Abstract
Xylose reductase (XR) is an intracellular enzyme, which catalyzes xylose to xylitol conversion in the microbes. It has potential biotechnological applications in the manufacture of various commercially important specialty bioproducts including xylitol. This study aimed to prepare XR from adapted strain of Candida tropicalis and to characterize it. The XR was isolated from adapted C. tropicalis, cultivated on Meranti wood sawdust hemicellulosic hydrolysate (MWSHH)-based medium, via ultrasonication, and was characterized based on enzyme activity, stability, and kinetic parameters. It was specific to NADPH with an activity of 11.16 U/mL. The enzyme was stable at pH 5–7 and temperature of 25–40 °C for 24 h and retained above 95 % of its original activity after 4 months of storage at −80 °C. The K m of XR for xylose and NADPH were 81.78 mM and 7.29 μM while the V max for them were 178.57 and 12.5 μM/min, respectively. The high V max and low K m values of XR for xylose reflect a highly productive reaction among XR and xylose. MWSHH can be a promising xylose source for XR preparation from yeast.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Xylose reductase (XR) is a member of the aldose reductase family (EC 1.1.1.21) of enzymes and is an oxidoreductase enzyme generally found in yeast and fungi. This intracellular enzyme occurs in the cytoplasm of xylose-assimilating microbes, where it mediates the first step of xylose metabolism by reducing xylose to xylitol with the concomitant oxidation of NAD(P)H [1, 2]. XR has widespread applications in the bio-based production of ethanol, xylitol, and sorbitol from xylose [3, 4], which make the enzyme a focus of interest. The use of high-priced commercial xylose limits the large-scale production of XR as well as its industrial application for manufacturing xylitol and other value-added bioproducts. This issue has encouraged the authors to work toward the development of improved techniques to lower the costs of XR preparation. Hence, the utilization of lignocellulosic hydrolysate as a source of carbon for XR preparation from yeasts can be interesting from an economic viewpoint.
Xylose, an aldopentose, is a predominant sugar of hemicellulose, which represents one third of the polysaccharide contents of the renewable plant biomass. Meranti wood sawdust (MWS), a lignocellulosic biomass (LCB), is a sawmill waste that is available at low cost throughout the year. The MWS contained high amount of hemicellulose (30.64 %), which makes this biomass adequate for xylose extraction [5, 6]. The hemicellulose part of MWS is easily and selectively hydrolyzed by dilute acid to produce xylose-rich MWS hemicellulosic hydrolysate (MWSHH) that can be used as a potential substrate for microbial growth and for the production of various specialty chemicals, mainly xylitol. The use of MWSHH as carbon and energy source for the cultivation of organisms has a dual benefit: the reduction of commercial xylose utilization and the preparation of industrially important enzyme XR that enhances the economy of the bioprocess. Xylitol, a naturally occurring sugar alcohol, is a functional sweetener that has raised global demand because of its potential application in the pharmaceutical, food, health, and cosmetic sectors [7]. It is mainly used as an ideal sweetener for people suffering from diabetes, obesity, and glucose-6-phosphate dehydrogenase deficiency because of its insulin-independent metabolism and low calories [7, 8]. It is also used commercially as a natural sweetener in various food products (e.g., chewing gum, ice cream, and bakery products). XR is not commercially available despite a large number of reports found in the literature on the important use of this enzyme, as well as a description of downstream processing to separate it from yeasts [3, 4].
Yeast under the genus Candida is still regarded as the best source of XR among the microorganisms. As a result, XR from Candida yeast has been isolated and characterized [1, 2, 4, 9–12]. XR usually prefers NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) as coenzyme (monospecific XR; msXR) [12], but in some cases, it utilizes both NADH and NADPH (dual specific XR; dsXR) [9, 13]. The variation in coenzyme specificities is proposed to regulate the redox potential balance among nicotinamide coenzymes under different growth conditions of yeasts [10]. Most of the XR is monomeric, but the enzymes from some xylose-fermenting yeasts consist of two identical [3, 14]. In this study, XR was isolated from adapted yeast Candida tropicalis because it is one of the most efficient xylitol producers [15, 16]. The biochemical properties of XR in yeasts vary between species and strains. Therefore, it is crucial to produce and characterize XR from the potential yeast in order to explore its maximum efficiency on xylose bioconversion. Besides, the determination of XR properties is necessary to understand its relative roles in the bioconversion of xylose to xylitol. The use of XR may offer an economic interest over the chemical and microbial production of xylitol. Although XR from different yeasts was characterized, the enzyme has not yet been prepared and studied from adapted yeast. This study focused on the XR preparation from adapted strain of C. tropicalis cultivated on MWS hydrolysate medium and the biochemical characterization of the enzyme.
