Abstract
Abiotic stress in the form of gamma irradiation has been shown a potent inducer of oxidative stress in plant cell cultures which produce higher amounts of commercially important secondary metabolites. In the present study, the impact of low doses of gamma irradiation on growth and accumulation of 20-hydroxyecdysone of Sesuvium portulacastrum was investigated. Shoot cultures were established on Murashige and Skoog medium supplemented with indole-3-acetic acid (0.5 mg L−1) and N 6-benzylaminopurine (2.0 mg L−1). Mutations were induced in tissue culture by treating multiple shoots at low doses of gamma irradiation in the range from 5 to 40 Gy. In vitro cell growth and 20-hydroxyecdysone were assessed during M1T1, M1T2, M1T3, and M1T4 generations. A gamma radiation dose of 20 Gy was calculated as 50% of the lethal value (LD50). The survival rates of multiple shoot cultures exposed to high doses were gradually reduced in the course of increased order of generations. High-gamma irradiation doses were harmful to growth and 20-hydroxyecdysone production. The accumulation of 20-hydroxyecdysone of 0.139 mg/plant dry weight was significantly two-fold higher than non-irradiated shoot cultures. The stressed shoots increased 13-fold 20-hydroxyecdysone at 20 Gy during the M1T4 generation compared to the yield of the M1T1 generation. The ex vitro plants produced 0.321 mg/plant dry weight of 20-hydroxyecdysone which was remarkably greater than the untreated control. The present study postulated that gamma radiation induced metabolic changes and easy-to-achieve putative mutant comprised with the high amount of 20-hydroxyecdysone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytoecdysteroids (PE) are triterpenoids that possess enormous biomedical potential against various diseases (Gorelick-Feldman and others 2008). 20-hydroxyecdysone (20HE) is commonly found in numerous plant families and has potential in human medicine (Lafont and Dinan 2003). Other rarer ecdysteroid analogs may also possess interesting biological properties (Bathori and Pongracz 2005). Current evidence has proven that lipophilic Leuzea carthamoides root extracts include a major phytoecdysteroid, 20HE-inhibited human breast adenocarcinoma MCF-7 cells (Gaube and others 2008). Toth and others (2008) reported the importance of 20HE as a curative agent for muscle atrophy and it increased body mass and muscle fiber growth. Ho and others (2015) reported that Microsorum grossum extracts containing the main bioactive components of 20HE can be used as innovative active cosmetic ingredients providing UV-protective effects for human dermal fibroblasts.
Many plants contain significant amounts of the 20HE, which has potential therapeutic value in human medicine. A perennial herb, Rhaponticum carthamoides, has been used for centuries in eastern parts of Russia and is known for its rich source of 20HE as the most abundant ecdysteroid in various parts of the plant in the range of 0.03–1.51% (Kokoska and Janovsk 2009). Rufaie and others (2011) reported that considerable amounts of 20HE were isolated from Taxus wallichiana, Cupressus tularosa, and Datura stramonium plant extracts and the highest quantity was obtained in T. wallichiana. It has been recently found that Chenopodium quinoa macerated seeds secreted 491 µg g−1 of 20HE and showed the highest level of biological activity (Graf and others 2014). Seasonal and geographical conditions influence the content of 20HE in different tissue types of wild Ajuga bracteosa (Kayani and others 2014). Fronds and rhizomes of Microsorum grossum extracts contain 20HE as the main bioactive molecule and can be used as an adaptogenic compound to protect the skin against oxidative stresses (Ho and others 2015). Similarly, fronds of the fern Microsorum scolopendria are a rich source of 20HE (0.20%) and widely used in traditional medicine (Snogan and others 2007). 20HE is the common and major ecdysteroid in plants and the biosynthetic pathway has been studied in Achyranthes japonica which suggested that biosynthesis of 20HE is not restricted to a particular organ or growth stage of plants (Bakrim and others 2008; Boo and others 2010) and hairy root cultures of Ajuga sp. (Fujimoto and others 2000). The biosynthetic and metabolic transformations of ecdysones have been studied by the amalgamation of mevalonic acid and cholesterol into the Sesuvium portulacastrum plant (Sipahimalani and others 1972). Bathori and others (1999) achieved the highest concentrations of 2.3% of 20HE in Serratula coronate during the flowering period. 20HE is metabolically stable and found in a variety of plant families and participates in plant defenses against herbivorous insects, rather than as a phytohormonal role (Schmelz and others 2000; Dinan 2001).
Gamma rays are ionizing radiation, and high doses are harmful due to the generation of free radicals which cause serious damage to the cells. Conversely, low doses of gamma irradiation have been used to improve cell growth and enhance production of high-value life-saving drugs in plant cell cultures (Kim and others 2013; Fulzele and others 2015). The morphological, biological, and physiochemical changes caused by gamma radiation exposure in a large range of plants have been studied by many researchers (Kovacs and Keresztes 2002; Kim and others 2004; Wi and others 2005). Apparently, low-dose gamma irradiation improved varieties of many crops, that is, papaya, banana, sugarcane, potato, and strawberry (Hang and Chau 2010; Gloria and Adao 2013; Oloriz and others 2011; Das and others 2000; Breitfellner and others 2002) have been developed. Also, gamma radiation has been used for the selection of putative mutants in vitro and ex vitro for crop improvement and nowadays mutation induction has become a well-known tool in plant breeding programs that can deliver the existing germplasm and improve cultivars in certain specific traits (Hung and Johnson 2008; Sun and others 2007). The putative mutant OASA2 of rice is a variety enriched with a high level of amino acids developed from irradiated callus cultures (Kim and others 2005). In an earlier study, gamma irradiation significantly stimulated shikonin production and increased total shikonin yields by 400% at 16 Gy in suspension cultures of Lithospermum erythrorhizon (Chung and others 2006). Fulzele and others (2015) demonstrated that the application of low doses of gamma rays acts as abiotic stress to plant tissue leading to the beneficial effects of enhanced anticancer drug camptothecin production in cell cultures of Nothapodytes foetida. Hasbullah and others (2012) reported that cell and organ cultures of Gerbera jamesonii were strongly influenced by the gamma radiation dose. Low doses of gamma irradiation increased the number of shoots in Cucumis melo, whereas the number of shoots decreased with increasing doses of irradiation (15 and 20 kRs) (Venkateshwarlu 2008).
