Abstract
It has been well established that gamma rays at low doses have stimulatory effects on plant growth and development. However, our knowledge regarding the molecular mechanism underlying the growth stimulation remains limited. In this study, we report the role of reactive oxygen species (ROS) and abscisic acid (ABA) in the growth stimulation using irradiated Arabidopsis seeds. The results indicated that 50 Gy gamma irradiation presented maximal beneficial effects on germination index, root length, and fresh weight. The contents of hydrogen peroxide (H2O2) and activities of antioxidant enzymes under gamma irradiation were markedly higher than those of controls. ROS scavenging significantly suppressed the growth of the irradiated plants. Furthermore, endogenous ABA was induced under low-dose gamma irradiation. The growth stimulation and elevated H2O2 level were affected in the irradiated ABA-deficient mutant aba2-1 compared with the mutant control. Transcriptional expression analysis of selected genes revealed that several genes for ABA biosynthesis were upregulated, and the genes for ABA catabolic pathway and transport were differentially regulated in response to low-dose gamma irradiation. Our results suggest that ROS and ABA signaling play an essential role in the stimulatory effects of low-dose gamma irradiation and that ROS, as secondary molecules, mediate ABA signal transduction under irradiation in response to stress factors during plant growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The current research in the field of ionizing radiation, which focuses on the power of gamma ray penetration, facilitates its wide application in techniques for the breeding and improvement of various plant species [1, 2]. The effects of gamma irradiation on the morphology changes and biological responses of plants are dependent on radiation doses [3, 4]. It has been proved that the use of gamma rays at low doses has positive effects on a range of plants. Various growth parameters, such as the stem length, diameter, and leaf area, were stimulated in red pepper (Capsicum annuum L.) plants treated with a low dose of gamma irradiation [5]. The growth and development of lettuce, such as germination index and root and shoot lengths, were improved by the exposure of dry seeds to low doses of gamma irradiation (2–30 Gy) [6]. Furthermore, irradiation with low gamma rays has been shown to significantly improve growth traits in crop, such as seedling/plant height, panicle number and length, and number of seeds per panicle in rice (Oryza sativa L.) [7]. Recently, the stimulatory effects of low-dose gamma irradiation on plant growth have received considerable attention.
Gamma irradiation can induce physiologic changes as well as a variety of biochemical responses at the cellular level. One of the common effects of ionizing radiation on plants is the induction of the formation of reactive oxygen species (ROS), such as superoxide anion (O2 ·−), hydroxyl radicals (·OH), singlet oxygen (1O2), and hydrogen peroxide (H2O2) [8]. In plants, H2O2 is the predominant reactive oxygen species, and this species can be transformed by other free radicals and has a relatively long life and high permeability across membranes [9, 10]. Excess ROS can lead to irreparable metabolic dysfunction and even cell death by directly attacking photosynthetic pigments, membrane lipids, proteins, and nucleic acids [11]. To cope with the adverse effects caused by ROS, plants have evolved a comprehensive and integrated enzymatic defense system like superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) to control the ROS balance [12]. SOD rapidly dismutates O2 ·− to produce H2O2 and O2, and POD and CAT are enzymes that catalyze the conversion of H2O2 to water and O2 [13]. It has been reported that the activities of these antioxidant enzymes are generally increased in plants in response to treatment with various ionizing radiation [14–16]. Although it is well established that ROS play an important role in the physiological metabolism of plants and mediate the defense responses to abiotic stress, the regulatory role of cellular ROS in the stimulatory effects of low-dose gamma radiation remains unknown.
The phytohormone abscisic acid (ABA) is defined as a stress hormone due to its rapid accumulation in response to various stresses and its regulation of stress responses. ABA homeostasis is regulated by its biosynthesis as well as by its degradation or inactivation pathways [17]. Recently, a comprehensive microarray analysis of the transcriptome features of the promoted-growth rice seedlings induced by low-energy N+ beam radiation revealed that genes associated with ABA synthesis and signaling were highly upregulated in response to the nitrogen ion beam [18], indicating that the ABA signaling system may play an important role in the regulation of plant responses to ionizing radiation. The role of ABA signaling in the stimulatory effects of low-dose gamma radiation has yet to be investigated. Previous studies have shown that ROS function as signal molecules in ABA signaling. Gene expression analysis revealed that H2O2 promoted ABA catabolism and gibberellin (GA) biosynthesis, which control the dormancy and germination of Arabidopsis seeds [19]. H2O2 also performs a crucial function in the induction of α-amylase production in barley aleurone cells by modulating the expression of genes related to GA and ABA signaling [20]. In addition, the analysis of the global gene expression profiles of Arabidopsis seedlings subjected to ABA or H2O2 treatment showed substantial shared transcriptional responses to these two treatments, suggesting that ABA and H2O2 regulate most of their downstream genes in a coordinated manner [21]. Taken together, these results highlight the importance of ROS signaling with respect to the ABA signal transduction networks in plants.
In this study, an effort was made to reveal the possible roles of ROS and ABA signaling in the biopositive responses of the model plant Arabidopsis thaliana (Columbia ecotype) to low-dose gamma irradiation. Thus, various plant growth parameters, the H2O2 content, the activities of antioxidant enzymes, the transcriptional level of NADPH oxidase genes, the endogenous ABA content, and the expression of genes related to ABA biosynthesis, metabolism, and homeostasis, were assessed with Arabidopsis seeds exposed to or not exposed to low-dose gamma irradiation. Our results extend the available knowledge of the molecular mechanism underlying the growth stimulation induced by low-dose gamma irradiation.
Materials and Methods
Plant Materials and Growth Conditions
Seeds of wild-type Arabidopsis and ABA-deficient mutant aba2-1 were used for gamma irradiation. For growing seedlings on agar-containing plates, Arabidopsis seeds were pretreated with 70 % ethanol for 1 min, surface sterilized in 2.5 % bleach for 10 min, and washed with distilled water at least five times. The seeds were planted on 1/2 Murashige and Skoog medium (Sigma) supplemented with 1 % (w/v) sucrose, 1 % (w/v) agar (pH 5.8), and placed at 4 °C in the dark for 48 h before germination. Growth conditions were at 23 °C with a 16-h light/8-h dark cycle. For ROS scavenging, the seedlings were grown in agar-containing plates supplemented with 0.5 % dimethylsulfoxide (DMSO; Sigma). All of the seeds used for experiments were Arabidopsis Columbia-0.