Materials and Methods
Preparation of MWS Hydrolysate
The wood biomass, MWS, was collected from a local sawmill (Seng Peng Sawmills Sdn Bhd, Malaysia), and its sample was prepared for subsequent hydrolysis. MWS hydrolysis was carried out at 124 °C with 3.26 % (w/w) H2SO4 for 80 min using a solid to liquid ratio (w/w) of 1:8 based on the results of previous studies [5, 6]. The solid and liquid phases were separated by filtration, and the resulting filtrate was named as MWSHH. The MWSHH was neutralized with CaO powder to pH 6, and its composition was determined by analytical methods. It was stored at 4 °C and used in subsequent adapted yeast cultivation and XR preparation experiments.
Microorganism, Media, and Maintenance
The microorganism, C. tropicalis IFO 0618, used in the current research was obtained from the ATCC, USA. The yeast strain was incubated on yeast extract peptone dextrose (YPD) agar plate at 30 °C for 30 h and maintained at 4 °C. The YPD agar medium has the following composition [12]: 2 % glucose, 0.5 % yeast extract, 0.5 % peptone, 0.1 % KH2PO4, 0.5 % MgSO4·7H2O, and 2 % agar (w/v) in ultrapure water. The synthetic growth medium (SGM) composed (%, w/v) of three xylose, 0.3 yeast extract, 0.3 K2HPO4, and 0.1 MgSO4·7H2O in water [17]. The YP-hydrolysate (YPH) agar medium was prepared from MWSHH containing 1.88 % xylose instead of using glucose, and the rest of the media ingredients were the same as the YPD agar medium as mentioned above. The pH of all the media used was adjusted to 6 with 1 M HC1 before autoclaving at 120 °C and 15 psi for 20 min.
Preparation of Adapted C. tropicalis
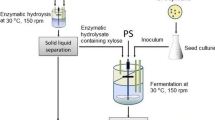
The adapted strain of C. tropicalis was prepared by sequentially transferring and growing cells in media containing increasing concentrations (10 to 100 % (v/v)) of MWSHH supplemented with (%, w/v) required amount of xylose, 0.3 yeast extract, 0.3 K2HPO4, and 0.1 MgSO4·7H2O to yield the same composition used for SGM. Cells were collected by centrifugation at 4,000 rpm (Eppendorf centrifuge 5810 R, Germany) for 10 min at 4 °C and then resuspended in fresh hydrolysate growth medium (HGM). Six successive batch cultures were carried out with HGM at 30 °C for 24 h at 150 rpm to obtain adapted C. tropicalis. Adapted strains were maintained on plates made from YPH agar medium and utilized in subsequent experiments.
Inoculum Preparation and Culture Conditions
To prepare inoculum, a single colony of adapted C. tropicalis (grown at 30 °C for 36 h on YPH agar) was transferred to an Erlenmeyer flask (250 mL) containing 50 mL of HGM (1.88 % xylose, 0.3 % yeast extract, 0.3 % K2HPO4, and 0.1 % MgSO4·7H2O). The inoculated flask was incubated at 30 °C in an incubator shaker for 24 h at 150 rpm.
