are confirmed or suspected carcinogens. Thus, the Environmental Protection Agency classifies them as priority pollutants, making their removal from polluted environments critical [5]. Therefore, it is necessary to establish effective methods to clean up BTEX compounds to protect the environment [6].
Many researchers have focused their studies on the isolation and identification of BTEX compounds-degrading microorganisms, such as Pseudomonas, Pseudoxanthomonas, Burkholderia, Sphingomonas, Thauera, Dechloromonas, Rhodococcus, Janibacter, and Acinetobacter [6–12]. Nevertheless, current studies are mostly focused on the metabolism pathways of BTEX compounds, as well as the genes and enzymes involved, and rarely on the development of an immobilization method for bioremediation [10, 13, 14]. The use of immobilized microorganisms rather than free cells in biotransformation is advantageous to enhance the stability of the biocatalyst and to facilitate its recovery and reuse [15]. These advantages have encouraged researchers to investigate the application of immobilized cells in the biodegradation of toxic compounds, such as phenol, pyridine, carbazole, and dibenzothiophene [16–19]. However, mass transfer limitation involved in substrate diffusion to the reaction system is still the major drawback in the application of an entrapment technique [20]. Recently, nanoparticles that represent a new generation of environmental remediation technologies have been used in the studies of immobilized microbial cells, which could reduce the mass transfer resistance of traditional immobilization processes, especially for magnetic nanoparticles [20–23]. Thus, it is necessary and significant to attempt to remove BTEX by immobilized BTEX degraders with magnetic nanoparticles.
In this study, the bioremediation of BTEX by a new immobilized bacterium Comamonas sp. JB in magnetic gellan gum was investigated. The selection of magnetic nanoparticles and degradation of BTEX compounds by magnetically immobilized cells of strain JB were studied. The qPCR assays were also investigated. Meantime, ecotoxicological assessment of the treated effluent was also carried out. In addition, the recycling of r-Fe2O3-magnetically immobilized cells and nonmagnetically immobilized cells coupling with activated zeolite on the treatment of petrochemical wastewater containing BTEX compounds was also tested.
Materials and Methods
Chemicals
Benzene, toluene, ethylbenzene, and o-, m-, and p-xylenes were purchased from J&K Scientific Ltd. (China). Fe3O4 nanoparticle (diameter <20 nm, 98 %), a-Fe2O3 nanoparticle (diameter <20 nm, 99.9 %), and r-Fe2O3 nanoparticle (diameter <20 nm, 99.9 %) were purchased from DKnano Scientific Ltd. (China). Activated zeolite as the ammonium exchangers was obtained from Zhejiang Shengshi Mining Industry Co., Ltd (China). All other commercially available chemicals were of analytical grade.
Bacterial Strain and Cultivation Conditions
Comamonas sp. JB was routinely grown in mineral salt medium (MSM), which contained KH2PO4 3.7 g L−1, K2HPO4·3H2O 5.2 g L−1, NH4Cl 2.0 g L−1, Na2SO4 1.0 g L−1, MgSO4 0.1 g L−1, and 1 mL L−1 of trace metal solution as previous described [24]. Yeast extract (40 mg L−1) was added to the MSM. Benzene, toluene, ethylbenzene, and o-, m-, and p-xylene were dissolved in dimethyl sulfoxide and added to the MSM at a suitable concentration. All cultures or cell suspensions were incubated at 30 °C on a reciprocal shaker at 150 rpm. Cell suspensions of BTEX-grown JB were prepared separately by centrifugating the cultures in late exponential phase at 10,000×g for 5 min, washing cell pallets twice with MSM, and resuspending cells in MSM.
Preparation of Gel Beads and Immobilized Cells
Immobilization of cells was performed using a method as described previously [20]. The gellan gum (1 % wt/vol) and cell suspension of strain JB with a turbidity at 660 nm of 2.5 were mixed at ratio of cell wet weight to dry polymers powder of 3 (wt/wt). Nonmagnetically immobilized cells were formed by extruding the mixture through a syringe into 0.2 M CaCl2 and letting it solidify for 2 h. For preparing magnetically immobilized cells, 80 mg L−1 of Fe3O4 nanoparticle, a-Fe2O3 nanoparticle, and r-Fe2O3 nanoparticle suspension were added to the abovementioned mixture of gellan gel and cell suspension, and the procedure was the same as that for nonmagnetically immobilized cells. Nonmagnetically immobilized inactive cells and magnetically immobilized inactive cells were prepared as described above.