The commercial market of phytoecdysteroids has proven the immense importance of their therapeutic applications, specific biological activities, and clarifying claims of anabolic potency. Also, it has been used as a muscle enhancer in various commercial botanical supplements available in the global markets such as Magnum Thrust from Magnum Nutraceuticals (Canada) and Ecdysten from ThermoLife (USA). Phytoecdysteroid improves the quality and quantity of silk production at the commercial level in the sericulture industry (Keshan and others 2000; Trivedy and others 2006). The administration of low concentrations of ecdysone influenced silk gland cells and caused a beneficial effect on silk yield and suggested that phytoecdysteroid treatment, if applied at an appropriate concentration, could boost the sericulture industry as well as the economy of silkworm rearing (Srivastava and Upadhyay 2013). Recently, plant extracts of Cupressus tularosa contain phytoecdysteroid (β-ecdysone) have been applied in sericulture industry as a crop saver (Rufaie and others 2015). Furthermore, in plants, 20HE is a major molting hormone of invertebrates and acts as a defensive substance against insects and plant-parasitic nematodes (Soriano and others 2004).
The progressive demand of 20HE in dietary supplements, for crop saving and for applications in the sericulture industry, is continuously increasing in the global markets. Therefore, it is obligatory to fulfill the global demand of 20HE by alternative and attractive systems. The use of low doses of gamma irradiation to enhance the secondary metabolite production level has previously been investigated by other researchers. However, the effects of low doses of gamma irradiation on shoot cultures of S. portulacastrum to achieve cell lines containing high amounts of 20HE have not been studied methodically earlier. In the present investigations, we have systematically studied the influence of low doses of gamma irradiation stress on improvement of 20HE production in in vitro cell cultures during different generation periods.
Materials and Methods
Plant Materials
Plant materials of S. portulacastrum were collected from a coastal area of Navi Mumbai, India. Plant specimens were authenticated from the Botanical Survey of India, Pune, India. Axillary buds were used as a source of explants for initiation of shoot cultures. Explants were kept under running tap water for 30 min before disinfection. After 30 min, plant materials were treated with Dettol for 3 min and subsequently surface-sterilized with 70% ethyl alcohol (v/v) for 3 min followed by HgCl2 (0.1% w/v) solution for 2 min. Finally, surface-sterilized plant materials were rinsed several times with sterile water and transferred aseptically onto solid Murashige and Skoog (MS) (Murashige and Skoog 1962) medium.
Culture Media and Conditions
MS medium was supplemented with indole-3-acetic acid (0.5 mg L−1), N 6-benzylaminopurine (2.0 mg L−1), and 3% w/v sucrose. The pH of the medium was adjusted to 5.8 with 0.1 N NaOH/HCl before autoclaving. Culture medium was autoclaved at 121 °C at 15 lbs for 20 min. Cultures were incubated at 25 ± 1 °C under 16/8 h light and dark photoperiod conditions (40 μmol m−2 s−1, cool white fluorescent tubes). The cultures were maintained on similar media compositions and subcultured after every 4 weeks. After a period of almost 6 subcultures, established shoot cultures were taken up for further study.
Gamma Irradiation
Four-week-old in vitro shoot cultures were irradiated at different doses in the range of 5, 10, 15, 20, 25, 30, 35, and 40 Gy. Shoots of uniform length 3–4 cm having 5–6 nodes were inoculated in plant tissue culture bottles (n = 6 per bottle). The irradiation was carried out in a uniform source of 60Cobalt 1.164 Gy min−1 (Gamma chamber, Bhabha Atomic Research Centre, Mumbai, India) at room temperature. The schematic pictorial representation is shown in Fig. 1. The non-irradiated shoot cultures were taken as controls. After being exposed at different irradiation doses, the cultures were transferred onto MS medium of the same media constituents and maintained at 25 ± 1 °C for 16 h with light provided by cool white fluorescent tubes to provide 40 μmol m−2 s−1 light intensity. To study the effect of gamma irradiation, irradiated shoot cultures were subcultured every 4 weeks, and the growth characteristics and 20HE contents for four subcultures denoted as M1T1, M1T2, M1T3, and M1T4 generations were evaluated. As for observation of irradiated shoot cultures, at the end of the 4-week culture period, total fresh weights, dry weights, and quantification of 20HE of the shoots were determined and recorded. All experiments were repeated thrice with the same number of replicates and values are mean ± S.E. of three independent experiments.
Determination of Radiation Sensitivity Test
The radiation sensitivity test was executed on multiple shoot cultures of S. portulacastrum. The survival rate of irradiated and non-irradiated shoot cultures was measured after subsequent fourth subcultures.
Acclimation of Plantlets
Almost 100 plantlets obtained from each generation were transferred to polyethylene bags containing garden soil:sand:cow dung (3:1:0.5). The plants were grown under the shed at 24 ± 1 °C for 4 weeks. After a month, plants were harvested and evaluated for their heights, biomass, and subsequently analysis for 20HE. A hundred shoot cultures were used in each treatment and experiments were repeated thrice.
Extraction of 20-Hydroxyecdysone
In vitro shoots of the M1T1, M1T2, M1T3, and M1T4 generations were harvested and analyzed separately for 20HE contents. 20HE was extracted by a microextraction process to minimize loss of sample. In brief, harvested biomass materials were dried in an oven at 55 °C for 16 h and powdered by a Wiley Mill (Model No. 4276, Thomas Scientific, USA). The dried powdered material (100 mg) was transferred to polypropylene microcentrifuge (Eppendorf) tubes and mixed with methanol (1 ml) followed by sonication at 33 KHz for 10 min. After sonication, the samples were centrifuged at 12,000×g for 5 min. The supernatant was transferred to clean glass vials and applied directly to a high-performance liquid chromatography (HPLC).
