Abstract
Caulobacter crescentus (NA1000 strain) are aquatic bacteria that can live in environments of low nutritional quality and present numerous genes that encode enzymes involved in plant cell wall deconstruction, including five genes for β-xylosidases (xynB1–xynB5) and three genes for xylanases (xynA1–xynA3). The overall activity of xylanases in the presence of different agro-industrial residues was evaluated, and it was found that the residues from the processing of corn were the most efficient in inducing bacterial xylanases. The xynA1 gene (CCNA_02894) encoding a predicted xylanase of group 10 of glyco-hydrolases (GH10) that was efficiently overexpressed in Escherichia coli LMG194 using 0.02 % arabinose, after cloning into the vector pJet1.2blunt and subcloning into the expression vector pBAD/gIII, provided a fusion protein that contained carboxy-terminal His-tags, named XynA1. The characterization of pure XynA1 showed an enzymatic activity of 18.26 U mL−1 and a specific activity of 2.22 U mg−1 in the presence of xylan from beechwood as a substrate. XynA1 activity was inhibited by EDTA and metal ions such as Cu2+ and Mg2+. By contrast, β-mercaptoethanol, dithiothreitol (DTT), and Ca2+ induced recombinant enzyme activity. Kinetic data for XynA1 revealed K M and V max values of 3.77 mg mL−1 and 10.20 μM min−1, respectively. Finally, the enzyme presented an optimum pH of 6 and an optimum temperature of 50 °C. In addition, 80 % of the activity of XynA1 was maintained at 50 °C for 4 h of incubation, suggesting a thermal stability for the biotechnological processes. This work is the first study concerning the cloning, overexpression, and enzymatic characterization of C. crescentus xylanase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The industrial revolution favored global economic growth and the largest consumption of raw materials and fossil fuels, leading to the depletion of these resources in nature and the negative impact of their use on the environment. Residues derived from plant cell walls can be considered the renewable energy source that has the greatest potential to contribute to the current and future energy needs. The use of lignocellulosic biomass to produce, for instance, second-generation ethanol requires optimizing the enzymes involved in the breakdown of plant cell walls [1].
Lignocellulosic biomass is made up of three main fractions of polymers: cellulose (45–55 %), hemicellulose (25–35 %), and lignin (20–30 %), which are joined together by covalent bonds. This structure forms a rigid complex structure that is difficult to degrade due to the molecular arrangement being resistant to microbial attack [2].
Hemicellulose is, after cellulose, the second most abundant renewable polysaccharide in nature and is present in abundance in the woods and residues from the agro-industry. Hemicellulose is primarily formed by xylan, a linear homopolysaccharide of β-d-xylanopyranose united by links of type β-1,4, and is located at the interface between cellulose and lignin in all layers of the plant cell wall. Structurally, hemicelluloses are formed by residues of sugars such as hexoses (d-mannose, l-rhamnose, l-arabinose, l-fucose, d-galactose, and l-galactose) and pentoses (d-xylose and l-arabinose) can also produce d-glucuronic acids such as 4-O-methyl-d-glucuronic acid and d-galacturonic acid, and the O-acetyl groups or esters of ferulic and p-coumaric acid are bonded via units of l-arabinose to the main chain [3].
The complete enzymatic hydrolysis of hemicellulose involves an enzyme complex composed of endo-xylanases (EC 3.2.1.8) that function in the main chain of xylan to release xylo-oligosaccharides (XOS) that act as targets for β-xylosidases (E.C. 3.2.1.37) and other auxiliary enzymes that function as side chains in α-glucuronidase (EC 3.2.1.139), α-l-arabinofuranosidase (EC 3.2.1.55), and acetyl xylan esterase (EC 3.1.1.72) [4].
The xylanases are glycoside hydrolases (GHs) with different molecular structures, hydrolytic activity, and physico-chemical properties. Microbial xylanases have applicability for various biotechnological industries because they can be frequently employed for the degradation of carbon sources such as low-cost lignocellulosic materials in agro-industrial waste, thus leading to the recovery of the energy capacity of compounds that were previously disposed of in nature. Xylanases can be used as animal feed supplements in the baking industry to improve the texture of bread; in the food and beverage industry, xylanases are used in large volumes of dough to increase the quality of the product and to produce the low-molecular-weight sugar xylitol; in the textile industry, xylanases are used in the bleaching of pulp cellulose. Another aspect of xylan-degrading enzymes that can bring benefits to the overall economy is processing lignocellulosic materials to generate biofuels and chemicals [5].
In fact, the present and future strategies for the optimization of biotechnological processes are to explore each challenge more effectively and efficiently using the energy capacity of the hemicelluloses present in agro-industrial waste and optimize them by using a class of molecular tools called recombinant DNA technology. Thus, the study of both specific xylanase genes and microorganisms that produce still-unknown xylanases remains an interesting alternative to economically identify enzymes that have industrially applicable characteristics and to develop cleaner industrial processes.
Caulobacter crescentus is a non-pathogenic Gram-negative bacterium that is found mainly in aquatic environments and in many types of soils. It is currently considered the best model for the analysis of the cell cycle and cell differentiation of bacteria due to its asymmetric life cycle [6]. The analysis of the genome of C. crescentus, the first free-living proteobacteria alpha class to be sequenced [6], showed the presence of eight genes that are directly involved in the degradation of xylan: five of these genes encode enzymes with beta-xylosidase activity, and three have endo-xylanase activity. Two genes (xynB1 and xynB2) encoding β-xylosidases have been previously studied [7–9].