Petrochemical Wastewater Treatment by Immobilization Cells
The petrochemical wastewater used in this study was from the petrochemical wastewater treatment plant (WWTP) located in northeast China. The raw wastewater quality is shown in Table S1. The raw wastewater was diluted 1:1 (v/v) in deionized water, and phenol and BTEX compounds were added to give prominence to degradation; the final concentrations of them are shown in Table S1. This high phenol- and BTEX-concentrated petrochemical wastewater was used in the following study. Study on the degradation of this petrochemical wastewater by three magnetically immobilized cells, nonmagnetically immobilized cells, and free cells of strain JB coupling with activated zeolite were carried out. The concentration of r-Fe2O3 nanoparticle (40 to 240 mg L−1) in magnetically immobilized cells on degradation of the petrochemical wastewater was studied. In the recycling experiments, after each biodegradation batch, r-Fe2O3-magnetically immobilized cells were collected and then were washed once with MSM. After the MSM was drained, 50 mL of petrochemical wastewater containing BTEX were added to repeat the cycle. Samples were taken at intervals to monitor the concentrations of BTEX and the acute toxicity of effluent and influent samples were also tested by Microtox bioassays as described below. All experiments were performed in triplicate.
Quantitative Real-Time PCR Assays
The concentration of strain JB in nonmagnetically immobilized cells, a-Fe2O3-magnetically immobilized cells, Fe3O4-magnetically immobilized cells, and r-Fe2O3-magnetically immobilized cells was selected for qPCR assays, which were conducted in triplicate using PCR Thermal Cycler Dice Real Time System (TaKaRa, China) with the primer set 16sFn (5′-TGGCAGATTAGGTAGTTGGTGG-3′) and 16sRn (5′-CAAAAGCAGTTTACAACCCGAG-3′). The qPCR mixture (25 μL−1) contained 12.5 μL−1 SYBR Premix Ex Taq (TaKaRa, China), 1 μL−1 of each primer (10 μM), and 2 μL template DNA. The thermal profile included 30 s of initial denaturation at 95 °C, followed by 40 cycles of 5 s at 95 °C and 30 s at 60 °C. The amplicons were visualized and check by electrophoresis on agarose gel (1.5 %, wt vol−1).
Analytical Methods
After each batch of biodegradation, the samples were extracted with two volume of methylene chloride for at least 1 h by inversion. The concentrations of BTEX were analyzed by gas chromatography with an HP-5 capillary column (Agilent Technologies, 6890 N) as the previously described [6]. The gas chromatography oven was programmed to increase from 60 °C (held for 1 min) to 220 °C at 10 °C min−1, after which 220 °C was held for 3 min. The gas flow to the detector contained H2 (40 mL min−1) and synthetic air (450 mL min−1), the detector temperature was 300 °C, the injection port temperature was 250 °C, and the 1 μL samples were loaded with an auto sampler with a split mode (5:1). The profiles of m-xylene and p-xylene mirrored each other, because they had the same retention time on the gas chromatography analysis chromatogram, thus the concentration of m/p-xylene was calculated by dividing the mixture concentrations of m-xylene and p-xylene by 2 [6]. Phenol concentration was analyzed using high-performance liquid chromatography (HPLC) system (Shimadzu LC20A; Thermo Hypersil ODS-2 column, 5 μm, 250 × 4.6 mm) as previously described [16]. NH3-N concentration was obtained via a Nessler’s reaction using UV/Vis spectrophotometer [15]. The acute toxicity of effluent and influent samples was assessed by Microtox bioassays using the luminescent bacteria Vibrio fischeri (NRRL B-11177) as the previously described [16]. The morphology of cells immobilized in gel beads was determined using a scanning electron microscope (SEM) (S-570; Hitachi, Japan).