Quantification of 20-Hydroxyecdysone
An isocratic analytical HPLC was performed on a JASCO liquid chromatography (PU-2080 Plus, Japan) equipped with an autosampler injector (AS-2055 plus Japan) with a 20-µl sample loop and a variable wavelength detector (Model No. UV-2075 plus, Japan). The HPLC column was a Merck Millipore RP C8 (particle size 5 µm, 4.6 mm × 150 mm) packed column. The mobile phase for 20-hydroxyecdysone elution was methanol:water (60:40, v/v) at a flow rate of 1 ml min−1 and UV detection at 245 nm. Data collection and integration were accomplished using Chrompass software. A 1 mg ml−1 stock solution of standard 20HE in methanol was prepared. For the calibration curve, 2–10 µl of standard solution of 20HE was applied in triplicate onto the HPLC. The peak areas were recorded and a calibration curve of 20HE was prepared by plotting peaks area against concentrations. The slope and intercept value for the calibration curve was Y = 22,358 X + 994.76 (R 2 = 0.999). Linearity was studied by least square linear regression analysis of peak areas obtained after application of different concentrations of standard 20HE solution in linearity ranges between 0.1 and 10 µg. No interference with 20HE was observed by using the methanol:water solvent as a mobile phase (Fig. 2). This method is sensitive and accurate with good reproducibility. Validation of the quantitative method was performed with the samples for five times. The results of the five injections from the same samples at the five concentrations (0.1–0.5 µg) showed similar retention time. A retention time of 20-hydroxyecdysone was 4.21 min. Peak identification was carried out on the basis of the standard sample of 20HE (Sigma, USA).
Growth Measurement
Fresh weights (FW) and dry weights of the cultures were determined by following the methods as described elsewhere (Fulzele and others 1995). In brief, after harvest, shoot cultures were gently pressed on filter papers (Whatman No. 1) to remove excess water for the determination of fresh weight (FW). Cultures were dried to constant weight in an oven at 55 °C for 16 h to obtain the dry weight (DW).
Statistical Analysis
The differences of mean values on growth and 20HE yield values were determined by one-way analysis of variance (ANOVA). All experiments were carried out in triplicate and values are mean ± S.E. of three independent experiments.
Results and Discussion
Radiation Sensitivity Test
The radiation sensitivity results were based on the survival percentage of irradiated and non-irradiated in vitro cell cultures. Shoot cultures exposed to various dosages of gamma irradiation or non-irradiated shoots showed a 100% survival rate at the first in vitro subcultures (M1T1 generation). We observed a significant percentage reduction in the survival rate of in vitro shoots with increased gamma irradiation doses during the M1T1, M1T2, M1T3, and M1T4 generations. In the present study, the lethal values (LD50) were observed based on the reduction of the survival rate of the shoot cultures after exposure to different doses of gamma irradiation and compared with non-irradiated cultures. The survival percentage of irradiated shoots to reach 50% as lethal values for gamma irradiation was 20 Gy as interpolated from the graph in Fig. 3. These results were in accordance with the radiation sensitivity test performed by Kiong and others (2008) who reported that gamma doses also decreased the survival percentage of plantlets of Orthosiphon stamineus. The optimum irradiation dose that induced maximum mutation with minimum damage to the shoot cultures, in the present work, LD50 value was 20 Gy. In accordance with our results, the lethal values have been studied by Kangarasu and others (2014) whereby LD50 was determined by reduction of survival cuttings of Manihot esculenta crantz at different measurements on gamma-treated material compared to untreated control.
Effects of Gamma Irradiation on Regeneration of Shoot Numbers and Shoot Length During the M1T1 Generation
A steady decline was found in shoot heights and numbers of multiple shoots regenerated from irradiated shoot cultures exposed at different irradiation doses. The maximum numbers of shoots were regenerated when exposed to 10 Gy of irradiation. Non-irradiated shoot cultures produced 3.88 multiple shoots, whereas irradiated shoots at 10 Gy regenerated 6.6 shoots in 1 month (M1T1 generation). Conversely, abnormalities were formed in the shoot when shoot cultures were exposed in the range from 25 to 40 Gy. As such, the results of the current study indicated that high-gamma doses totally reduced regeneration of shoots and formed stunted and abnormal shoots, whereas low doses improved shoot regeneration 70% more compared to non-irradiated shoots (Fig. 4). The results in the present study were supported by Sakr and others (2013), who reported that gamma irradiation at low doses increased the number of shoot cultures of Dracaena surculosa. Likewise, a similar observation was reported by Hasbullah and others (2012), on the effects of gamma irradiation on shoots regenerated from irradiated cultures of G. jamesonii. Authors have broadly studied and observed that the number of shoots was drastically reduced when cultures were exposed to high irradiation doses, whereas low doses influenced regeneration and obtained 6.6 regenerated shoots. Also, lower doses of gamma irradiation influenced shoot regeneration efficiency in multiple shoots of Cucumis melo, whereas high doses reduced the shoot formation indicating their sensitivity to gamma radiation (Venkateshwarlu 2008). Although the irradiation doses for the in vitro cell cultures varied, Kumari and others (2013) reported that shoot regeneration decreased gradually with the increase in radiation dose and the shoots that developed were small, less vigorous with retarded growth similar to the results obtained in the present study. Similarly, gamma irradiation augmented plant regeneration capability in cultures of Eleusine coranna (Pius and others 1994) and improved shoot organogenesis in Helianthus annuus (Encheva and others 1993). Recently, Al-Safadi and Elias (2011) reported that low doses of gamma irradiation influenced shoot growth, whereas exposure at high doses showed an adverse effect.
Effects of low doses of gamma irradiation on shoot regeneration and shoot height of S. portulacastrum in MS medium supplemented with indole-3-acetic acid (0.5 mg L−1) and N 6-benzylaminopurine (2 mg L−1). The observation was obtained from triplicate determinations. These results were representative from three independent replicates (±SE)
As shown in Fig. 4, a stimulation of shoot length was observed when shoot cultures of S. portulacastrum were exposed at different low doses of gamma irradiation. This study revealed that the shoot length was 5.64 cm at 10 Gy, which is 54% more than that of non-irradiated shoot cultures. At high doses of gamma irradiation, the lengths of shoots were reduced by 45% compared to non-irradiated cultures. These results were in a parallel with Al-Safadi and Elias (2011) who reported that shoot length of Capparis spinosa was significantly increased at 10 Gy of gamma irradiation. A stimulatory effect of low doses of gamma irradiation on shoot cultures of S. portulacastrum was observed in this study and is a well-corroborated phenomenon that has previously been reported by many researchers in several other crops. George and Rao (1980) reported that shoot growth of Brassica juncea was stimulated at the low dose of gamma irradiation. Borzouei and others (2010) observed stimulating effects on the shoot length of Triticum aestivum following gamma irradiation. Shoot length declined up to 46% as the radiation dose increased.