In the present study, the biochemical characterization of the first recombinant xylanase from C. crescentus was performed by the cloning and expression of the xynA1 gene (CCNA_ 02894) in Escherichia coli that encodes the predicted endo-1,4-beta-xylanase (EC 3.2.1.8), which belongs to the GH10 family. The pure recombinant xylanase and its various biochemical parameters were characterized. Although C. crescentus presents three candidate genes for xylanase GH10, two of which are more likely candidates than the third, no xylanase of C. crescentus has been studied. Therefore, the current study presents the first record of the study of one of them.
Materials and Methods
Bacterial Strains and Culture Conditions
The C. crescentus NA1000 strain was grown at 30 °C in PYE medium (peptone and yeast extract) to extract genomic DNA. The overall xylanase activity of C. crescentus was assessed after the growth of bacteria on minimal medium supplemented with 0.2 % (w/v) glucose and 2 % (w/v) of different agro-industrial residues. For cloning, subcloning, and induction, DH5α, TOP10, and LMG194 E. coli strains were used, respectively. These strains were grown in Luria-Bertani (LB) media containing ampicillin (100 μg mL−1) at 37 °C.
Production of Xylanase by C. crescentus in Different Agro-industrial Wastes
For the analysis of global xylanase activity by C. crescentus, the bacteria cells were grown in the presence of different agro-industrial residues: corn straw, hemicellulose from corn straw, rice straw, soybean straw, passion fruit fiber, crushed barley, coconut fiber, sugarcane bagasse, and corn cobs; all of these were obtained from the agro-industries of the Western Paraná region (Brazil), a large agricultural region. All of the residues were prepared as described in Corrêa et al. [9]. Corn straw hemicelluloses were prepared by pre-treatment using auto-hydrolysis, in which 1 g of corn straw was added to a tube with 10 mL of water and subjected to heating at 200 °C for 1 h in the digester tube. After this time, the tube was immediately transferred to an ice bath and cooled. The liquid phase was filtered through a filter paper, and the hemicellulose present in the solution was precipitated by the addition of 3 volumes of absolute ethanol (20 °C, 24 h). After this period, the liquid fraction was carefully removed by inversion, and the precipitate was then oven-dried at 50 °C for 24 h.
Xylanase production was carried out in a medium containing 2 % (w/v) of different residues as a carbon source in 5 mL of minimal medium M2 containing 0.2 % glucose (w/v). Agricultural residues were sterilized at 121 °C for 15 min and cooled, and then the minimum medium was sterilized by vacuum filtration. The pre-inoculum of C. crescentus was performed in cell growth medium containing PYE for 12 h at 30 °C and 120 rpm. The medium containing agricultural waste and minimal medium was used to dilute the inoculum of C. crescentus cells in the stationary phase for an optical density (OD) value at λ = 600 nm equal to 0.150. The cultures were incubated at 30 °C with agitation (120 rpm) for 24 h. After this period, aliquots of 1 mL of growth solution were centrifuged at 15,000×g for 5 min at room temperature. After centrifugation, the supernatant was reserved for the analysis of extracellular xylanase, and the cell pellets were dried and frozen for the determination of intracellular xylanase. The frozen cell pellet was lysed with 350 μL of 50 mM phosphate buffer pH 6.0 under vigorous vortexing until it was thawed. The sample was kept on ice, and the overall activity dosage levels of the xylanolytic bacteria were determined.
Cloning of the C. crescentus xynA1 Gene
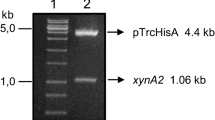
The primers xynA1-EcoRI-Forw (5′aagaattctgcgcgtttttcttggagcg3′) and xynA1-HindIII-Rev (5′tataagcttcccgcgccggcgcggccttcag3′) were synthesized according to the DNA sequence of the gene xynA provided by sequencing of the genome of C. crescentus (accession number: CCNA_02894) [10]. As this was the first of three genes encoding xylanases in C. crescentus that was studied by our laboratory, we named it xynA1. Gene amplification was performed using 50 pmol of each primer in a 50-μL PCR volume containing 0.2 μg of genomic DNA from C. crescentus, 5 μL of DMSO, and 1 U of Platinum Taq DNA Polymerase (Invitrogen®). Amplification was performed with 35 cycles of 94 °C for 30 s, 55 °C for 30 s, and 68 °C for 2 min. The PCR product was resolved by 1 % agarose (Sigma®) 1× TAE gel electrophoresis. The fragment of 1158 bp corresponding to the xynA1 gene was removed from the gel and treated with polynucleotide kinase (Fermentas®) to produce blunt ends and was then ligated to the cloning vector pJET1.2/blunt (Fermentas®). After confirmation of the recombinant cloning and sequencing, pJET1.2/blunt-xynA1 was digested with EcoRI/HindIII, and the fragment generated was cloned in frame into the vector pBAD/gIIIA EcoRI/HindIII (Invitrogen) to generate a fusion protein with a histidine tag at the carboxy-terminal region protein, named XynA1. The recombinant plasmids pJET1.2/blunt-xynA1 and pBAD/gIIIA-xynA1 were sequenced by the sequencing service of the Chemistry Institute of São Paulo University, São Paulo, SP, Brazil.