Results and Discussion
Selection of Magnetic Nanoparticles for Immobilization
In this study, three kinds of magnetic nanoparticles (a-Fe2O3, r-Fe2O3, and Fe3O4 nanoparticles) were evaluated as the magnetic nanoparticle for the immobilization of strain JB in gellan gum. The biodegradation of petrochemical wastewater containing phenol and BTEX compounds was conducted by free cells, nonmagnetically immobilized cells, and magnetically immobilized cells (a-Fe2O3, r-Fe2O3, and Fe3O4 nanoparticles) coupling with activated zeolite, respectively. As shown in Fig. 1a, phenol and all BTEX compounds were completely consumed within 8 to 32 h by free cells. The activity of nonmagnetically immobilized cells was lower than that by free cells; phenol and all BTEX compounds were completely consumed within 12 to 36 h by nonmagnetically immobilized cells (Fig. 1b). The SEM image of nonmagnetically immobilized cells is shown in Fig. S1a. It indicated that strain JB cells could be clearly observed, and the sheets of gellan gum matrix were tightly bound together, which may be resulted in impeding of the mass transfer of substrate from the environment to the central reaction site [20]. Additionally, no decrease of phenol and BTEX compounds content was observed when nonmagnetically immobilized inactive cells and gellan gel beads without cells served as biocatalysts, which confirmed that the removal of phenol and BTEX compounds was due to biodegradation by strain JB (data not shown).
Biodegradation of petrochemical wastewater containing phenol, benzene, toluene, ethylbenzene, o-xylene, m/p-xylene, and NH3-N by free cells (a), nonmagnetically immobilized cells (b), magnetically immobilized cells (a-Fe2O3, c), magnetically immobilized cells (r-Fe2O3, d), and magnetically immobilized cells (Fe3O4, e)
In contrast, high biodegradation activities for phenol and BTEX compounds were obtained when magnetically immobilized cells served as the biocatalyst (Fig. 1c–e). Among these three magnetic nanoparticles, the highest biodegradation activities for phenol, benzene, toluene, ethylbenzene, o-xylene, and m/p-xylene were presented by r-Fe2O3-magnetically immobilized cells, 100 mg L−1 phenol could be degraded completely in 6 h, 30 mg L−1 and benzene, toluene, ethylbenzene, o-xylene, and m/p-xylene could be degraded completely within 12, 16, 20, 20, and 8, respectively. Magnetically immobilized cells with a-Fe2O3 and Fe3O4 nanoparticle showed slightly lower biodegradation activities for phenol and all the BTEX compounds than that with r-Fe2O3 nanoparticle, and phenol and all the BTEX compounds could be degraded completely within 6 to 24 h. Meanwhile, the SEM images of magnetically immobilized cells indicated that the sheets of gellan gum matrix were loosely bound together, and many pores existed between the sheets of gellan gum matrix in magnetic gellan gel beads, especially for immobilization with r-Fe2O3 nanoparticle (Fig. S1 b–d). It confirmed that the existence of nanoparticle could reduce or eliminate mass transfer problems to improve the biodegradation activities of magnetically immobilized cells. Therefore, r-Fe2O3 nanoparticle was chosen as the most suitable magnetic nanoparticle in the subsequent experiments. Because of the ion exchange adsorption of activated zeolite, all the NH3-N could be completely removed within 2 h.
Bioremediation of Petrochemical Wastewater by r-Fe2O3-Magnetically Immobilized Cells
The effects of different concentrations of r-Fe2O3 nanoparticle (40, 80, 120, and 240 mg L−1) on the activity of immobilized cells were studied. Figure 2 shows that the biodegradation rate was highest at an r-Fe2O3 nanoparticle concentration of 120 mg L−1, 100 mg L−1 phenol could be degraded completely in 4 h, and 30 mg L−1 benzene, toluene, ethylbenzene, o-xylene, and m/p-xylene could be degraded completely within 8, 12, 16, 16, and 6, respectively. The equivalent amount of phenol, benzene, toluene, ethylbenzene, o-xylene, and m/p-xylene at r-Fe2O3 nanoparticle concentration of 80 mg L−1 could be degraded completely within 6, 12, 16, 20, 20, and 8, respectively. While the biodegradation rate was lower at an r-Fe2O3 nanoparticle concentration of 40 and 240 mg L−1, phenol, benzene, toluene, ethylbenzene, o-xylene, and m/p-xylene could be degraded completely within 6, 16, 20, 24, 28, and 12 h, respectively. These results revealed that the biodegradation activity of the immobilized strain JB cells was significantly enhanced by adding 120 mg L−1 r-Fe2O3 nanoparticle, which may be due to the reduction or elimination of mass transfer problems.