Gamma Irradiation
Uniform seeds were randomly divided into two groups: (1) nonirradiated seeds for the controls and (2) seeds exposed to gamma irradiation. Gamma irradiation was conducted using a 60Co [Cobalt-60] gamma source at a dose rate of 8.5 Gy/min. The doses of exposure used in this study were 25, 50, 75, 100, and 150 Gy.
Morphological Observations
Three replicates of 100 seeds each were used for the seed germination test. The seeds were considered germinated when they exhibited a radical extension of 0.2 cm. The germinated seeds were counted daily for a period of 3 days to determine the germination index.
The germination index is a quantitative expression of germination that relates the daily germination rate to the maximum germination value. The germination index (GI) was calculated using the following formula:
where N 1, N 2, N 3,…, N n represent the number of seeds that germinated on days 1, 2, 3…,n. Each result represents the mean of three biologic replicates.
The root length and fresh weight of the control and irradiated samples were measured on the fifth day after vernalization for a period of 2 days. The root length of 30 individual seedlings was measured, and fresh weight was determined from 300 seedlings. All of the data represent the mean of three biologic replicates.
Measurement of ROS Content
The hydrogen peroxide concentration in the seedlings was measured according to Patterson et al. (1984) [22]. Five-day old seedlings (0.50 g of fresh weight (FW)) were homogenized in 5 mL of cold acetone, and the homogenate was centrifuged at 10,000g and 4 °C for 15 min. The supernatant was collected and added into a concentrated hydrochloric acid solution of 0.1 mL 20 % TiCl4 and 0.2 mL concentrated ammonia. After a 5-min reaction at 25 °C, the reaction mixture was centrifuged at 8000g and 4 °C for 10 min. The pellets were washed with cold acetone twice and added into 3 mL 1 M H2SO4. The absorption at 410 nm was measured, and the concentration of H2O2 was determined using a standard curve plotted with known concentrations of H2O2.
Determination of Antioxidant Enzyme Activity
Five-day-old seedlings (0.20 g of FW) were homogenized in a mortar and pestle with 2 mL of 50 mM ice-cold phosphate buffer (pH 7.8) containing 4 % polyvinylpyrrolidone (PVP) and 1 mM ethylene diamine tetraacetic acid (EDTA). The homogenate was centrifuged at 10,000 rpm/min and 4 °C for 15 min. The supernatant was used for assaying the activities of SOD, POD, and CAT. All of the procedures were conducted at 4 °C.
The superoxide dismutase (SOD, EC1.15.1.1) activity was determined by monitoring the inhibition of the photochemical reduction of nitroblue tetrazolium (NBT), as described by Giannopolitis and Ries (1977) [23]. The reaction mixture (3 mL) contained 50 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, 130 mM methionine, 0.75 mM NBT, 0.02 mM riboflavin, and 0.1 mL enzyme extract. Riboflavin was added as the last component, and the reaction mixtures were illuminated for 15 min at a light intensity of 5000 lx. Non-illuminated and illuminated reactions without the supernatant served as calibration standards. One unit of SOD activity was defined as the amount of enzyme required to cause 50 % inhibition of the reduction of NBT as monitored at 560 nm.
The peroxidase (POD, EC 1.11.1.7) capacity was measured using guaiacol [24]. The enzyme extract (0.02 mL) was added to the reaction mixture containing 0.02 mL guaiacol solution and 0.01 mL hydrogen peroxide solution in 3 mL of phosphate buffer solution (pH 7.0). The addition of enzyme extract started the reaction, and the increase in absorbance was recorded at 470 nm for 5 min.
The catalase (CAT, EC 1.11.1.6) activity was determined using the method as described [25], which involves measuring the initial rate of disappearance of H2O2. The reaction solution (3 mL) consisted of 50 mM phosphate buffer (pH 7.0), 20 mM H2O2, and 0.1 mL enzyme extract. The reaction was initiated by adding the enzyme extract. The decrease of H2O2 was monitored at 240 nm for at least 3 min.
ABA Quantification
Five-day-old Arabidopsis seedlings germinated from gamma-irradiated or nonirradiated seeds under normal growth conditions or treated with 0.5 % DMSO were used for the measurement of the endogenous ABA contents. Fresh samples of approximately 500 mg of whole seedlings were homogenized in 2 mL extraction buffer (80 % methanol containing 0.5 g L−1 citric acid monohydrate and 0.1 g L−1 butylated). The homogenate was incubated in 10 mL extraction buffer for 24 h at 4 °C in the dark on a rotary shaker. The ABA in the supernatants was further purified using a C18 column (Sep-Pak Vac 3-cc [500-mg] C18 Cartridges, Waters). The purified fraction was evaporated in a freeze dryer. The residue was then dissolved in 1 mL methanol for analysis. The endogenous ABA levels were quantified using the Phytodetek ABA enzyme immunoassay test kit (Agdia) with an ELISA reader according to manufacturer’s instructions.
RNA Isolation and Real-Time Quantitative RT-PCR
The total ribonucleic acid (RNA) from 5-day-old seedlings germinated from seeds treated with or without 50 Gy gamma irradiation was extracted using the SV Total RNA Isolation Kit (Promega), and the complementary DNA (cDNA) was synthesized with SuperScript III Reverse Transcriptase (Invitrogen). Polymerase chain reactions were performed in triplicate with a Mastercycler ep realplex thermal cycler (Eppendorf, Hamburg, Germany). The Mastercycler® ep realplex 2.2 software was used to compile the PCR protocols and to define the plate setups. The reaction system was as followed: 10 μL 2 × SYBR® Premix Ex Taq TM (Takara Bio, Japan), 1 μL of the reverse transcription reaction (1:5 diluted), and 200 nM of each gene-specific primer in a final volume of 20 μL. The following standard thermal profile was used for all PCRs: pre-denaturation at 95 °C for 2 min, denaturation at 95 °C for 15 s, annealing at 58 °C for 15 s, and prolongation at 72 °C for 20 s, 40 cycles. Data were analyzed using the realplex 2.2 software (Eppendorf) to calculate cycle threshold (CT) values. The data were analyzed using the comparative Ct (2−∆∆Ct) method [26]. To compare the data from different PCR runs or cDNA samples, the Ct values for the genes were normalized to the Ct value of 18 sRNA, which was a housekeeping gene included in each PCR run. The accession numbers and primers of the genes used for real-time quantitative reverse transcription-PCR (RT-PCR) are listed in Supplementary Table 1.