Growth Profile of Adapted C. tropicalis
A 10 % inoculum of adapted C. tropicalis (3.34 ± 0.40 g/L CDW) from a 24 h culture was aseptically added to the HGM for starting growth profile experiments. The cells were continuously cultivated in Erlenmeyer flasks (1 L) containing 250 mL of culture at 30 °C and 150 rpm. The cell growth was observed by measuring culture absorbance and cell dry weight (CDW). For growth analysis, 5 mL culture was withdrawn at various time intervals. The absorbance of cell culture was measured at 600 nm (A 600) in a quartz cuvette against a blank consisting of sterile medium with a UV–vis spectrophotometer. The initial concentration of inoculum in all experiments was 0.42 ± 0.05 g/L CDW, which corresponds to a culture turbidity of 0.60 ± 0.06. Cell fresh weight (CFW) was measured from 20 h cultured broth by centrifugation at 8,000 rpm for 20 min. The centrifuge tubes were preweighed, and after centrifugation, the supernatant was decanted, and the pellet weight was calculated. The correlation between xylose consumption and cell biomass production was determined. Unless otherwise stated, all experiments were done in triplicate and mean values ± standard deviations (SD) were recorded throughout the article. The error bars represent the respective SDs of the mean values.
Determination of Cell Dry Weight
The CDW was determined by dry weight method as described hereafter. To prepare filter disk, the glass filter (Grade GF/C, Whatman) was placed on an aluminum weighing dish as a support and dried in an oven at 105 °C for 1 h. The heated dish with filter disk was cooled in desiccators for 30 min, weighed before the experiment, and recorded as the initial weight of the dish and filter (W 1). The disk was inserted in filtration apparatus, placed the marked weighing dish to the side, and then, suction was begun by a vacuum pump. The cell suspension was mixed well and pipetted 5 mL of it onto the seated glass filter. The filter was washed with three successive double volumes of sterile distilled water. The filter was carefully removed from filtration apparatus and transferred to the marked dish. Then, the dish was dried at 105 °C for 12 h. After the drying, dish was removed from oven and cooled as before. Weighed the dish again along with filter disk and recorded as the final weight of the dish and filter disk plus dried cells (W 2). The CDW was calculated by Eq. (1) and was expressed as g/L.
where W 1 is the weight of dish and filter disk (g); W 2 is the weight of dish and filter disk + dried cell (g), and V is the volume of cell culture used (mL).
XR Preparation
Ten percent (v/v) of the adapted C. tropicalis inoculum (24 h; 3.34 g/L CDW) was introduced into loosely cotton plugged flask (1 L) containing 250 mL of HGM and incubated as described before. Cells were collected at the end of the log growth phase (20 h; 4.87 ± 0.39 g/L CDW) by centrifugation at 8,000 rpm for 20 min at 4 °C. The cell pellet was rinsed with ice-cold potassium phosphate buffer (0.1 M, pH 7) and centrifuged. The rinsed pellet was resuspended in the required volume of same buffer resulting in a CFW to buffer ratio (w/v) of 1:2. The cell suspension was submitted to disruption with a cell homogenizer (Omni Ruptor 4000, USA) by 20-kHz sonication at pulsing/resting cycles of 1 s for 45 min [4]. The resulting cell homogenate was then centrifuged as before to obtain a supernatant solution. For further clarification, the supernatant was centrifuged again at 12,000 rpm for 30 min. All operations were done on ice during cell disruption. The refined supernatant was stored at −80 °C and used as crude enzyme.
XR Activity Determination
XR activity was assayed by measuring the amount of NADPH oxidized after a timed reaction coupled with the reduction of xylose to xylitol in a quartz cuvette (1 cm path) at 25 °C following the method outlined by Yokoyama et al. [12]. The reaction mixture contained 0.2 mL of 0.1 M phosphate buffer (pH 7), 0.2 mL of 0.1 M 2-mercaptoethanol (2-ME), 0.1 mL crude XR, 0.1 mL of 3.4 mM NADPH, and 1.2 mL of sterile water. 2-ME was added in the reaction mixture to inhibit protease activity if present in the XR or reaction mixture. The reaction mix was allowed to stand for 1 min, and then, the reaction was initiated by adding 0.2 mL of 0.5 M D-xylose. The preboiled XR was added to the control instead of live XR. The rate of NADPH oxidation was measured at 340 nm by UV–vis spectrophotometer at 1 min intervals for 5 min. One unit (U) of XR is defined as the amount of enzyme required to catalyze the oxidation of 1 μmol of NADPH per min at pH 7 and 25 °C. The activity of XR was determined using the extinction coefficient of NADPH through Eqs. (2) and (3) [18] and expressed as U/mL. Specific activity was expressed as units of the enzyme per milligram of protein.
where ΔA 340/min is the rate of decrease in absorbance at 340 nm per min; V a is the volume of assay (mL); V e is the volume of enzyme (mL), DF is the dilution factor, and E mM is the millimolar extinction coefficient of NADPH (6.22 mM−1 cm−1).