Biodegradation of petrochemical wastewater containing phenol, benzene, toluene, ethylbenzene, o-xylene, m/p-xylene, and NH3-N by r-Fe2O3-magnetically immobilized cells at different concentrations of r-Fe2O3 nanoparticle. a r-Fe2O3-magnetically immobilized cells at an r-Fe2O3 nanoparticle concentration of 40 mg L−1, b r-Fe2O3-magnetically immobilized cells at an r-Fe2O3 nanoparticle concentration of 80 mg L−1, c r-Fe2O3-magnetically immobilized cells at an r-Fe2O3 nanoparticle concentration of 120 mg L−1, and d r-Fe2O3-magnetically immobilized cells at an r-Fe2O3 nanoparticle concentration of 240 mg L−1
Reuse of r-Fe2O3-Magnetically Immobilized Cells in the Bioremediation of Petrochemical Wastewater
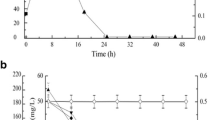
In an industrial bioremediation process, the recycling of the biocatalysts could be an important factor that determines the effectiveness of degradation over time. The activities of r-Fe2O3-magnetically immobilized cells and nonmagnetically immobilized cells coupling with activated zeolite in the bioremediation of petrochemical wastewater were tested repeatedly. As shown in Fig. S2a and S3, from the first to the fifth cycle, r-Fe2O3-magnetically immobilized cells exhibited high biodegradation activity, and all the compounds were completely consumed in 20 h; from the sixth to the eighth cycle, the same amount of all the compounds were completely consumed in only 16 h. In contrast, from the first to the third cycle, all the compounds were completely consumed in 36 h by nonmagnetically immobilized cells (Fig. S2b and Fig. S4); from the fourth to eighth cycle, the degradation rate of phenol and m/p-xylene was also 100 %, while the degradation rate of benzene, toluene, ethylbenzene, and o-xylene was decreased from 99 to 90 %, 98 to 84 %, 98 to 83 %, and 95 to 75 %, respectively. For the removal of NH3-N, the NH3-N removal rate was decreased from 100 to 30 % during initial three cycles, while the removal rates of NH3-N were decreased from 30 to 0 % from the fourth to eighth cycle might be due to the saturated adsorption ability to NH3-N by activated zeolite. Figure S1e is the SEM image of r-Fe2O3-magnetically immobilized cells after eight cycles of the biodegradation experiments. As shown in Fig. S1e, the sheets of gellan gum matrix were also loosely bound together. Moreover, the number of pores that existed between the sheets of gellan gum matrix evidently increased, which could further reduce or eliminate of mass transfer problems to improve the biodegradation activities of magnetically immobilized cells after eight cycles of the biodegradation experiments. Similar results were obtained in previous studies on the degradation of carbazole by Fe3O4-magnetically immobilized cells [20]. All these results indicated that r-Fe2O3-magnetically immobilized cells should be a promising biocatalyst used in biodegradation of petrochemical wastewater.
Quantitative Real-Time PCR Assays
In order to investigate the concentration of strain JB in nonmagnetically immobilized cells and magnetically immobilized cells, qPCR was done using the 16s V3 genes from strain JB as the standard. As shown in Fig. S5, the concentrations of strain JB in a-Fe2O3-magnetically immobilized cells (sample 2), Fe3O4-magnetically immobilized cells (sample 3), and r-Fe2O3-magnetically immobilized cells (sample 4) after first cycle of degradation experiment were almost the same as those in nonmagnetically immobilized cells (sample 1). It confirmed that high biodegradation activities for phenol and BTEX compounds obtained by magnetically immobilized cells might be supported by the existence of nanoparticles, the loose binding of the sheets of gellan gum matrix, and the existence of many pores between the sheets of gellan gum matrix (Fig S1). The concentration of strain JB in nonmagnetically immobilized cells (sample 5) and r-Fe2O3-magnetically immobilized cells (sample 6) after eighth cycle of degradation experiment was also investigated, and the concentration of strain JB was 438982 and 510852 copies ng L−1DNA, respectively. The concentration of strain JB in r-Fe2O3-magnetically immobilized cells after eighth cycle of degradation experiment was significantly increased, which indicated that high biodegradation activity for phenol and BTEX compounds might by be supported by the good growth of cells in the magnetic gellan gel bead. These results were also consistent with a previous report that the growth of cells in the magnetic gellan gel beads was considered to have enhanced the biodegradation activity for carbazole in the recycling experiments [20].