Hasbullah and others (2012) reported that a significant decline was observed in the length of irradiated shoot cultures of G. jamesonii exposed at high doses when compared to non-irradiated cultures. The similar observation has been observed in the present study in that 54% length of S. portulacastrum shoots at 10 Gy dose of gamma irradiation and then shoot length was reduced at high doses. In addition, high doses of gamma irradiation were reported to be harmful in several studies such that of Tshilenge-Lukanda and others (2013) who reported that high doses of gamma irradiation reduced plant height and the number of leaves of Arachis hypogaea.
In the present study, we observed that high doses of gamma irradiation molded deformed leaves, whereas low doses favored positive effects (Fig. 5). Similarly, Hung and Johnson (2008) reported that increased doses of irradiation at 80 Gy produced deformed leaves and treatment of low doses showed no major differences in shoot growth of Wasabia japonica. In fact, as previously mentioned by many researchers, high doses of gamma radiation retarded growth and development; mutagenic effectiveness was increased with the increased dose of gamma radiation and low doses, in general, were found to be more efficient in causing less cell damage (Rohani and others 2012; Caro-Melgarejo and others 2012). According to the results obtained in the present study, shoot morphology, shoot height, and number of shoots were impaired by high doses of gamma irradiation, which were consistent with the research of El-Shakhs and others (2007) and Smelkova and others (1999) who reported that high doses of gamma rays showed reduced stunted leaves and reduced plant heights of Picea abies, Larix deciduas, and Pinus sylvestris, whereas low doses of gamma rays favored most positive effects upon growth.
The phenotypes of the control and shoot cultures of S. portulacastrum exposed to different doses of gamma irradiation: 1-month-old cultures after gamma irradiation treatment and the effects of low doses of gamma irradiation on growth of S. portulacastrum shoot cultures on MS medium supplemented with indole-3-acetic acid (0.5 mg L−1) and N 6-benzylaminopurine (2 mg L−1) during the M1T1 generation. The observation was obtained from triplicate determinations. These results were representative from three independent replicates (±SE)
The abiotic stress of gamma radiation at different doses can alter or damage plant cells and even affect the morphology, anatomy, biochemistry, and physiology of plants (Wi and others 2007). In recent years, a number of articles have addressed that low doses of gamma irradiation could be used as harmless and stimulatory effects to improve growth, whereas higher doses of ionizing radiation reduced the mitotic activity (Mohajer and others 2014). Kiong and others (2008) found that radiation intensified plant sensitivity to gamma rays and decreased the number of endogenous growth regulators via either breakdown or lack of synthesis due to radiation. In the present study, enhancement of shoot numbers and shoot heights were stimulated at low doses of gamma irradiation intensity, whereas similar effects were retarded above at 25 Gy onwards. From the results of the present study, it can be concluded that the effects of gamma irradiation on shoot height and number of shoots varied between different dose treatments, and shoot cultures have the ability to tolerate high doses of gamma irradiation.
Effects of Gamma Irradiation on Multiple Shoot Cultures During the M1T1 Generation
The efficiency of shoot growth showed variation when exposed to low doses of gamma irradiation in the range from 5 to 40 Gy. A significant stimulation of growth rate was observed at low doses in the range from 5 to 20 Gy and thereafter drastically reduced the growth rate when exposed to high doses during the M1T1 generation. Maximum biomass was obtained when the shoots were exposed to the 5 Gy radiation dose compared to non-irradiated shoot cultures. In the present study, low doses favored shoot growth and achieved 2.1 g FW biomass, which is higher than non-irradiated shoot cultures. However, biomass of 0.52 and 0.51 g FW obtained from shoots were irradiated at 25 and 40 Gy, respectively, which is almost 67% less than that of non-irradiated shoot cultures (Fig. 5). In addition, there were no significant morphological abnormalities observed in the irradiated cultures at low doses and non-irradiated shoot cultures. Similar results were reported by Ling and others (2010), whereby cell cultures of Orthosiphon stamineus exposed to low doses were not affected in growth rate and showed the highest increase in fresh weight (202%), whereas cultures exposed at higher doses exhibited the lowest increase in fresh weight. Earlier many researchers have investigated that treatment with higher gamma irradiation doses was harmful, whereas low doses showed stimulatory effects. As observed in the present study, it has been reported by Ling and others (2008) that plant growth increased at low doses in Citrus sinensis, whereas inhibition occurred when radiation intensities increased. The present results were in parallel with Al-safadi and others (2011) who reported that gamma irradiation at 10 Gy stimulated the growth of shoots up to 200% and increased shoot rooting percentage from 75 to 100%. According to Bajaj (1970), high doses of gamma irradiation caused inhibition of tissue culture growth along with the failure of RNA synthesis and subsequently the failure of protein synthesis. Gamma rays belong to ionization radiation, which generates free radicals and high doses pose harmful effects on plant morphology, anatomy, biochemistry, and physiology depending on the irradiation level. Our results are in line with Venkateshwarlu (2008) who reported that the number of shoots and root lengths of Cucumis melo decreased with the increasing dosage of gamma irradiation and no significant changes were found in the morphology of phenotypes of plants when irradiated with relatively low doses of gamma rays.
Effects of Gamma Irradiation on 20HE Production During M1T1 to M1T4 Generations
The production of valuable commercial products through plant cell culture technology has been a challenging subject for many researchers. The ecdysteroid 20HE is a steroid hormone and valuable secondary metabolite produced abundantly in S. portulacastrum. Thus, to enhance the production of 20HE, selection of a putative mutant using gamma-irradiated shoots was subsequently subjected to the estimation of secondary metabolites during different generations. By comparing the production, significant variation has been observed during the successive four in vitro subcultures (M1T1, M1T2, M1T3, and M1T4 generations) and it was found that low doses of gamma irradiation enhanced the production of 20HE (Fig. 6).