Overexpression and Purification of the C. crescentus XynA1 Recombinant
For induction of the expression of the recombinant xylanase of C. crescentus, E. coli LMG194 cells containing the plasmid pBAD/gIIIA-xynA1 were grown in LB medium containing ampicillin (100 μg mL−1) at 37 °C and 180 rpm for 12 h. When the E. coli cells reached the stationary phase, the culture was diluted 100 times and again subjected to the same growth conditions until the mid-log phase. After this period, the culture was induced with 0.02 % (v/v) L(+)-arabinose (Sigma®) for 4 h. Cells were lysed using Fast Break Cell Lysis solution (Promega®) containing 20 mg mL−1 lysozyme (Sigma®) and protease inhibitors (10 μL mL−1) (Sigma®). The mixture containing the induced E. coli culture was incubated at 25 °C at 90 rpm for 30 min. Next, the lysate was centrifuged at 15,000 × g for 10 min at 4 °C. The supernatant was transferred to pre-packed His-SpinTrap nickel-Sepharose columns (GE Healthcare®) to purify the recombinant fusion protein by affinity chromatography. The proteins adhered to the column were eluted with 20 mM of phosphate buffer (pH 7.4) containing 500 mM imidazole. The purified samples were dialyzed against deionized water and analyzed by 9 % SDS-PAGE.
Determination of Xylanase Activity and Total protein
The reactions to verify xylanase activity were performed using xylan from beechwood (Sigma®) in sodium phosphate buffer (pH 6.0) as a substrate, followed by incubation at 50 °C, and the reducing sugars were measured using the 3,5-dinitrosalicylic acid (DNS) method [10]. Xylanase activity was defined as the amount in millimoles of xylose per milliliter of enzyme produced in 1 min under the assay conditions (U mL−1). The protein concentrations were measured using the Bradford reagent from Bio-Rad with BSA as the standard.
The Effect of pH and Temperature on XynA1 Activity and Stability
The optimum pH was checked using buffer McIlvaine (pH 3–10) at 50 °C. To determine the pH stability, the purified xylanase was incubated at different pH values (3–10) at 4 °C for 24 h, and the residual activity of the protein was verified according to a standard protocol. To determine the optimum temperature, the enzyme at the optimum pH was subjected to standard tests by changing the temperature range from 30 to 80 °C. The thermostability was assessed by incubating the enzyme at the optimum pH found among the top three temperatures for 0–240 min, followed by a standard enzyme activity analysis. The optimum pH and temperature and the thermostability were expressed as the activity relative to the highest activity found (100 %).
The Effect of Different Compounds on XynA1 Activity
The effect of different compounds on C. crescentus XynA1 activity was verified by diluting the enzyme into 2 mM of the different compounds, followed by incubation for 15 min on ice and subsequent enzyme activity dosage calculation at the optimum temperature. The results were expressed as the percentage of residual activity compared with the dosage control sample in the absence of the compounds.
Determination of the Kinetic Parameters of C. crescentus XynA1
To verify the effect of the concentration of xylan from beechwood in the reaction, the substrate concentration was varied (4–14 mg mL−1) in the assays optimized for xylanase measurement using the method described by Miller [10]. Kinetic parameters, such as the Michaelis-Menten constant (K M) and the maximum reaction rate (V max), were measured for data plotting and verification of the double-reciprocal linear equation.
Statistical Analysis
The experiments were performed in triplicate, with repetitions for inconsistent data. The graphical Origin® 8.5 statistical program (Graph and Technical Data Analysis) was used for the statistical analysis of the data by analyzing the dispersion medium and for plotting the graphs.
Comparative Analysis of C. crescentus Xylanase with Xylanases from Other Bacteria
The sequence analysis of the predicted xylanase obtained from the C. crescentus xynA1 gene deposited in GenBank was performed using the algorithm NCBI BLAST (Basic Local Alignment Search Tool) and program Blastx. The isoelectric point and molecular size of the protein were determined according Kozlowski [11]. The sequence of the XynA1 and other bacterial xylanases belong to the GH10 group according to the classification of CAZY (Carbohydrate-Active Enzymes Database—http://www.CAZY.org). We constructed a phylogenetic tree using the neighbor-joining method and bootstrap using the MEGA program 6:06. The protein sequence of the bacterial xylanase GH11 group was used as the outgroup for analyses of kinship.
Results and Discussion
Effect of Various Agro-wastes on the Production of Xylanase
The C. crescentus cells were grown in minimal medium containing 0.2 % (w/v) glucose supplemented with various agro-industrial residues as a carbon source. The C. crescentus cells exhibited a clear preference for corn derivatives to induce global C. crescentus xylanase activity (Fig. 1). The intracellular production of xylanase was higher in the presence of the corn straw hemicelluloses (1.4 U mL−1) followed by corn cobs (1.39 U mL−1). The other agro-industrial residues showed lower xylanase production; for example, the fiber of the passion fruit showed the worst performance as an inducer of intracellular xylanase activity (1 U mL−1). The best extracellular xylanase induction occurred in the presence of corn straw hemicelluloses (5.60 U mL−1), followed by maize straw grass (3.53 U mL−1) and the fiber of the passion fruit (1.87 U mL−1).