Toxicity Assessment (Microtox Test)
In this study, ecotoxicity estimation was conducted by using the Microtox test (bacterium V. fischeri) to determine the change in effluent toxicity during the bioremediation of petrochemical wastewater by r-Fe2O3-magnetically immobilized cells coupling with activated zeolite as previous described [15]. The influent contained high levels of phenol, benzene, toluene, ethylbenzene, o-xylene, and m/p-xylene and had high toxicity against strain V. fischeri as indicated by IR value that exceeded 96 % (Fig. 3). As shown in Fig. 3a, from the first to the second cycle, the IR value of effluent was to be 25 % (moderate toxicity) after treatment by r-Fe2O3-magnetically immobilized cells coupling with activated zeolite; from the third to the eighth cycle, the IR value of effluent was increased slightly (from 28 to 32 %). In contrast, from the first to the second cycle, the IR value of effluent was to be 35 % (moderate toxicity) after treatment by nonmagnetically immobilized cells coupling with activated zeolite and from the third to the eighth cycle, the IR value of effluent was increased significantly (from 40 to 57 %). These results further confirmed that r-Fe2O3-magnetically immobilized cells coupling with activated zeolite exhibited higher biodegradation activity on the petrochemical wastewater than that by nonmagnetically immobilized cells coupling with activated zeolite and could led to less toxicity than the untreated petrochemical wastewater.
Conclusions
In this study, the bioremediation of petrochemical wastewater by r-Fe2O3-magnetically immobilized cells coupling with activated zeolite was investigated. r-Fe2O3 nanoparticle was chosen as the most suitable magnetic nanoparticle, and the optimal concentration was 120 mg L−1. The recycling experiments demonstrated that the degradation activity of r-Fe2O3-magnetically immobilized cells was still high after eight cycles. According to these findings, magnetically immobilized cells of strain JB should be a promising biocatalyst used in biodegradation of petrochemical wastewater.
References
Prenafeta-Boldú, F. X., Vervoort, J., Grotenhuis, J. T. C., & van Groenestijn, J. W. (2002). Substrate interactions during the biodegradation of benzene, toluene, ethylbenzene, and xylene (BTEX) hydrocarbons by the fungus Cladophialophora sp. strain T1. Applied and Environmental Microbiology, 68, 756–762.
Saeed, T., & Al Mutairi, M. (1999). Chemical composition of the water- soluble fraction of the leaded gasolines in seawater. Environmental International, 25, 117–129.
Littlejohns, J. V., & Daugulis, A. J. (2008). Kinetics and interactions of BTEX compounds during degradation by a bacterial consortium. Process Biochemistry, 43, 1068–1076.
Costa, A. S., Romão, L. P., Araújo, B. R., Lucas, S. C., Maciel, S. T., Wisniewski, A., Jr., & Alexandre, M. R. (2012). Environmental strategies to remove volatile aromatic fractions (BTEX) from petroleum industry wastewater using biomass. Bioresource Technology, 105, 31–39.
Dean, B. J. (1985). Recent findings on the genetic toxicology of benzene, toluene, xylenes and phenols. Mutation Research, 154, 153–181.
Kim, J. M., Le, N. T., Chung, B. S., Park, J. H., Bae, J. W., Madsen, E. L., & Jeon, C. O. (2008). Influence of soil components on the biodegradation of benzene, toluene, ethylbenzene, and o-, m-, and p-xylenes by the newly isolated bacterium Pseudoxanthomonas spadix BD-a59. Applied and Environmental Microbiology, 74, 7313–7320.
Chakraborty, R., O’Connor, S. M., Chan, E., & Coates, J. D. (2005). Anaerobic degradation of benzene, toluene, ethylbenzene, and xylene compounds by Dechloromonas strain RCB. Applied and Environmental Microbiology, 71, 8649–8655.
Kim, D., Kim, Y. S., Kim, S. K., Kim, S. W., Zylstra, G. J., Kim, Y. M., & Kim, E. (2002). Monocyclic aromatic hydrocarbon degradation by Rhodococcus sp. strain DK17. Applied and Environmental Microbiology, 68, 3270–3278.
Shinoda, Y., Sakai, Y., Uenishi, H., Uchihashi, Y., Hiraishi, A., Yukawa, H., Yurimoto, H., & Kato, N. (2004). Aerobic and anaerobic toluene degradation by a newly isolated denitrifying bacterium, Thauera sp. strain DNT-1. Applied and Environmental Microbiology, 70, 1385–1392.
Zylstra, G. J., & Kim, E. (1997). Aromatic hydrocarbon degradation by Sphingomonas yanoikuyae B1. Journal of Industrial Microbiology and Biotechnology, 19, 408–414.
Jin, H. M., Choi, E. J., & Jeon, C. O. (2013). Isolation of a BTEX-degrading bacterium, Janibacter sp. SB2, from a sea-tidal flat and optimization of biodegradation conditions. Bioresource Technology, 145, 57–64.