Effects of low doses of gamma irradiation on plant height and accumulation of 20-hydroxyecdysone in shoot cultures of Sesuvium portulacastrum in MS medium supplemented with indole-3-acetic acid (0.5 mg L−1) and N 6-benzylaminopurine (2 mg L−1) during M1T1, M1T2, M1T3, and M1T4 generations. The observation was obtained from triplicate determinations. These results were representative from three independent replicates (±SE)
In the M1T1 generation, 20HE production was not correlated with increased doses of gamma irradiation. An inhibitory effect was observed at the intermediate gamma radiation dose of 25 Gy, whereas there was a significant stimulation of production at lower doses in the range of 5 to 20 Gy and even at high doses of 30 to 40 Gy during the M1T1 generation. Data in Fig. 6 showed that maximum 20HE production of 0.078 mg/plant dry weight was recorded in non-irradiated shoots, whereas at 25 Gy, the minimum concentration of 0.0041 mg/plant dry weight of 20HE obtained which was 19-fold less compared to control. The high-gamma irradiation dose of 40 Gy showed a concentration of 20HE of 0.026 mg/plant dry weight, which was 36% lower than that of non-irradiated shoot cultures. However, in the M1T1 generation, the gradual increase of the concentration of 20HE was not associated with increased doses of gamma irradiation. This study found that the significant variation of 20HE produced at different doses of gamma irradiation during the first subculture in the M1T1 generation was consistent with the research of Kiong and others (2008) who have demonstrated a similar type of variation using gamma irradiation of Orthosiphon stamineus at various doses. The authors further documented that the concentration of rosmarinic acid production was variable at different doses of gamma irradiation and the maximum rosmarinic acid concentration was achieved at 30 Gy, whereas the variation in the yield of 34, 32 and 23% at 10, 60, and 70 Gy was not parallel with increased doses of gamma irradiation. From the present study, it can be concluded that the free radicals generated in plants during irradiation may act as stress signals and may elicit stress responses in cell cultures and therefore variations in yield were obtained at different doses of gamma irradiation. The results of this study showed abiotic stress during the first subculture after irradiation which activated stress conditions by the impact of different radiation doses.
HPLC was performed after the irradiation and subsequent subcultures after each generation of a month; the level of 20HE in the irradiated shoot cultures was significantly lower than the control in the M1T1 generation. However, the 20HE concentration of irradiated shoots was increased during M1T2, M1T3, and M1T4 generations and becomes higher than the control as non-irradiated shoot cultures. Maximum 20HE was produced by irradiated shoots at 20 Gy (0.139 mg/plant); non-irradiated shoot cultures produced 0.064 mg/plant dry weight (Fig. 6). The stressed shoots had 13-fold increases in 20HE at the irradiation dose of 20 Gy in the M1T4 generation, compared to the yield at 20 Gy in the M1T1 generation. This phenomenon is probably due to the instant oxidation of the compounds, thus playing an antioxidant role by reducing the free radicals and the reactive oxygen species induced by irradiation (Larson 1988; Urbain 1996). Data obtained by other authors like Al-Safadi and others (1990) showed that the stimulating effect of gamma radiation on product synthesis was prompt because it increased the mitotic division of cultured cells. The positive effect of low doses of gamma irradiation on 20HE production by shoot cultures was due to the stimulatory effects of cell division or the alteration of metabolic processes affecting synthesis of phytohormones or nucleic acid (Hanan and others 2011). In addition, irradiated shoot cultures synthesized maximum amounts of 20HE at the optimal dose of 20 Gy of gamma irradiation, which was relatively higher than seeds and seedlings in terms of showing the stimulatory effects of gamma radiation because seeds and seedlings are generally more sensitive to gamma irradiation than in vitro cell cultures (Bajaj and others 1970; Borzouei and others 2010). There were no significant changes in plant height between different irradiation treatments compared to the control in the M1T3 and M1T4 generations. Similar results were recorded with in vitro shoot tips of Dracaena surculosa (Sakr and others 2013). The present study revealed that the production of the target compound was influenced by gamma irradiation and achieved a high amount of 20HE compared to non-irradiated shoot cultures, which was consistent with the report by El-Beltagi and others (2011) indicating that irradiated callus cultures of Rosmarinus officinalis enhanced the secondary metabolite production of rosemary compared to untreated callus cultures. Likewise, Chung and others (2006) reported that shikonin production was the highest at the gamma irradiation dose of 20 Gy, with recorded production greater than non-irradiated callus cultures of Lithospermum erythrorhizon.
Improvement of secondary metabolites by using low doses of gamma irradiation has previously been reported by several researchers. Fulzele and others (2015) reported that the 20 Gy dose level of gamma irradiation influenced cell growth by two-fold and also product synthesis and achieved 0.098% dry weight camptothecin and 0.0043% dry weight 9-methoxy camptothecin, which is 20-fold more than that of non-irradiated callus cultures of Nothapodytes foetida. Likewise, results of the present study of increased 20HE content in shoots due to exposure to ionization radiation is in agreement with the findings of Jaisi and others (2013) who reported a fourfold increase in plumbagin by root cultures of Plumbago indica when exposed to gamma radiation at a dose level of 20 Gy. Recently, Khalil and others (2015) observed that a low dose of gamma irradiation to callus cultures of Stevia rebaudiana enhanced stevioside content.
In the present study, the concentration of 20HE in the M1T1 generation was less as compared to in vitro shoots in the M1T4 generation. The experimental data showed that the accumulation of 20HE in irradiated shoots was not successively constant in all the generations studied. However, irradiated shoot cultures increased the level of 20HE constantly during subsequent generations. Our results are in agreement with Urbain (1996) who reported that the synthesis of polyphenolic acids of irradiated plants increased after a few days and this phenomenon occurred probably due to the immediate oxidation of polyphenolic acids.
Effect of Gamma Irradiation on Ex Vitro Growth and 20HE Production
After four subsequent in vitro subcultures of gamma irradiation, shoots were transferred to paper cups to evaluate the content of 20HE in putative mutants ex vitro. To evaluate the plant height of ex vitro plants of S. portulacastrum, plant height of irradiated shoots was two-fold less than non-irradiated shoots, whereas the concentration of 20HE was found in the increased order at low doses of gamma irradiation. The concentrations of 20HE of the ex vitro plants obtained from non-irradiated shoots produced 0.214 mg/plant dry weight 20HE, whereas the ex vitro plants obtained from irradiated shoots exposed to 20 Gy comprised 0.321 mg/plant dry weight 20HE which was 66.6% more than controls and 30-fold more than that of the M1T1 generation (Fig. 7). From the present results, we have achieved a putative mutant, which contained high amounts of 20HE and large-scale propagation is under shade house. This study revealed that the maximum amount of 20HE was produced by ex vitro shoots irradiated at 20 Gy, which was consistent with the study of Hung and Johnson (2008) who reported that isothiocyanate levels of ex vitro plants from shoots with 20 Gy of gamma rays were significantly greater than those of the plants derived from non-irradiated shoot cultures of Wasabia japonica. Also, Ocimum basilicum mutants obtained by using different doses of gamma radiation showed variation in rosmarinic acid and total protein concentrations (Guirgis and others 2007). Mutagenesis by means of gamma rays has played an important role in constructing putative mutants with improved properties which can produce higher amounts of commercially important metabolites (Sanada 1988). In accordance with our results, it could be suggested that low doses of gamma irradiation are a promising tool to achieve putative mutants comprised with high concentrations of secondary metabolites.