Effect of various agro-industrial wastes in the production of xylanases by C. crescentus. CS corn straw, CSH corn straw hemicellulose, RS rice straw, SS soy straw, PFF passion fruit fiber, BB barley bagasse, CF coconut fiber, SCB sugarcane bagasse, CC corn cob. The fermentation medium consisted of 2 % of agro-industrial residues (w/v) in M2 minimal medium containing 0.2 % glucose (w/v). The inoculum was performed by diluting cells in the stationary phase for an OD 600 nm of 0.15 in the same culture media containing different carbon sources. Bacterial growth occurred at 30 °C for 24 h with shaking at 120 rpm
The use of alternative sources for the production of xylanase, such as agro-industrial residues, has been the subject of various studies because the substrates are low cost, easily accessible, and profitable. Pure xylan, due to its high cost, is not accessible for xylanase production on an industrial scale [4]. Examples of this mode of application of residues for xylanase production by bacteria are Bacillus altitudinis DHN8 that produced xylanase induced by sorghum straw, wheat straw, rice straw, and corn straw [12]. The Geobacillus stearothermophilus KIBGE-IB29 strain showed xylanase induction with orange peel, barley, sago, and wheat bran [13]. Xylanase from Streptomyces sp. CS428 was induced by corncob and wheat bran [14]. Poorna and Prema [15] showed that the production of xylanase by Bacillus pumilus can be induced with a medium containing rice straw and soybean flakes. Because of the induction of xylanases by waste pretreated with Bacillus circulans D1, xylanase production was obtained from sugarcane bagasse (8.4 U mL−1) and grass hydrolysates (7.5 U mL−1) [16]. A similar result was found for xylanase production when C. crescentus was grown in the presence of hydrolyzed corn straw as a carbon source.
The higher production of xylanase in this study was obtained with the growth of the bacteria in the presence of hemicellulose obtained form corn straw. One hypothesis to explain this finding is that derived from corn crop residues are richer in hemicellulose and thus can effectively activate more specifically the production of xylan-degrading enzymes. In favor of this hypothesis has been the work of Corrêa et al. [8], which used corncob as an excellent inducer of C. crescentus β-xylosidase activity. According Adhyaru et al. [12], the induction of xylanases can be considered a complex phenomenon, and the level of response to different inducers varies according to the strain. The present study indicated that the production of the intracellular xylanase of C. crescentus was better in the presence of corncob; the best extracellular xylanase production was induced with corn straw. Corn straw hemicelluloses were effective for the production of intra- and extracellular C. crescentus xylanase, but their feasibility is limited to be further analyzed on an industrial level.
Cloning of the Gene xynA1 and Purification of the Recombinant Enzyme
The fragment of 1158 bp containing the xynA1 gene encoding an endo-1,4-beta-xylanase of C. crescentus was amplified by PCR using genomic DNA as the template and the primers xynA1-EcoRI-Forw and xynA1-HindIII-rev. The PCR product generated was ligated into the cloning vector pJET1.2/blunt and subsequently subcloned into the reading frame of a carboxy-terminal His-tag of the expression vector pBAD/gIIIA digested with EcoRI/HindIII restriction enzymes. The recombinant pBAD/gIIIA-xynA1 was sequenced, and the predicted protein of 385 amino acids of 43 kDa in size had 100 % identity to that encoded by the C. crescentus xynA1 gene (CCNA_02894). The pBAD/gIIIA-xynA1 was incubated with 0.02 % arabinose (v/v) for xylanase recombinant expression; subsequently, the enzyme was purified, and its expression was verified by polyacrylamide gel electrophoresis.
The E. coli cells overexpressing the xynA1 gene were lysed, and the recombinant protein was purified from the cells using pre-packaged nickel-Sepharose columns. A single band of approximately 50 kDa by SDS-PAGE (Fig. 2) was observed that was in agreement with the predicted molecular weight considering additional amino acids from the carboxy-His-tag. Assays of enzymatic activity of purified C. crescentus xylanase showed an activity of 18.26 U mL−1 and a specific activity of 2.22 U mg−1 using xylan from beechwood as a substrate of the reaction. To explore the bacterial enzymes for industrial applications, overexpression of these enzymes is desirable. There are several reports describing E. coli as a heterologous host. In C. crescentus, for example, interesting data have been obtained for β-xylosidase I [7] and β-xylosidase II enzymes [8] that were overexpressed in E. coli and fully characterized. In the present study, the xynA1 gene cloned and expressed in E. coli resulted in an activity of 18.26 U mL−1, representing an increase of approximately 13- and 3-fold in xylanase production by the intracellular (1.41 U mL−1 ) and extracellular (5.6 U mL−1) NA1000 parental strain of C. crescentus grown in minimal medium containing 2 % (w/v) corn straw hemicellulose. After purification, the enzyme was characterized biochemically such that, using the knowledge of its properties, further studies of its biotechnological applications can be performed.