Kim, J. M., & Jeon, C. O. (2009). Isolation and characterization of a new benzene, toluene, and ethylbenzene degrading bacterium, Acinetobacter sp. B113. Current Microbiology, 58, 70–75.
Assinder, S. J., & Williams, P. A. (1990). The TOL plasmids: determinants of the catabolism of toluene and the xylenes. Advances in Microbial Physiology, 31, 1–69.
Choi, E. J., Jin, H. M., Lee, S. H., Math, R. K., Madsen, E. L., & Jeon, C. O. (2013). Comparative genomic analysis and benzene, toluene, ethylbenzene, and o-, m-, and p-xylene (BTEX) degradation pathways of Pseudoxanthomonas spadix BD-a59. Applied and Environmental Microbiology, 79, 663–671.
Shi, S. N., Qu, Y. Y., Ma, F., & Zhou, J. T. (2014). Bioremediation of coking wastewater containing carbazole, dibenzofuran, dibenzothiphene and naphthalene by a naphthalene-cultivated Arthrobacter sp. W1. Bioresource Technology, 166, 79–86.
Shi, S. N., Qu, Y. Y., Ma, F., & Zhou, J. T. (2014). Bioremediation of coking wastewater containing carbazole, dibenzofuran and dibenzothiphene by immobilized naphthalene-cultivated Arthrobacter sp. W1 in magnetic gellan gum. Bioresource Technology, 164, 28–33.
Dwyer, D. F., Krumme, M. L., Boyd, S. A., & Tiedje, J. M. (1986). Kinetics of phenol biodegradation by an immobilized methanogenic consortium. Applied and Environmental Microbiology, 52, 345–351.
Lee, S. T., Rhee, S. K., & Lee, G. M. (1994). Biodegradation of pyridine by freely suspended and immobilized Pimelobacter sp. Applied Microbiology and Biotechnology, 41, 652–657.
Li, F. L., Xu, P., Feng, J. H., Meng, L., Zheng, Y., Luo, L. L., & Ma, C. Q. (2005). Microbial desulfurization of gasoline in a Mycobacterium goodii X7B immobilized-cell system. Applied and Environmental Microbiology, 71, 276–281.
Wang, X., Gai, Z., Yu, B., Feng, J., Xu, C., Yuan, Y., Lin, Z., & Xu, P. (2007). Degradation of carbazole by microbial cells immobilized in magnetic gellan gum gel beads. Applied and Environmental Microbiology, 73, 6421–6428.
Fernando Bautista, L., Morales, G., & Sanz, R. (2010). Immobilization strategies for laccase from Trametes versicolor on mesostructured silica materials and the application to the degradation of naphthalene. Bioresource Technology, 101, 8541–8548.
Qiu, H., Lu, L., Huang, X., Zhang, Z., & Qu, Y. (2010). Immobilization of horseradish peroxidase on nanoporous copper and its potential applications. Bioresource Technology, 101, 9415–9420.
Liu, Y., Zeng, Z., Zeng, G., Tang, L., Pang, Y., Li, Z., Liu, C., Lei, X., Wu, M., Ren, P., Liu, Z., Chen, M., & Xie, G. (2012). Immobilization of laccase on magnetic bimodal mesoporous carbon and the application in the removal of naphthaleneic compounds. Bioresource Technology, 115, 21–26.
Jiang, B., Zhou, Z.C., Dong, Y., Tao, W., Wang, B., Jiang, J.W., & Guan, X.Y., 2014. Biodegradation of benzene, toluene, ethylbenzene, and o-, m-, and p-xylenes by the newly isolated bacterium Comamonas sp. JB. Applied Biochemistry and Biotechnology.
Acknowledgments
This work was supported by grants from Science and Technology Project of Liaoning Province (2014203006), Ocean & Fisheries Project of Liaoning Province (201301), Public Science and Technology Research Funds Projects of Ocean (201205012–7), Science and Technology Project of Dalian City (2012J21DW029).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 7575 kb)
Rights and permissions
About this article
Cite this article
Jiang, B., Zhou, Z., Dong, Y. et al. Bioremediation of Petrochemical Wastewater Containing BTEX Compounds by a New Immobilized Bacterium Comamonas sp. JB in Magnetic Gellan Gum. Appl Biochem Biotechnol 176, 572–581 (2015). https://doi.org/10.1007/s12010-015-1596-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1596-0