Statistical Analysis
The experiments were completely random designs with at least three replications. For each experiment described above, the results are expressed as the means ± standard errors. One-way analysis of variance (ANOVA) was applied using SPSS software to determine the significance of the results. A P value of less than 0.05 was considered to denote a statistically significant difference, a P value less than 0.01 was considered to denote a highly significant difference, and a P value less than 0.001 was considered to denote an extremely significant difference.
Results
Effects of Gamma Irradiation on Seed Germination and Plant Growth
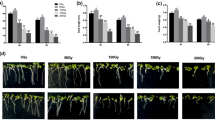
Figure 1 shows the growth responses of 5-day-old Arabidopsis seedlings developed from seeds irradiated with different doses of gamma ray radiation. The germination potential of the irradiated seeds, which is expressed as the germination index (GI), was promoted with increasing gamma ray doses of up to 100 Gy (Fig. 1a). Exposure to doses of 25 and 75 Gy resulted in 15 and 20 % increases in the GI, respectively, compared with the control. The highest stimulation of the germination process, i.e., 36 %, was recorded with a dose of 50 Gy. The GI obtained with a dose of 100 Gy was slightly increased, whereas that obtained with 150 Gy was decreased by 10 %.
Analysis of the growth parameters in response to a range of gamma irradiations. Arabidopsis seeds were irradiated with 25, 50, 75, 100, or 150 Gy. 0 Gy was used as the control. a Effect of gamma irradiation on the germination index. b Effect of gamma irradiation on the root length. c Effect of gamma irradiation on the fresh weight. The difference in the biological effects obtained in response to gamma irradiation was determined through comparisons with the control. *Significant difference (P < 0.05) compared with the control. **Highly significant difference (P < 0.01) compared with the control. ***Extremely significant difference (P < 0.001) compared with the control. Note that gamma irradiation at the low dose of 50 Gy resulted in the maximal growth stimulation
The biometric measurements of the roots that emerged from the irradiated seeds (25-75 Gy) showed an increase in the root length. (Fig. 1b). At the doses of 25 and 75 Gy, the root length was increased by 3.9 and 5.8 %, respectively. The maximum increase of 19 % was recorded with the dose of 50 Gy. An absorbed dose of 100 Gy had no effects on the root length, while with the higher dose of 150 Gy, the radicular system severely decreased compared with the control.
The fresh weight of the seedlings was enhanced after gamma treatment with doses ranging from 25 to 75 Gy. Compared with the control, the fresh weight increased from 9.5 (25 Gy) and 15 % (75 Gy) to the maximum of 31 % (50 Gy). The results showed that there was almost no increase in the fresh weight in response to 100 Gy irradiation. However, exposure to the dose of 150 Gy caused a significant reduction of 17 % compared with the normal growth (Fig. 1c). Based on the analysis above, it can be concluded that gamma ray treatment with a dose of 50 Gy promotes maximal seeding development in terms of the overall growth parameters in comparison with other doses. Thus, we used seeds that were irradiated with the dose of 50 Gy for the subsequent studies.
ROS Level and Antioxidant Enzyme Activities in Response to Low-Dose Gamma Irradiation
As an abiotic stress, gamma irradiation stimulates the production of reactive oxygen species. As shown in Table 1, irradiation with 50 Gy increased the H2O2 concentration in seedlings significantly (by 22 %) compared with the control. We then analyzed the gene expression of Arabidopsis RBOHs (respiratory burst oxidase homologs), which encode the NADPH oxidases [27]. The transcript levels of Atrbohs analyzed in this study were obviously enhanced in response to 50 Gy gamma irradiation (Fig. 2). High expression levels of AtrbohB and AtrbohF were observed with the low gamma ray treatment, i.e., these were increased by 25 and 26 % (P < 0.05), respectively, compared with the levels observed in control plants. The maximal increase in the AtrbohD expression level of 33 % (P < 0.01) was obtained. In contrast, the transcription of the AtrbohC gene was less induced with this treatment compared to the control, indicating that Atrbohs genes are differentially regulated at the transcriptional level in response to low-dose gamma irradiation.
Expression patterns of genes encoding NADPH oxidases in response to low-dose gamma irradiation. The difference in gene expression was determined through comparisons of the expressions obtained in the control (0 Gy) and the 50 Gy gamma-irradiated samples. *Significant difference (P < 0.05) compared with the control. **Highly significant difference (P < 0.01) compared with the control
A high ROS concentration usually triggers antioxidant enzyme activities. As shown in Table 1, the POD activity in control seedlings was measured as 1.09 ± 0.09 (U min−1 g−1), while the POD activity in response to 50 Gy gamma irradiation was increased to 1.65 ± 0.07 (U min−1 g−1); this increase was statistically highly significant (P < 0.01). The analysis of SOD activity revealed an increase from 22.49 ± 0.19 to 35.66 ± 0.27 (U min−1 g−1) compared with the level observed in the control. Similarly, the CAT activities were found to be greatly increased (P < 0.01).
Reducing ROS Affects the Growth Stimulation Induced by Low-Dose Gamma Irradiation
To determine the role of ROS level in the growth stimulation induced by low-dose gamma irradiation, we analyzed the effects of ROS reduction with 0.5 % dimethylsulfoxide (DMSO), which is an ROS scavenger, on seedling development. The results in Fig. 3a showed that DMSO treatment caused a highly significant decrease (P < 0.01) in the germination index of seeds irradiated with 50 Gy gamma ray compared with the control plants. The germination index of nonirradiated seeds was not affected obviously by treatment with 0.5 % DMSO. The analysis of the root length revealed a marked decline of 17 % (P < 0.01) in the irradiated plantlets subjected to DMSO treatment compared with the nontreated samples. The root length of the nonirradiated plantlets was not affected by DMSO treatment (Fig. 3b). In addition, the fresh weight of the irradiated seedlings was also influenced by DMSO treatment. Compared with the irradiated control plants, DMSO treatment induced a significant decrease (P < 0.05) in the fresh weight of the irradiated seedlings but had no apparent influence on the fresh weight of the nonirradiated samples (Fig. 3c). Our results imply that ROS production is related to the stimulatory effects of low-dose gamma irradiation.