SDS-PAGE profile of the cell lysate of recombinant E. coli LMG194, indicating the overexpression of the heterologous gene xynA1 and purification of the recombinant C. crescentus xylanase (lanes): 1 marker broad range protein molecular weight markers (Promega®); 2 total proteins extracted from strain LMG194; 3 total proteins of the strain LMG194 containing the construct pBAD/gIII A-xynA1 after 4 h of induction with 0.02 % arabinose (w/v) at 37 °C; 4 aliquot from the pellet after cell lysis; 5–6 buffer obtained after washing the columns; 7–8 eluate of the nickel-Sepharose columns with buffer containing 500 mM imidazole showing the purified xylanase of approximately 50 kDa
Biochemical Characterization of Xylanase C. crescentus
The optimal pH for XynA1 activity was determined as pH 6 followed by pH 7 (Fig. 3). However, concerning pH stability, it was observed that, at pH 6, the enzyme lost 50 % of its activity in 24 h which was not observed with pH 7, where 60 % of the activity remained, indicating that the latter was the best condition to maintain the stability of enzyme activity. Other pH ranges tested showed lower activities. Additionally, it is worth mentioning that the isoelectric point based on protein structure was 9, and then the baseline activity was in this range because the protein precipitated on this pI. Other bacterial xylanases have also shown pH 6 to be as efficient: Bacillus spp. SPS-strain 0 [13], Streptomyces sp. CS624 [14], and the extremophile bacterium Caldicellulosiruptor kronotskyensis [15]. However, it is possible to check a wide pH range for bacterial xylanases because of the slightly acidic xylanase from the Bacillus sp. strain BP-23 (pH 5.5) [16] to the alkaline Bacillus sp. 41 M-1 strain (pH 9.0) [17]. C. crescentus xylanase was shown to be most stable at neutral pH 7, suggesting a specificity of these conditions to pH, unlike most other xylanases that exhibit a stability range such as that of xylanase C. kronotskyensis at pH 5.5–7.5 [15]. The determination of the optimum pH and pH stability of enzymes is important for enzyme biotechnology because they indicate the conditions under which the enzyme remains active in high yield. Moreover, these data contribute to the formulation of enzyme cocktails in which the synergism of the enzymes and yield depend on the characteristics of each. Considering these factors that XynA1 from C. crescentus presented as ideal activity––i.e., characteristic of neutral to slightly acidic conditions and this enzyme presented an optimum temperature at 50 °C (Fig. 4a). The xylanase activity decreased to approximately 30 and 40 % at 55 and 60 °C, respectively. When tested at extreme temperatures, 30 and 80 °C, the enzyme showed less than 30 % activity. Bacteria with xylanase activity at the same optimum temperature were observed for Cellulosimicrobium sp. MTCC 10645 [18] and Jonesia denitrificans [19]. Xylanases were found at extreme temperatures in the thermophilic Bacillus spp. strain SPS-0 [13] (75 °C), in the extremophile Thermotoga thermarum (95 °C) [20], and in the cold-active Flavobacterium johnsoniae (30 °C) [21].
The effect of pH on the enzymatic activity of recombinant xylanase (black circles) and stability at various pH values (white circles). The xylanase activity was verified by incubating the purified enzyme with xylan from beechwood in buffers at different pH (3–10) for 10 min at 50 °C. In parallel, aliquots of the purified enzyme were incubated at 4 °C for 24 h under the same varying conditions of pH; thereafter, the xylanase activity was assessed to verify its stability. The enzymatic activity was expressed as a percentage (relative activity) of the maximum value at pH 6. The stability at different pH was expressed as the residual activity of the enzyme activity value obtained at zero time incubation
a Effect of temperature and b thermal stability of xylanase activity from C. crescentus. The optimum temperature was verified by the standard measurement of enzyme activity at pH 6 and different temperatures (30–80 °C). Assays to verify the thermostability were performed with incubation of the XynA1 (b) at pH 6 and at 45 °C (black circles), 50 °C (black squares), and 55 °C (black triangles) for 0 to 240 min. Next, the samples were used for the determination of the activity pattern. The optimum temperature was presented as the activity relative to the highest value, and the thermal stability and residual activity were compared with the activity at the zero time of incubation
Thermostability assays (Fig. 4b) demonstrated that the hydrolytic activity of the enzyme was maintained for a longer period at 50 °C, losing only 20 % of its activity at 4 h of incubation. The other temperatures tested for thermostability, 45 and 55 °C, resulted in a decrease by 50 % of the activity in 4 and 1 h, respectively. Similarly, xylanases from Bacillus subtilis [22] and J. denitrificans [19] were also stable at 50 °C, maintaining 85 % (3 h) and 70 % (4 h) of their activities, respectively.
The characteristics of pH, pH stability, temperature, and thermostability provide important factors for the industrial application of xylanases. Similar to C. crescentus XylA1, the xylanase from the Bacillus sp. strain BP-23 showed similar characteristics of optimum pH and temperature (pH 5.5 and 50 °C). Additionally, chemical bleaching experiments using pulp generated a savings of 38 % compared with the consumption of chlorine dioxide [16], making it a possible biotechnological application to be tested for C. crescentus XylA1.