Accumulation of 20-hydroxyecdysone and plant height of 1-month-old putative mutant ex vitro plants obtained from in vitro shoot cultures of Sesuvium portulacastrum in MS medium supplemented with indole-3-acetic acid (0.5 mg L−1), N 6-benzylaminopurine (2 mg L−1) exposed to low doses of gamma irradiation. The observation was obtained from triplicate determinations. These results were representative from three independent replicates (±SE)
Effects of Gamma Irradiation on Survival Rate of In Vitro and Ex Vitro Shoot Cultures
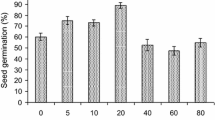
The putative mutants derived from shoot cultures exposed at different doses were acclimatized. The present results revealed that the survival rates of multiple shoots exposed at higher doses of gamma irradiation were gradually reduced in the course of increased order of in vitro generation. Multiple shoots subjected to exposure at various doses of gamma irradiation showed variations in survival rate. The survival rate of irradiated shoots was decreased with increased doses of gamma irradiation. However, all irradiated shoots at various doses showed 100% survival in the M1T1 generation (Fig. 6). Multiple shoots exposed at higher doses in the range from 30 to 40 Gy were not able to survive in the M1T2 generation, whereas shoots subjected to exposure at 5 to 20 Gy exhibited significant survival rates during consequent in vitro generation and ex vitro conditions (Fig. 8). It can be concluded that the impact of higher doses could not be tolerated by shoot cultures for a long time and caused in vitro shoots to become yellow and die. To evaluate the survival rate of ex vitro plants under shade, around 80% of the ex vitro plants originated from 20 Gy survived, whereas 96, 96, 92, and 88% survival percentages of plants were obtained from control, 5, 10, and 15 Gy, respectively.
Survival rate of gamma-irradiated shoots of S. portulacastrum. a In vitro cultured shoots of S. portulacastrum were irradiated at increasing doses of gamma irradiation. b Survival rate of 1-month-old ex vitro plants obtained after exposure of in vitro shoot cultures at low doses of gamma irradiation. The observation was obtained from triplicate determinations. These results were representative from three independent replicates (±SE)
Unlike our evaluation, Sakr and others (2013) reported that 100% in vitro survival of Dracaena surculosa plants was recorded at low doses and the survival rate decreased at high doses of gamma irradiation. Likewise, increasing the intensity of gamma irradiation was found to negatively affect the survivability of callus and plant regeneration (Hossain and Alam 2001) and plantlets of Anubias congensis (Tangpong and others 2009). This study indicated that the survival rate of ex vitro plants increased with decreased doses of gamma irradiation. Similar results have been demonstrated by Kangarasu and others (2014) who reported a reduction in the survival percentage of Manihot esculenta crantz stem cuttings with the increased doses of gamma irradiation.
In conclusion, low doses of gamma irradiation have proven to be feasible, environmentally friendly, and a low-cost technology that can lead to the efficient selection of putative mutants to enhance secondary metabolites. In the present study, a low dose of gamma irradiation was found to be an effective and resourceful tool to improve the yield of ecdysteroid 20-hydroxyecdysone. The LD50 dose based on the percent reduction in survival after exposure with gamma irradiation at different doses was 20 Gy for the in vitro multiple shoot cultures of S. portulacastrum. The content of ecdysteroid 20-hydroxyecdysone was 66% more in ex vitro plants obtained from shoots exposed to 20 Gy than control. Our findings have established a high-yielding putative mutant, which contained high amounts of 20HE and we are presently beginning propagation on a large scale under a shade house for commercial use. These results proved to be encouraging for future research on the selection of putative mutants capable of enhancing the product yield of target compounds of important medicinal plants containing high-value bioactive compounds.
References
Al-Safadi B, Elias R (2011) Improvement of caper (Capparis spinosa L.) propagation using in vitro culture and gamma irradiation. Sci Hortic 127:290–297
Al-Safadi B, Simon PW (1990) The effects of gamma irradiation on the growth and cytology of carrot (Daucus carota L.) tissue culture. Environ Exp Bot 30:361–371
Bajaj YPS (1970) Effect of gamma-irradiation on growth, RNA, protein, and nitrogen contents of bean callus cultures. Ann Bot 34:1089–1096
Bakrim A, Maria A, Sayah F, Lafont R, Takvorian N (2008) Ecdysteroids in spinach (Spinacia oleracea L.): biosynthesis, transport and regulation of levels. Plant Physiol Biochem 46:844–854
Bathori M, Pongracz Z (2005) Phytoecdysteroids-from isolation to their effects on humans. Curr Med Chem 12:153–172
Bathori M, Kalasz H, Csikkelne SA, Mathe I (1999) Components of Serratula species; screening for ecdysteroid and inorganic constituents of some Serratula plants. Acta Pharm Hung 69:72–76
Boo KH, Lee D, Jeon GL, Ko SH, Cho SK, Kim JH, Park SP, Hong Q, Lee SH, Lee DS, Riu KZ (2010) Distribution and Biosynthesis of 20-Hydroxyecdysone in plants of Achyranthes japonica nakai. Biosci Biotechnol Biochem 74:2226–2231
Borzouei A, Kafi M, Khazaei H, Naseriyan B, Majdabadi AA (2010) Effects of gamma radiation on germination and physiological aspects of wheat (Triticum Aestivum L.) Seedlings. Pak J Bot 42:2281–2290
Breitfellner F, Solar S, Sontag G (2002) Effect of gamma irradiation on flavonoids in strawberries. Eur Food Res Technol 215:28–31
Caro-Melgarejo DP, Estupinan-Rincon SY, Rache-Cardenal LY, Pacheco-Maldonado JC (2012) Effect of gamma rays on vegetative buds of Physalis peruviana L. Acta Agron 61:305–314
Chung BY, Lee YB, Baek MH, Kim JH, Wi SG, Kim JS (2006) Effects of low-dose gamma-irradiation on production of shikonin derivatives in callus cultures of Lithospermum erythrorhizon S. Radiat Phys Chem 75:1018–1023
Das A, Gosal SS, Sidhu JS, Dhaliwal HS (2000) Induction of mutations for heat tolerance in potato by using in vitro culture and radiation. Euphytica 114:205–209
Dinan L (2001) Phytoecdysteroids: biological aspects. Phytochemistry 57:325–339
El-Beltagi HS, Ahmed OK, El-Desouky W (2011) Effect of low doses†¯of gamma irradiation on oxidative stress and secondary metabolites production of rosemary (Rosmarinus officinalis L.) callus culture. Radiat Phys Chem 80:968–976.