Effects of the ROS scavenger DMSO on a the germination index, b root length, and c fresh weight of Arabidopsis seedlings germinated from irradiated or nonirradiated seeds. Seedlings grown under normal growth condition were used as the control. The differences in biological effects treated with 0.5 % DMSO treatment were determined through comparisons with the respective controls. *Significant difference (P < 0.05) compared with the control. **Highly significant difference (P < 0.01) compared with the control
The Induction of ABA by Low-Dose Gamma Irradiation IS Associated with Growth Stimulation
To determine whether there is a link between the phytohormone ABA and plant response to the stress of low-dose gamma irradiation, we measured the endogenous ABA concentrations in seedlings. As shown in Fig. 4, the ABA level in the nonirradiated control seedlings was 105 ± 2.64 pmol g−1 FW, and exposure to gamma irradiation at the dose of 50 Gy highly increased the ABA concentration to 139.67 ± 3.05 pmol g−1 FW, which was significantly higher than that of nonirradiated seedlings (P < 0.05). This finding indicates that low-dose gamma irradiation triggers ABA accumulation in Arabidopsis.
Effects of low-dose gamma irradiation and DMSO treatment on the endogenous ABA content. The ABA contents in 5-day-old Arabidopsis seedlings germinated from seeds irradiated with or without 50 Gy gamma irradiation, grown under normal growth conditions, or treated with 0.5 % DMSO were quantified. Seedlings grown under normal conditions were used as the controls. The means denoted by the same letter are not significantly different (P > 0.05)
To further demonstrate whether ABA accumulation is involved in the growth stimulation induced by low-dose gamma irradiation, we irradiated seeds from wild-type and aba2-1 (a mutant deficient in ABA biosynthesis) plants [28, 29] and compared their growth parameters. Because the ABA level controls seed dormancy, we first tested the effects of ABA reduction on the germination index. As shown in Fig. 5a, irradiation with the dose of 50 Gy significantly increased the germination index of the wild-type plants compared to the wild-type control. In contrast, the germination index of the mutant lines exposed to the same irradiation was almost equivalent to that of the nonirradiated mutant plants, even though the aba2-1 mutation promotes seed germination, as reported previously [28]. The quantitative analysis demonstrated that the average length of the newly grown primary roots of wild type irradiated with 50 Gy gamma rays was 1.42 ± 0.02 cm, which is obviously longer than that of the wild-type control (1.19 ± 0.03 cm, P < 0.01). In contrast, the primary root length of aba2-1 mutant was not significantly different from that of the mutant control seedlings (Fig. 5b). Furthermore, the ABA-deficient mutant inhibited the stimulatory effect on fresh weight, because the fresh weight of the irradiated aba2-1 mutant seedlings was indistinguishable from that of the controls. However, low-dose gamma treatment significantly increased the fresh weight of the wild-type plants compared to the wild-type controls (Fig. 5c).
ABA-deficient mutant suppresses the stimulatory effects of low-dose gamma irradiation. The a germination index, b root length, c fresh weight, and d H2O2 content were not promoted in the ABA biosynthesis mutant aba2-1 germinated from seeds irradiated with 50 Gy gamma irradiation compared with the mutant control (0 Gy). The wild-type plants subjected to the same gamma irradiation showed the expected stimulatory effects compared with the wild-type control (0 Gy). *Significant difference (P < 0.05) compared with the control. **Highly significant difference (P < 0.01) compared with the control
Increased ROS Level Is Induced by the Promotion of Endogenous ABA Under Low-Dose Gamma Irradiation
A cause-effect relationship between ABA and ROS production has been documented. To determine whether the increase in the ROS level in response to low-dose gamma irradiation is related to the induction of ABA, the H2O2 concentration in seedlings germinated from irradiated or nonirradiated seeds of wild-type and aba2-1 mutant plants was measured and compared. The wild-type plants treated with 50 Gy gamma irradiation exhibited markedly higher amounts of H2O2 than the wild-type control. In contrast, no variation in the H2O2 concentration was observed between the irradiated aba2-1 mutant lines and the corresponding control seedlings (Fig. 5d). To further determine the regulatory role of ABA in ROS production, we examined whether the turnover of ROS influences the ABA level in response to gamma irradiation. The results showed that the reduction of ROS by DMSO treatment had no obvious effects on the ABA induction in response to low-dose gamma irradiation and on the steady-state level of ABA in nonirradiated plants (Fig. 4).
Expression Analysis of Genes Related to ABA in Response to Low-Dose Gamma Irradiation
The ABA level is known to be induced under stress condition, and this induction is mainly due to the induction of genes encoding the enzymes responsible for the maintenance of ABA homeostasis. We investigated the expression of selected genes that are known to be involved in the biosynthesis, catabolism, and transport of ABA in response to low-dose gamma irradiation using real-time quantitative reverse transcription-PCR (RT-PCR) (Table 2). Several genes associated with ABA biosynthesis (ABA2, ABA4, and AAO3) presented higher relative levels of gene expression in 50 Gy irradiation samples compared with the controls; however, the transcript level of ABA1 was relatively unaffected by low-dose gamma irradiation. In ABA biosynthesis, xanthoxin generation represents the key step and is catalyzed by 9-cis-epoxycarotenoid dioxygenase (NCED). Under low gamma ray treatment, the NCED2 mRNA levels were upregulated compared with the control. Similarly, the NCED3 transcript was also induced under the same growth conditions.