The Effect of Compounds on the Activity of Xylanase
Purified xylanase was subjected to activity enzyme assays in the presence of different compounds at a concentration of 2 mM, for instance, metal chelators such as ethylenediamine tetraacetic acid (EDTA), various metals, and reducing agents, including β-mercaptoethanol and DTT (Table 1). Xylanase activity was strongly inhibited by the metals Cu2+ (61 %) and Mg2+ (64 %) and the chelating agent EDTA (60 %). The inhibition by copper metal has been found for the xylanase of Paenibacillus campinasensis BL11 (80 %) [23] and Bacillus mojavensis FK-UEB [24] (100 %). Xylanase of F. johnsoniae showed a 36 % inhibition by magnesium [21], while Streptomyces sp. CS624 xylanase showed a 60 % inhibition by magnesium [14]. One explanation for the metal inhibition may be due to the interaction between the metal ions and amino acids of the catalytic domain of the enzyme [14]. Inhibition of xylanase activity in the presence of EDTA indicates that some metal is required for the enzyme reaction, and the enzyme most likely has cysteine residues included in its catalytic area [14, 25]. This inhibitory effect has also been observed for xylanase from Streptomyces sp. CS624 (65 %) [14] and P. campinasensis BL11 (50 %) [23]. The Zn 2+ (18 %) and Fe 2+ (24 %) ions moderately affected the activity in these experiments. The reducing agents β-mercaptoethanol (10 %) and DTT (30 %), as well as the ion Ca2+ (11 %), induced the activity of the enzyme. According to Mander et al. [14], β-mercaptoethanol has been known as an enhancer of xylanase activity by some bacteria, and this induction has been reported for xylanase from P. campinasensis BL11 (58 %) [23] and Bacillus subtilis (123 %) [22]. This finding is justified by the power of this reducing agent to neutralize the oxidative effects of disulfide bonds, most likely from cysteine residues, providing enzyme stabilization or stimulation [23]
.
Chen et al. [21] also showed a stimulation of xylanase activity in the presence of DTT in F. johnsoniae (36 %) and commented that the thiol grouping reduced the stability/activity of the enzyme. Ca2+ ion can influence the enzymatic activity required by the enzyme to cause changes in its conformation or to bind to active sites [25]. The stimulation of calcium has also been reported for xylanase from Streptomyces sp. CS624 (27 %) [14]. Other metals tested did not affect the activity of C. crescentus XylA1.
Kinetic Parameters
For an enzyme to be used industrially, its kinetic parameters should be appropriate and, with it, its functional role should be determined. The Michaelis-Menten constant was determined using various concentrations of beechwood xylan (4–14 mg mL−1). The values of K M and V max were 3.77 mg mL−1 and 10.20 μM min−1. These data indicate that the enzyme has a low affinity and reduced reaction speed to beechwood xylan. Xylanases with lower affinity to the substrate have also been reported such as those from P. campinasensis BL11 (K M 6.78 mg mL−1) [23], Streptomyces sp. CS624 (K M 9.79) [14], and Bacillus circulans BL53 (K M 9.9 mg mL−1 and V max 25.25 min−1 mM) [26]. Similar to C. crescentus XynA1, kinetic data were found for the xylanase from F. johnsoniae with a K M equal to 5 and a V max of 13.23 μmol mg−1 min−1 [21] indicating that the affinity for this substrate may be poor for some bacterial xylanases. Another common feature between these xylanases is that both bacteria are found in aquatic environments and live in oligotrophic freshwater habitats. The values reported are also consistent with those reported by Beg et al. [4] for microbial xylanases.
Analysis of the XynA1 Sequence
The predicted sequence for the C. crescentus xylanase from the xynA1 gene annotated as a probable GH10 in GenBank [6] was compared with other xylanases that belong to the same group of glyco-hydrolases according CAZY (http://www.cazy.org/Glycoside-Hydrolases.htmL). A phylogenetic tree was assembled using the neighbor-joining method and bootstrap in the MEGA 6.06 program. The GH11 xylanase was also used for comparison and was defined as an outgroup.
Figure 5 shows the phylogenetic tree for the xylanases of the bacterial species studied by multiple different approaches according to the literature. Bacteria that showed greater similarity between the proteins analyzed were those of the genus Caulobacter. The high similarity among the strains C. crescentus NA1000 and C. crescentus CB15 was expected because the NA1000 strain is a mutant derivative of CB15 [6]. The sample with the highest similarity was AGU12220, which showed an evolutionary proximity with C. crescentus and was observed even at 90 % similarity to a precursor of an endo-1,4-beta-xylanase (GH10) of Brevundimonas abyssalis that was isolated from the sediments of the deep sea floor in Japan. Brevundimonas abyssalis belongs to the Caulobacteraceae family of the class Alpha-proteobacteria and is ranked among the Brevundimonas and Caulobacter gender [27]. Among the randomly selected GH10 xylanases in CAZY, those from the bacteria belonging to Sphingomonas sanxanigenens DSM 19645 showed the highest similarity (63 %) of the product of the C. crescentus xynA1 gene, followed by endo-1,4-beta-xylanase (GH10) from Agrobacterium sp. H13-3. Additionally, C. crescentus, S. sanxanigenens, and Agrobacterium are both Alpha-proteobacteria, justifying the similarity of their xylanase sequences.