Encheva J, Ivanov P, Tsvetkova F, Nikolova V (1993) Development of a new initial breeding material in sunflower (Helianthus annuus L.) using direct organogenesis and somatic embryogenesis. Euphytica 68:181–185
Fujimoto Y, Ohyama K, Nomura K, Hyodo R, Takahashi K, Yamada J, Morisaki M (2000) Biosynthesis of sterols and ecdysteroids in Ajuga hairy roots. Lipids 35:279–288
Fulzele DP, Heble MR, Rao PS (1995) Production of terpenoid from Artemisia annua L. plantlet cultures in bioreactor. J Biotechnol 40:139–143
Fulzele DP, Satdive RK, Kamble SN, Singh SA, Singh S (2015) Improvement of anticancer drug camptothecin production by gamma irradiation on callus cultures of Nothapodytes foetida. Intl J Pharm Res Allied Sci 4:19–27
Gaube AF, Wolfl S, Pusch L, Werner U, Kroll TC, Schrenk D, Hartmann RW, Hamburger M (2008) Effects of Leuzea carthamoides on human breast adenocarcinoma MCF-7 cells determined by gene expression profiling and functional assays. Planta Med 74:1701–1708
George L, Rao PS (1980) In vitro regeneration of mustard plants (Brassica juncea var. RAI-5) on cotyledon explants from non-irradiated, irradiated and mutagen-treated seed. Ann Bot 46:107–112
Gloria MBA, Adao RC (2013) Effect of gamma radiation on the ripening and levels of bioactive amines in bananas cv. Prata. Radiat Phys Chem 87:97–103
Gorelick-Feldman J, MacLean D, Ilic N, Poulev A, Lila MA, Cheng D, Raskin I (2008) Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J Agric Food Chem 56:3532–3537
Graf BL, Poulev A, Kuhn P, Grace MH, Lila MA, Raskin I (2014) Quinoa seeds leach phytoecdysteroids and other compounds with anti-diabetic properties. Food Chem 163:178–185
Guirgis AA, El-Kawi MAA, Abbas HN, Araffa AMS, Maksoud AI (2007) High rosmarinic acid content in induced mutants and in in vitro elicited sweet basil (Ocimum basilicum L.) callus. Asian J Plant Sci 6:1058–1064
Hanan HL, Abdalla MA, Farag SA (2011) Radio-stimulation of phytohormons and bioactive components of Coriander seedlings. Turkish. J Biochem 36:230–236
Hang NTN, Chau NM (2010) Radiation induced mutation for improving papaya variety in Vietnam. Acta Hortic 851:77–80
Hasbullah NA, Taha RM, Saleh A, Mahmad N (2012) Irradiation effect on in vitro organogenesis, callus growth and plantlet development of Gerbera jamesonii. Hortic Bras 30:252–257
Ho R, Teai T, Meybeck A, Raharivelomanana P (2015) UV-protective effects of phytoecdysteroids from Microsorum grossum extracts on human dermal fibroblasts. Nat Prod Commun 10:33–36
Hossain MF Alam MS (2001) Effect of gamma irradiation on the callus developed from India Rice. Pak J Bio Sci 4:670–671
Hung CD, Johnson K (2008) Effects of ionizing radiation on the growth and allyl isothiocyanate accumulation of Wasabia japonica in vitro and ex vitro. In Vitro Cell Dev Biol-Plant 44:51–58.
Jaisi A, Sakunphueak A, Panichayupakaranant P (2013) Increased production of plumbagin in Plumbago indica root cultures by gamma ray irradiation. Pharm Biol 51:1047–1051
Kangarasu S Ganeshram S John Joel A (2014) Determination of lethal dose for gamma rays and ethyl methan sulphonate induced mutagenesis in Cassava (Manihot esculenta crantz). Int J Sci Res 3:2–6
Kayani WK, Rani R, Ihsan-Ul-Haq, Mirza B (2014) Seasonal and geographical impact on the morphology and 20-hydroxyecdysone content in different tissue types of wild Ajuga bracteosa Wall. ex Benth. Steroids 87:12–20
Keshan B, Ray AK (2000) Estradiol-1 in Bombyx mori: Possible significance and its effect on silk production. J Insect Physiol 46:1061–1068
Khalil SA, Ahmad N, Zamir R (2015) Gamma radiation induced variation in growth characteristics and production of bioactive compounds during callogenesis in Stevia rebaudiana (Bert). New Negat Plant Sci 1:1–5
Kim JH, Baek MH, Chung BY, Wi SG, Kim JS (2004) Alterations in the photosynthetic pigments and antioxidant machineries of red pepper (Capsicum annuum L.) seedlings from gamma-irradiated seeds. J Plant Biol 47:314–321
Kim JH, Chung BY, Kim JS, Wi SG (2005) Effects of gamma-irradiation on growth, photosynthesis, and antioxidative capacity of red pepper (Capsicum annuum L.) plants. J Plant Biol 48:47–56
Kim DS, Song M, Kim SH, Jang DS, Kim JB, Ha BK, Kim SH, Lee KJ, Kang SY, Jeong IY (2013) The improvement of ginsenoside accumulation in Panax ginseng as a result of gamma-irradiation. J Ginseng Res 37:332–340
Kiong ALP, Lai AG, Hussein S, Harun AR (2008) Physiological responses of Orthosiphon stamineus plantlets to gamma irradiation. Am-Eurasian J Sustain Agric 2:135–149
Kokoska L, Janovska D (2009) Chemistry and pharmacology of Rhaponticum carthamoides: a review. Phytochemistry 70:842–855
Kovacs E, Keresztes A (2002) Effect of gamma and UV-B/C radiation on plant cells. Micron 33:199–210
Kumari V, Chaudhary HK, Prasad R, Kumar A, Jambhulkar S, Sharma S (2013) Effect of gamma radiations on in vitro regeneration in Brassica carinata A. Braun. Int J Sci Res Publ 3:1–6