The cellular ABA levels can be reduced through two catabolic pathways: hydroxylation and conjugation [30]. In A. thaliana, the major ABA catabolic pathway is mediated by four members of the CYP707A family of cytochrome P450 monooxygenases, which catalyze the hydroxylation of ABA at C′-8 to form the unstable 8′-hydroxy ABA that is subsequently converted to phaseic acid (PA) by spontaneous isomerization [31, 32]. All four CYP707A genes were differentially expressed in response to low-dose gamma irradiation. Notably, CYP707A2 expression was significantly enhanced by 1.7-fold compared to the control. In contrast, exposure to low-dose gamma irradiation obviously repressed the transcription of CYP707A4 and had no effects on the expression levels of CYP707A1 and CYP707A3. In Arabidopsis, UGT71B6 encodes ABA glycosyltransferase which mediates the ABA conjugation with glucose to form an inactive ABA glucose ester (ABA-GE) [33]. The results shown in Table 2 demonstrate that the expression of UGT71B6 was 2.2-fold higher in 50-Gy-irradiated plants than in the nonirradiated samples. Similar expression level of AtBG1, which encodes β-glucosidase that releases free ABA from the glucosyl ester form [34], was recorded in the irradiated plants compared with the control.
ABA signaling also requires translocation from ABA-producing cells via cellular transporters to allow its rapid distribution into neighboring tissues, a process that is mediated by ABA-importing and ABA-exporting transporters encoded by AtABCG40 and AtABCG25, respectively [35, 36]. We detected a strong induction of AtABCG40 expression to a level 4.3-fold higher than that observed in the control. In contrast, AtABCG25 expression was downregulated, which may contribute to the increased ABA content observed in response to 50 Gy gamma irradiation. Taken together, our results indicate that ABA metabolism and homeostasis are differentially regulated in response to low-dose gamma irradiation in Arabidopsis.
Discussion
It is generally accepted that ionizing radiation differentially affects the morphology, anatomy, biochemistry, and physiology of plants in a dose-dependent manner. Luckey (2003) defined radiation hormetic effects as stimulation by small doses of ionizing radiation and inhibition at large doses, and this definition has been validated in a variety of organisms, including microorganisms, invertebrates, experimental animals, and higher plants [37]. An increasing body of evidence shows that the growth and development of various plant species are obviously improved in response to gamma irradiation at relatively low doses. In the present study, we investigated the biological responses of Arabidopsis seedlings germinated from dry seeds irradiated with a range of Cobalt-60 gamma rays. The results showed that gamma irradiation (less than 100 Gy) notably stimulated the growth of seedlings, including the germination index, primary root length, and fresh weight compared with the nonirradiated samples (Fig. 1). In particular, the 50-Gy gamma treatment displayed the maximal positive effects on all of the growth parameters analyzed, and this finding is consistent with other reports. Seed germination was previously shown to be increased with increasing gamma ray doses (50, 80, and 110 Gy), but a dose of 150 Gy inhibited the germination percentage in comparison with the lower doses [38]. In addition, 50 Gy gamma irradiation increased flag leaf area, photosynthetic rate, stomatal conductance, and plant nutrition in wheat [39]. Based on a previously published mathematical model analyzed with hormetic-like data, Fornalski et al. (2012) proposed that hormesis is typically observed at doses less than 30 Gy, and the no observed adverse effect level (NOAEL) point may be located between 30 and 100 Gy for the irradiated seeds [40]. These findings are not inconsistent with our results, because an obvious inhibitory effect was detected at high doses (150 Gy) of gamma rays. Several hypotheses have been proposed to explain the stimulatory effects of low-dose gamma radiation. The exposure of plant tissues to low-dose atomic radiation was found to induce positive radiation, which was designated secondary biogenic radiation that stimulates the division of resting undamaged cells likely through the activation of functional membrane receptors [41]. Low-dose gamma irradiation may activate ribonucleic acid (RNA) or protein synthesis during the early stages of germination after seed irradiation, which stimulates seed germination [42]. Kovalchuk et al. (2007) reported that the auxin-responsive genes are directly or indirectly stimulated in plants exposed to acute ionizing radiation [43]. Furthermore, Fan et al. (2003) speculated that increased antioxidants in response to gamma irradiation may act as stress signals and trigger stress responses in plants [44]. In our study, we found that ROS and ABA signaling are essential for the growth stimulation induced by low-dose gamma irradiation (see discussion below).
After its absorption in biological tissue, gamma irradiation triggers the production of reactive oxygen species likely through interactions with atoms or molecules in the cell to generate short-lived free radicals and the predominant secondary ROS, hydrogen peroxide (H2O2) [45, 46]. The detection of H2O2 accumulation in plant tissue is critical to understanding the effects of gamma irradiation on plant growth. By the histochemical detection at the ultrastructural level, Wi et al. (2006) observed that H2O2 was deposited substantially in the plasma membranes and cell walls of all tissue types examined with the exception of cotyledons in pumpkin plants exposed to high-dose gamma rays [47], and this finding is similar to the results obtained in mesophyll cells from Arabidopsis leaves exposed to relatively high doses of gamma irradiation [48]. In the present study, we found that the H2O2 contents were increased in the Arabidopsis seedlings germinated from seeds irradiated with low-dose gamma irradiation compared with the nonirradiated samples (Table 1). We further examined the relative gene expression of Arabidopsis RBOHs that encode the plasma membrane NADPH oxidases involved in the generation of superoxide, which is subsequently catalyzed to H2O2 by dismutation [49, 50]. In response to 50 Gy gamma irradiation, the transcription levels of AtrbohB and AtrbohF were notably enhanced compared with the controls, and this treatment resulted in maximal AtrbohD expression and no changes in the expression of AtrbohC (Fig. 2). Recently, Sugimoto et al. (2014) reported that the RbohD and RbohF genes in space-grown mizuna are significantly upregulated by space radiation and other environmental factors [51]. Considering the link between the plasma membrane deposition of H2O2 in plant cells and the cellular localization of NADPH oxidase, we propose that the increase in the ROS level induced by low-dose gamma irradiation is partially due to the upregulation of Arabidopsis RBOH genes.