Phylogenetic analysis was performed using the MEGA6.06 program. The tree was assembled by the neighbor-joining method with the complete sequence of GH10 xylanases from C. crescentus (highlight) and those from other bacteria obtained from CAZY. A GH11 xylanase was also used as an outgroup to confirm the relationship. The percentage of identical trees, wherein the grouped rate was associated with the bootstrap test (500 replicates), are shown next to the branches. The tree is drawn to scale, with the lengths of the branches in the same units as those of evolutionary distances used to infer a phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and are in units of the number of amino acid substitutions per site. The analysis included 16 amino acid sequences. All of the positions containing gaps and missing data were eliminated. A total of 184 positions comprised the final data set
A lower degree of similarity with the xylanase from C. crescentus bacteria was found compared with GH10 endo-xylanase from Gram-positive bacteria such as Bacillus halodurans and Clostridium acetobutylicum ATCC 824. As expected, a low similarity was observed between GH10 xylanases and those that form the outgroup belonging to group 11 of glyco-hydrolases. The absence of similarity between proteins from different families (GHs) is due to diversification-related amino acid sequences, in which families can be defined based on the similarities between the amino acid sequences in their catalytic domains, whereas the members of the same family may share common structural folds and active site topology, as well as similar catalytic mechanisms [28]. The organization of enzymes by their similarities portrays how evolutionary events led to a broad structural diversity of the various groups, reflecting their specificities for different substrates or different catalytic patterns to distant groups. Unlike enzymes that have a similar arrangement in groups, others may show the conservation of amino acid residues in the catalytic domains and similar patterns of catalysis that may, in many cases, be even identical, suggesting that these enzymes differed little during the evolutionary process and arose from a common ancestor [28].
Conclusions
Although many xylanases have been characterized in fungi, only a few reports have demonstrated data concerning bacterial xylanases. Thus, the present study is the first study concerning the cloning, overexpression, and enzymatic characterization of C. crescentus xylanase. In addition, bacterial xylanases were induced in response to different agro-industrial residues. Functional analysis of the C. crescentus xynA1 gene by XynA1 overexpression in E. coli showed xylanase activity 13 times greater than the intracellular production of xylanase by the parental strain grown on minimal medium containing 2 % (w/v) of hemicellulose obtained from corn straw. Biochemical characterization demonstrated that recombinant XynA-1 showed a pH of 6.0 and an optimum temperature of 50 °C, respectively. In addition, the thermal stability was maintained for 4 h at 50 °C with only 20 % loss of activity. Analysis of the predicted protein encoded by the gene xynA1 showed higher similarity with other GH10 families of the Caulobacteraceae class of Alpha-proteobacteria.
References
Liu, T., Mcconkey, B., Huffman, T., Smith, S., Macgregor, B., Yemshanov, D., & Kulshreshtha, S. (2014). Potential and impacts of renewable energy production from agricultural biomass in Canada. Applied Energy, 130, 222–229.
Peng, P., & She, D. (2014). Isolation, structural characterization, and potential applications of hemicelluloses from bamboo: A review. Carbohydrate Polymers, 112, 701–720.
Ojeda, K., El-Halwagi, M., Kafarov (2013) V. Chapter 12: Design of a lignocellulosic feedstock biorefinery based on a biochemical processing platform using process integration methodologies and energy analysis. In: Integrated biorefineries: design, analysis, and optimization, Stuart, P. R.; El-Halwagi, M. M. CRC Press, p.370-391.
Beg, Q. K., Kapoor, M., Mahajan, L., & Hoondal, G. S. (2001). Microbial xylanases and their industrial applications: A review. Applied Microbiology and Biotechnology, 56, 326–338.
Zhong, C., Lau, M. W., Balan, V., Dale, B. E., & Yuan, Y. J. (2009). Optimization of enzymatic hydrolysis and ethanol fermentation from AFEX-treated rice straw. Applied Biochemistry and Biotechnology, 84, 667–676.
Marks, M. E., Castro-Rojas, C. M., Teiling, C. D. U. L., Kapatral, V., Walunas, T. L., & Crosson, S. (2010). The genetics basis of laboratory adaptation in Caulobacter crescentus. The Journal of Bacteriology, 192, 3678–3688.
Graciano, L., Corrêa, J. M., Gandra, R. F., Seixas, F. A. V., Kadowaki, M. K., Sampaio, S. C., Silva, J. L., Osaku, C. A., & Simão, R. C. G. (2012). The cloning, expression, purification, characterization and modeled structure of Caulobacter crescentus ß-xylosidase I. World Journal of Microbiology and Biotechnology, 28, 2879–2888.
Corrêa, J. M., Graciano, L., Abrahão, J., Loth, E. A., Gandra, R. F., Kadowaki, M. K., Henn, C., & Simao, R. C. G. (2012). Expression and characterization of a GH39 β-xylosidase II from Caulobacter crescentu. Applied Biochemistry and Biotechnology, 168, 2218–2229.
Corrêa, J. M., Mingori, M. R., Gandra, R. F., Loth, E. A., Seixas, F. A., & Simão, R. C. G. (2014). Depletion of the xynB2 gene upregulates β-xylosidase expression in C. crescentus. Applied Biochemistry and Biotechnology, 172, 1085–1097.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugars. Analytical Chemistry, 31, 426–428.
Kozlowski, L. P. (2013) Isoelectric point according different scales—isoelectric point calculator, available in: http://isoelectric.ovh.org
Adhyaru, D. N., Bhatt, N. S., & Modi, H. A. (2014). Enhanced production of cellulase-free, thermo-alkali-solvent-stable xylanase from Bacillus altitudinis Dhn8, its characterization and application in sorghum straw saccharification. Biocatalysis and Agricultural Biotechnology, 3, 182–190.