Lafont R, Dinan L (2003) Practical uses for ecdysteroids in mammals including humans: an update. J Insect Sci 3:7
Larson RA (1988) The antioxidants of higher plants. Phytochemistry 27:969–978
Ling A, Kiong P, Pick A, Ling K, Chia JY, Hussein S, Harun AR (2008) Physiological Responses of Citrus sinensis to gamma irradiation. World Appl Sci J 5:12–19
Ling A, Ong A, Hussein S, Harun A (2010) Morphological and physiological responses ofOrthosiphon stamineuscallus to gamma irradiation at different doses. World J Agri Sci 6:58–66
Mohajer S, Taha RM, Lay MM, Khorasani Esmaeili A, Khalili M (2014) Stimulatory effects of gamma irradiation on phytochemical properties, mitotic behavior, and nutritional composition of sainfoin (Onobrychis viciifolia Scop.). Sci World J 2014:1–9
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:475–479
Oloriz MI, Gil V, Rojas L, Veita N, Hofte M, Jimnez E (2011) Selection and characterisation of sugarcane mutants with improved resistance to brown rust obtained by induced mutation. Crop Pasture Sci 62:1037–1044
Pius J, George L, Eapen S, Rao PS (1994) Evaluation of somaclonal and mutagen induced variation in Fingermillet. Plant Breed 112:239–243
Rohani O, Samsul Kamal R, Rajinder S, Mohd-Nazir B (2012) Mutation induction using gamma irradiation on oil palm (Elaeis guineensis Jacq) cultures. J Oil Palm Res 24:1448–1458
Rufaie ZH, Munshi NA, Sharma RK, Ahmed K, Malik GN, Raja TA (2011) Occurrence of insect moulting hormone (β-ecdysone) in some locally available plants. Int J Adv Biol Res 2:104–107
Rufaie ZH, Baqual MF, Sharma RK, Nissar A, Ganie Mir MR (2015) Use of phytoecdysteroid (β-ecdysone) as a crop saver in sericulture industry. Int J Sci Nat 6:147–150
Sakr SS, El-Khateeb MA, Taha HS, Esmail SA (2013) Effect of gamma irradiation on in vitro growth chemical composition and anatomical structure of Dracaena surculosa L. J Appl Sci Res 9:3795–3801
Sanada T, Nishida T, Ikeda F (1988) Resistant mutant to black spot disease of Japanese pear “Nijisseiki” induced by gamma rays. Gamma Field Symposia 25:87–108
Schmelz EA, Grebenok RJ, Ohnmeiss TE, Bowers WS (2000) Phytoecdysteroid turnover in spinach: long-term stability supports a plant defense hypothesis. J Chem Ecol 26:2883–2896
Sipahimalani AT, Banerji A, Chadha MS (1972) Biosynthesis and interconversion of phytoecdysones in Sesuvium portulacastrum L. J Chem Soc Chem Commun 11:692–693
Smelkova L (1999) Effect of gamma rays on the germination of conifer seeds. Acta Fac For Zvolen Slovak 41: 81–90
Snogan E, Vahirua Lechat I, Ho R, Bertho G, Girault JP, Ortiga S, Maria A, Lafont R (2007) Ecdysteroids from the medicinal fern Microsorum scolopendria (Burm. f.). Phytochem Anal 18:441–450
Soriano IR, Riley IT, Potter MJ, Bowers WS (2004) Phytoecdysteroids: a novel defense against plant-parasitic nematodes. J Chem Ecol 30:1885–1899
Srivastava K, Upadhyay VB (2013) Effect of phytoecdysteroid on silk producing potential of multivoltine mulberry silkworm Bombyx mori L. Bioscan 8:43–47
Sun JY, Tu JD, Fan SW, Wu JG, Shi CH (2007) The screening of mutants induced by physical and chemical factors and construction of mutant population for Brassica napus L. Yi Chuan 29: 475–82
Tangpong P, Taychasinpitak T, Jompuk C, Jompuk P (2009) Effects of acute and chronic gamma irradiations on in vitro culture of Anubias congensis N.E. Brown. Kasetsart J Nat Sci 43:449–457.
Toth N, Szabo A, Kacsala P, Heger J, Zador E (2008) 20-Hydroxyecdysone increases fiber size in a muscle-specific fashion in rat. Phytomedicine 15:691–698
Trivedy K, Nirmal Kumar S, Dandi SB (2006) Phytoecdysteroid and its use in sericulture. Sericologia 46:57–78
Tshilenge-Lukanda L, Kalonji-Mbuyi AC, Nkongolo KK, Kizungu RV (2013) Effect of gamma irradiation on morpho-agronomic characteristics of groundnut. Am J Plant Sci 04:2186–2192
Urbain MW (1996) Fruits vegetables and nuts In Schweigert BS (ed) Food irradiation, Academic Press Inc., Orlando p 170–216
Venkateshwarlu M (2008) Effect of gamma rays on different explants of callus treatment of multiple shoots in Cucumis melo cv. Bathasa. J Environ Biol 29:789–792
Wi SG, Chung BY, Kim JH, Baek MH, Yang DH, Lee JW, Kim JS (2005) Ultrastructural changes of cell organelles in Arabidopsis stems after gamma irradation. J Plant Biol 48:195–200
Wi SG, Chung BY, Kim JS, Kim JH, Baek MH, Lee JW, Kim YS (2007) Effects of gamma irradiation on morphological changes and biological responses in plants. Micron 38:553–564
Acknowledgements
We thank for the grant offered by Board of Research in Nuclear Sciences, Mumbai, India (2013/35/17/BRNS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kapare, V., Satdive, R., Fulzele, D.P. et al. Impact of Gamma Irradiation Induced Variation in Cell Growth and Phytoecdysteroid Production in Sesuvium portulacastrum . J Plant Growth Regul 36, 919–930 (2017). https://doi.org/10.1007/s00344-017-9697-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-017-9697-3