As responses to ROS overproduction, plant cells activate endogenous enzymatic defense mechanisms against oxidative stress [13]. The major enzymes in these mechanisms, namely peroxidase (POD), superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX), provide highly efficient machinery for detoxifying O2 ·− and H2O2 in plant cells. Our results indicated that the enzymatic activities of POD, SOD, and CAT were all significantly increased to different extents in response to low-dose gamma irradiation compared with the controls. Moreover, CAT showed much higher activities than SOD in both the irradiated and nonirradiated samples (Table 1). Based on the function of the antioxidant enzymes, this finding suggests that H2O2 is the main free radical of the ROS induced by low gamma rays. Our results are supported by several previous reports. The stimulation of POD activity was observed in garlic bulbs irradiated with low doses of gamma rays (10 Gy) [52]. Higher levels of SOD and APX activities were measured in peppers exposed to low doses of gamma irradiation (from 2 to 8 Gy) [5]. A recent report on the responses of Arabidopsis plants to gamma irradiation at different growth stages revealed that POD activity is increased by 100 and 800 Gy gamma irradiation at both the vegetative and reproductive stages, that SOD activity is increased at the reproductive stage but decreased at the vegetative stage, and that CAT activities are differentially regulated depending on the irradiation doses and developmental stages [48], indicating that plants exhibit different ROS scavenging reactions in response to gamma irradiation depending on the doses of gamma rays and the developmental stages. Given that antioxidant enzymes control the ROS metabolism in plant cells, our results suggest that increased activities of POD, SOD, and CAT help scavenge the excess ROS produced under low-dose gamma irradiation and contribute to the maintenance of the relatively nontoxic level of ROS in cells.
In recent years, it has become apparent that ROS act as important signaling molecules in plants by regulating multiple processes, such as growth and development, programmed cell death, and the acclimatory responses to abiotic and biotic stresses [53, 54]. However, it is unclear whether the ROS level induced by low-dose gamma irradiation functions as signal molecules involved in the radiation-induced hormetic effects in plants. To test this hypothesis, we examined the effects of ROS reduction due to the presence of DMSO, which is used as an efficient ROS scavenger for ionizing radiation, on seedling growth [55, 56]. Our results showed that the germination index, primary root length, and fresh weight of the irradiated samples were significantly inhibited or reduced, whereas these growth parameters were not influenced in the nonirradiated samples by the 0.5 % DMSO treatment (Fig. 3). The concentration of DMSO used was verified by other studies in X-ray-irradiated animal cells [57] and low-energy-ion-irradiated plants [58, 59]. Deducing from the negative effects of ROS scavenging on the growth stimulation induced by low-dose gamma irradiation, our results imply that the relatively elevated ROS level likely triggers the signal transduction associated with a stimulatory effect as the response to low-dose gamma irradiation.
It is well known that plant hormone abscisic acid (ABA) signaling is essential for the modulation of a variety of physiological processes, including seed dormancy, stomata closure, senescence, and adaptive responses to stresses, such as cold, drought, and salinity [60]. The contents of hormones indole-3 acetic acid, gibberellic acid, and abscisic acid in coriander seedlings were increased treated with 60 to 80 Gy gamma irradiation, which stimulated both fresh and dry weights [61], implying a role of ABA in the response to gamma irradiation. In the present study, we found that gamma irradiation with the dose of 50 Gy notably induced ABA accumulation compared with the wild-type controls (Fig. 4). The responses of the ABA-deficient mutant line aba2-1 to low-dose gamma irradiation revealed that the germination index, primary root length, and fresh weight of 50 Gy gamma-irradiated mutant seedlings were not significantly different from those of the mutant control samples, whereas wild-type plants showed growth stimulation under the same irradiation conditions compared with the wild-type controls (Fig. 5). Thus, our results provide strong evidence for the role of ABA in low gamma ray-induced biopositive stress. Furthermore, we determined the relationship between the ROS level and ABA accumulation in response to low-dose gamma irradiation. Our results showed that H2O2 concentration was not increased by low gamma rays in the ABA-deficient mutant aba2-1 compared with the mutant control lines. Moreover, the scavenging of ROS with DMSO treatment had no obvious influence on the ABA concentration in both the aba2-1 and wild-type plants (Fig. 4). Similar results were reported previously. Pretreatment with the ABA biosynthesis inhibitor tungstate suppressed the increase in the generation of O2 ·− and H2O2 and the activities of antioxidant enzymes in the leaves of detached maize plants exposed to water stress [62]. In contrast, the water stress-induced ABA accumulation was not affected by ROS scavengers, such as DMSO, nor by direct treatment with H2O2 in excised maize leaves or leaves of detached plants [62, 63]. In addition, the H2O2 concentration was increased in the UV-B-irradiated leaves of wild-type maize but changed slightly in the leaves from vp14 maize, a mutant defective in ABA synthesis [64]. Taken together, our results suggest that ABA synthesis is likely involved in ROS production under low-dose gamma irradiation.
The genome-scale profiling of transcripts has shown that the ionizing radiation-responsive genes in Arabidopsis are distinctively regulated depending on the irradiation pattern and growth stage [43, 48, 65, 66]. Most of the altered genes are associated with cell growth, development and morphogenesis, photosynthesis, general metabolism, ROS scavenging, signal transduction, and defense/stress responses. A recent report on the transcriptome profiles of growth-promoted rice seedlings treated with a low-energy ion beam revealed that the expression of genes related to ABA synthesis and signal transduction was obviously upregulated, implicating the possible role of ABA signaling in the regulation of the encouraged stress [18]. However, it is unclear how low-dose gamma irradiation regulates the expression of genes responsible for the ABA homeostasis in vivo. In our study, we analyzed the transcription levels of genes encoding enzymes required for ABA synthesis, catabolism, and transport with and without 50 Gy gamma irradiation (Table 2). The real-time quantitative RT-PCR data showed that most of the ABA biosynthesis-related genes selected were highly upregulated in the irradiated samples compared with the controls. In particular, the increased mRNA level of NCED3 was highlighted, which is consistent with the well-established role of NCED3 as a rate-limiting enzyme in ABA biosynthesis in response to abiotic stress [67]. Among the CYP707A genes associated with ABA degradation, an increased expression level was only detected for CYP707A2, suggesting that the limited induction of CYP707A genes may be responsible for the elevated ABA levels obtained in response to gamma irradiation. Moreover, UGT71B6 and AtBG1, which are required for another important catabolic pathway of ABA, were both upregulated at similar levels, indicating that ABA inactivation and recovery are also essential for the maintenance of ABA accumulation in response to low-dose gamma irradiation. Interestingly, the expression of AtABCG40, which encodes an ABA-importing transporter, was significantly upregulated, whereas the expression of AtABCG25, which encodes an ABA-exporting transporter, was repressed. Thus, the vastly different transcriptional profiles that regulate ABA metabolism and turnover support the increase in ABA content as a result of low-dose gamma irradiation.