Bataillon, M., Cardinali, A.-P. N., Castillon, N., & Duchiron, F. (2000). Purification and characterization of a moderately thermostable xylanase from Bacillus sp. strain Sps-0. Enzyme and Microbial Technology, 26, 187–192.
Mander, P., Choi, Y. E., Pradeep, G. C., Choi, Y. S., Hong, J. H., Cho, S. S., & Yoo, J. C. (2014). Biochemical characterization of xylanase produced from Streptomyces sp. Cs624 using an agro residue substrate. Process Biochemistry, 49, 451–456.
Qiao, W., Tang, S., Mi, S., Jia, X., Peng, X., & Han, Y. (2014). Biochemical characterization of a novel thermostable gh11 xylanase with cbm6 domain from Caldicelluloriruptor kronotskyensis. Journal of Molecular Catalysis B: Enzymatic, 107, 8–16.
Blanco, A., Vidal, T., Colom, J. F., & Pastor, F. I. (1995). Purification and properties of xylanase a from alkali-tolerant Bacillus sp. strain Bp-23. Applied and Environmental Microbiology, 61, 4468–4470.
Nakamura, S., Wakabayashi, K., Nakai, R., Aono, R., & Horikoshi, K. (1993). Production of alkaline xylanase by a newly isolated alkaliphilic Bacillus sp. strain 41m-1. World Journal of Microbiology and Biotechnology, 9, 221–224.
Kamble, R. D., & And Jadhav, A. R. (2012). Production, purification and characterisation of alkali stable xylanase from Cellulosimicrobium sp. mtcc 10645. Asian Pacific Journal of Tropical Biomedicine, 2, 1790–1797.
Nawel, B., Said, B., Estelle, C., Hakim, H., & Duchiron, F. (2011). Production and partial characterization of xylanase produced by Jonesia denitrificans isolated in Algerian soil. Process Biochemistry, 46, 519–525.
Shi, H., Zhang, Y., Li, X., Huang, Y. J., Wang, L., Wang, Y., Ding, H., & Wang, F. (2013). A novel highly thermostable xylanase stimulated by Ca2+ from Thermotoga thermarum: Cloning, expression and characterization. Biotechnology for Biofuels, 6, 1–9.
Chen, S., Kaufman, M. G., Miazgowicz, K. L., Bagdasarian, M., & Walker, E. D. (2013). Molecular characterization of a cold-active recombinant xylanase from Flavobacterium johnsoniae and its applicability in xylan hydrolysis. Bioresource Technology, 128, 145–155.
Sá-Pereira, P., Mesquita, A., Duarte, J. C., Barros, M. R. A., & Costa-Ferreira, M. (2002). Rapid production of thermostable cellulase-free xylanase by a strain of Bacillus subtilis and its properties. Enzyme and Microbial Technology, 30, 924–933.
Ko, C. H., Tsai, C. H., Tu, J., Lee, H. I., Ku, L. T., Kuo, P. A., & Lai, Y. K. (2010). Molecular cloning and characterization of a novel thermostable xylanase from Paenibacillus campinasensis Bl11. Process Biochemistry, 45, 1638–1644.
Kallel, F., Driss, D., Bouaziz, F., Neifer, M., Ghorbel, R., & Chaabouni, S. E. (2014). Production of xylooligosaccharides from garlic straw xylan by purified xylanase from Bacillus mojavensis Ueb-Fk and their in vitro evaluation as prebiotics. Food and Bioproducts Processing. doi:10.1016/j.fbp.2014.07.012.
Murugan, S., Arnold, D., Pongiya, U. D., & Narayanan, P. M. (2011). Production of xilanase from Arthobacter sp. MTCC6915 using saw dust as substrate under solid state fermentation. Enzyme Research, 1, 1–7.
Heck, J. X., Soares, L. H. B., Hertz, P. F., & Ayub, M. A. Z. (2006). Purification and properties of a xylanase produced by Bacillus circulans Bl53 on solid-state cultivation. Biochemical Engineering Journal, 32, 179–184.
Tsubouchi, T., Shimane, Y., Usui, K., Shimamura, S., Mori, K., Hiraki, T., Tame, A., Uematsu, K., Maruyama, T., & Hatada, Y. (2013). Brevundimonas abyssalis sp. nov., a dimorphic prosthecate bacterium isolated from deep-subsea floor sediment. International Journal Of Systematic And Evolutionary Microbiology, 63, 1987–1994.
Henrissat, B., Callebaut, I., Fabrega, S., Lehn, P., Mornon, J. P., Davies, G. (1995). Conserved catalytic machinery and the prediction of a common fold for several families of glycosyl hydrolases. Proceedings of The National Academy of Sciences of The United States of America, 92, 7090-7094.
Acknowledgments
L. Graciano, J.M. Corrêa, F.G.N. Vieira, and A. Bosetto were fellows of the Coordination of Improvement of Higher Education Personnel (CAPES). R.C.G. Simão was partially supported by Araucaria Foundation (process 630/2014).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Graciano, L., Corrêa, J.M., Vieira, F.G.N. et al. Cloning and Expression of the xynA1 Gene Encoding a Xylanase of the GH10 Group in Caulobacter crescentus . Appl Biochem Biotechnol 175, 3915–3929 (2015). https://doi.org/10.1007/s12010-015-1560-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1560-z