Conclusions
The current study preliminarily explored the roles of ABA and ROS signaling in the responses of Arabidopsis to low-dose gamma irradiation. Our results clearly suggest that the exposure of plant tissues to low-dose gamma irradiation triggers the ABA accumulation, which induces an increased generation of ROS. The overproduction of ROS not only activates the antioxidant enzyme system to scavenge the excess ROS to maintain the optimal redox status in plants, but the ROS also act as secondary signal molecules to further amplify the associated defense signaling, thereby easily overcoming daily stress factors, such as fluctuations in the light intensity, temperature variations, and water loss, during plant growth. Our findings provide new information for the improvement of plant growth in agriculture through the use of gamma irradiation at low doses.
References
Bala, M., & Singh, K. P. (2013). Journal of Horticultural Science and Biotechnology, 88, 462–468.
He, S., Han, Y., Wang, Y., Zhai, H., & Liu, Q. (2009). Plant Cell, Tissue and Organ Culture, 96, 69–74.
Kim, J.-H., Baek, M.-H., Chung, B. Y., Wi, S. G., & Kim, J.-S. (2004). Journal of Plant Biology, 47, 314–321.
Wi, S. G., Chung, B. Y., Kim, J. S., Kim, J. H., Baek, M. H., Lee, J. W., & Kim, Y. S. (2007). Micron, 38, 553–564.
Kim, J.-H., Chung, B. Y., Kim, J.-S., & Wi, S. G. (2005). Journal of Plant Biology, 48, 47–56.
Marcu, D., Cristea, V., & Daraban, L. (2013). International Journal of Radiation Biology, 89, 219–223.
Maity, J. P., Mishra, D., Chakraborty, A., Saha, A., Santra, S. C., & Ch&a, S. (2005). Radiation Physics and Chemistry, 74, 391–394.
Calucci, L., Pinzino, C., Zandomeneghi, M., Capocchi, A., Ghiringhelli, S., Saviozzi, F., Tozzi, S., & Galleschi, L. (2003). Journal of Agricultural and Food Chemistry, 51, 927–934.
Neill, S., Desikan, R., & Hancock, J. (2002). Current Opinion in Plant Biology, 5, 388–395.
Yang, T., & Poovaiah, B. W. (2002). Proceedings of the National Academy of Sciences of the United States of America, 99, 4097–4102.
Bailey-Serres, J., & Mittler, R. (2006). Plant Physiology, 141, 311.
Mittler, R. (2002). Trends in Plant Science, 7, 405–410.
Mittler, R., Vanderauwera, S., Gollery, M., & Van Breusegem, F. (2004). Trends in Plant Science, 9, 490–498.
Cho, H. S., Lee, H. S., & Pai, H.-S. (2000). Journal of Plant Biology, 43, 82–87.
Moussa, H. R. (2008). Journal of New Seeds, 9, 89–99.
Zaka, R. V., Ecasteele, C. M., & Misset, M. T. (2002). Journal of Experimental Botany, 53, 1979–1987.
Seiler, C., Harshavardhan, V. T., Rajesh, K., Reddy, P. S., Strickert, M., Rolletschek, H., Scholz, U., Wobus, U., & Sreenivasulu, N. (2011). Journal of Experimental Botany, 62, 2615–2632.
Ya, H., Chen, Q., Wang, W., Chen, W., Qin, G., & Jiao, Z. (2012). Journal of Radiation Research, 53, 558–569.
Liu, Y., Ye, N., Liu, R., Chen, M., & Zhang, J. (2010). Journal of Experimental Botany, 61, 2979–2990.
Ishibashi, Y., Tawaratsumida, T., Kondo, K., Kasa, S., Sakamoto, M., Aoki, N., Zheng, S. H., Yuasa, T., & Iwaya-Inoue, M. (2012). Plant Physiology, 158, 1705–1714.
Wang, P., Du, Y., Guo, Y., Zhou, Y., Miao, C., & Song, C. (2006). Journal of Integrative Plant Biology, 48, 62–74.
Patterson, B. D., MacRae, E. A., & Ferguson, I. B. (1984). Analytical Biochemistry, 139, 487–492.
Giannopolitis, C. N., & Ries, S. K. (1977). Plant Physiology, 59, 309–314.
Zhang, J., & Kirkham, M. B. (1994). Plant and Cell Physiology, 35, 785–791.
Bergmeyer, N. (1970). Methoden der enzymatischen Analyse (p. 636). Berlin: Akademie Verlag.
Schmittgen, T. D., & Livak, K. J. (2008). Nature Protocols, 3, 1101–1108.
Sagi, M., & Fluhr, R. (2006). Plant Physiology, 141, 336–340.
Leon-Kloosterziel, K. M., Gil, M. A., Ruijs, G. J., Jacobsen, S. E., Olszewski, N. E., Schwartz, S. H., Zeevaart, J. A., & Koornneef, M. (1996). The Plant Journal, 10, 655–661.
Schwartz, S. H., Tan, B. C., Gage, D. A., Zeevaart, J. A., & McCarty, D. R. (1997). Science, 276, 1872–1874.
Nambara, E., & Marion-Poll, A. (2005). Annual Review of Plant Biology, 56, 165–185.
Kushiro, T., Okamoto, M., Nakabayashi, K., Yamagishi, K., Kitamura, S., Asami, T., Hirai, N., Koshiba, T., Kamiya, Y., & Nambara, E. (2004). The EMBO Journal, 23, 1647–1656.
Saito, S., Hirai, N., Matsumoto, C., Ohigashi, H., Ohta, D., Sakata, K., & Mizutani, M. (2004). Plant Physiology, 134, 1439–1449.
Priest, D. M., Ambrose, S. J., Vaistij, F. E., Elias, L., Higgins, G. S., Ross, A. R., Abrams, S. R., & Bowles, D. J. (2006). The Plant Journal, 46, 492–502.
Lee, K. H., Piao, H. L., Kim, H. Y., Choi, S. M., Jiang, F., Hartung, W., Hwang, I., Kwak, J. M., Lee, I. J., & Hwang, I. (2006). Cell, 126, 1109–1120.
Kang, J., Hwang, J. U., Lee, M., Kim, Y. Y., Assmann, S. M., Martinoia, E., & Lee, Y. (2010). Proceedings of the National Academy of Sciences of the United States of America, 107, 2355–2360.
Kuromori, T., Miyaji, T., Yabuuchi, H., Shimizu, H., Sugimoto, E., Kamiya, A., Moriyama, Y., & Shinozaki, K. (2010). Proceedings of the National Academy of Sciences of the United States of America, 107, 2361–2366.
Luckey, T. D. (2003). RSO Magazine, 8, 22–40.
Abdel-Hady, M. S., Okasha, E. M., Soliman, S. S. A., & Talaat, M. (2008). Australian Journal of Basic and Applied Sciences, 2, 401–405.
Singh, B., & Datta, P. S. (2010). Radiation Physics and Chemistry, 79, 819–825.
Fornalski, K. W., Adamowski, Ł., Turowski, T. W., & Wojnarowicz, J. (2012). Nukleonika, 57, 421–426.
Kuzin, A. M. (1997). Bulletin of Experimental Biology and Medicine, 123, 313–315.
Kuzin, A. M., Vagabova, M. E., & Revin, A. F. (1976). Radiobiology, 16, 259–261.
Kovalchuk, I., Molinier, J., Yao, Y., Arkhipov, A., & Kovalchuk, O. (2007). Mutation Research, 624, 101–113.
Fan, X., Toivonen, P. M. A., Rajkowski, K. T., & Sokorai, K. J. B. (2003). Journal of Agricultural and Food Chemistry, 51, 1231–1236.
Kovacs, E., & Keresztes, A. (2002). Micron, 33, 199–210.
Esnault, M.-A., Legue, F., & Chenal, C. (2010). Environmental and Experimental Botany, 68, 231–237.
Wi, S. G., Chung, B. Y., Kim, J.-S., Kim, J.-H., Baek, M.-H., & Lee, J.-W. (2006). Journal of Plant Biology, 49, 1–8.
Kim, D. S., Kim, J.-B., Goh, E. J., Kim, W.-J., Kim, S. H., Seo, Y. W., Jang, C. S., & Kang, S.-Y. (2011). Journal of Plant Physiology, 168, 1960–1971.
Sagi, M., & Fluhr, R. (2001). Plant Physiology, 126, 1281–1290.
Taylor, W. R., Jones, D. T., & Segal, A. W. (1993). Protein Science, 2, 1675–1685.
Sugimoto, M., Oono, Y., Gusev, O., Matsumoto, T., Yazawa, T., Levinskikh, M. A., Sychev, V. N., Bingham, G. E., Wheeler, R., & Hummerick, M. (2014). BMC Plant Biology, 14, 4.
Croci, C. A., Arguello, J. A., Curvetto, N. R., & Orioli, G. A. (1991). International Journal of Radiation Biology, 59, 551–557.
Gechev, T. S., Van Breusegem, F., Stone, J. M., Denev, I., & Laloi, C. (2006). Bioessays, 28, 1091–1101.
Mittler, R., Vanderauwera, S., Suzuki, N., Miller, G., Tognetti, V. B., Vandepoele, K., Gollery, M., Shulaev, V., & Van Breusegem, F. (2011). Trends in Plant Science, 16, 300–309.
DeLara, C. M., Jenner, T. J., Townsend, K. M., Marsden, S. J., & O'Neill, P. (1995). Radiation Research, 144, 43–49.
Miyazaki, T., Hayakawa, Y., Suzuki, K., Suzuki, M., & Watanabe, M. (1990). Radiation Research, 124, 66–72.
Kashino, G., Prise, K. M., Suzuki, K., Matsuda, N., Kodama, S., Suzuki, M., Nagata, K., Kinashi, Y., Masunaga, S., Ono, K., & Watanabe, M. (2007). Journal of Radiation Research, 48, 327–333.
Chen, H., Li, F., Yuan, H., Xiao, X., Yang, G., & Wu, L. (2010). Journal of Radiation Research, 51, 651–656.
Mei, T., Yang, G., Quan, Y., Wang, W., Zhang, W., Xue, J., Wu, L., Gu, H., Schettino, G., & Wang, Y. (2011). Journal of Radiation Research, 52, 159–167.
Raghavendra, A. S., Gonugunta, V. K., Christmann, A., & Grill, E. (2010). Trends in Plant Science, 15, 395–401.
Latif, H. H., Abdalla, M. A., & Farag, S. A. (2011). Turkish Journal of Biochemistry, 36, 230–236.
Jiang, M., & Zhang, J. (2002). Journal of Experimental Botany, 53, 2401–2410.
Jia, W., & Zhang, J. (2000). Plant, Cell and Environment, 23, 1389–1395.
Tossi, V., Lamattina, L., & Cassia, R. (2009). The New Phytologist, 181, 871–879.
Nagata, T., Yamada, H., Du, Z., Todoriki, S., & Kikuchi, S. (2005). Journal of Agricultural and Food Chemistry, 53, 1022–1030.
Sahr, T., Voigt, G., Schimmack, W., Paretzke, H. G., & Ernst, D. (2005). The New Phytologist, 168, 141–148.
Barrero, J. M., Rodriguez, P. L., Quesada, V., Piqueras, P., Ponce, M. R., & Micol, J. L. (2006). Plant, Cell and Environment, 29, 2000–2008.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (11405147, 11375154 and 31300163). The authors express great gratitude to Prof. Le Jie, Institute of Botany, Chinese Academy of Sciences, Beijing, China, for providing the ABA-deficient mutant (aba2-1) of A. thaliana.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wencai Qi and Liang Zhang contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Suppl. Table 1
Accession numbers and primers of the genes used for real-time quantitative RT-PCR in this study. (DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Qi, W., Zhang, L., Feng, W. et al. ROS and ABA Signaling Are Involved in the Growth Stimulation Induced by Low-Dose Gamma Irradiation in Arabidopsis Seedling. Appl Biochem Biotechnol 175, 1490–1506 (2015). https://doi.org/10.1007/s12010-014-1372-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1372-6