Optimum Temperature and pH of XR
The optimum temperature for the activity of crude XR was calculated by incubating the reaction mixture at different temperatures ranging from 20 to 80 °C for 5 min at pH 7. The optimum pH of the enzyme was determined by the XR activity assay in the following buffers (0.1 M): sodium acetate (pH 4–5), potassium phosphate (pH 6–8), and glycine-NaOH (pH 9–10) at 25 °C for 5 min. The XR assay procedure mentioned before was essentially followed during the study of pH and temperature effects on the enzyme.
Thermal, pH, Incubation Period, and Storage Stability of XR
Thermal stability of XR was examined by treating 0.1 mL of the enzyme with 0.2 mL of 0.1 M potassium phosphate buffer (pH 7) at different temperatures ranging from 20 to 80 °C for 1 h without substrate and then brought back to 25 °C. The pH stability was determined by incubating 0.1 mL of XR with 0.2 mL of different buffers at different pHs (4–10) at 25 °C for 1 h. To assess the impact of incubation period on the enzyme stability, 0.1 mL of XR was mixed with 0.2 mL of phosphate buffer (0.1 M, pH 7). The resulting enzyme mix was incubated at 25 °C without substrate for various incubation periods ranging from 1 to 48 h before the standard assay to determine XR activity. In all cases, the residual activity was measured by the standard assay method at 25 °C and pH 7. The relative activity of the thermal, pH, and incubation period-treated enzyme was calculated by comparing the activity with that of without thermal, pH, and incubation period treatment, respectively. To analyze the storagability, XR was prepared in phosphate buffer and stored at −80 °C in screw capped vials. The remaining activity of XR was monitored with storage time varying from 30 to 150 days following the standard assay method. The original activity of XR was regarded as a standard to calculate the relative activity, which was defined by (remaining activity) / (original activity) × 100.
Kinetic Parameters of XR
The kinetic parameters of XR namely K m and V max were measured to examine their impact on the production of xylitol. They were calculated using the Michealis-Menten (M-M) equation and Lineweaver-Burk (L-B) method [19]. The rate of xylitol production, V (μM/min), versus initial substrate (xylose or NADPH) concentration, [S] (mM or μM), was fitted to the following M-M equation (Eq. (4)):
where K m is the M-M constant (mM) and V max is the maximum reaction rate (μM/min). The V max value corresponds to the condition where all enzyme molecules are saturated with its substrate. K m defines the concentration of substrate at which the reaction velocity is half of its maximum value. The K m represents the affinity between enzyme and substrate. A lower K m value of enzyme indicates a higher affinity for the substrate. The M-M equation was further rearranged to Eq. (5), according to which a linear plot between 1/V and 1/[S] was attempted. This double reciprocal relationship is called L-B plot. The L-B equation is used to construct a straight line with a slope K m/V max, and an intercept on the y-axis is equal to 1/V max. The values of intercept and slope were measured by linear regression.
To determine the kinetic constants of XR-catalyzed reduction of xylose, a series of initial reaction rate (V) measurements were performed at a fixed and saturating concentration of coenzyme NADPH (300 μM) while the substrate xylose was varied over a range of 40–400 mM, and again, at a constant and saturating concentration of xylose (280 mM), the coenzyme was varied from 15 to 150 μM according to the method described by Fernandes et al. [20]. The reaction mixture had a total volume of 2 mL. Kinetic experiments were conducted at defined reaction conditions in 0.1 M K-phosphate buffer (pH 6.5) employing 3 % (v/v) of XR. After adding and mixing the last component of the reaction, a 100 μL mixture representing 0 h reaction was withdrawn and boiled in a water bath for 5 min to stop the reaction and stored at −20 °C until analysis. The remaining assay mixture (1.9 mL) was incubated for 12.25 h at 35 °C with agitation at 100 rpm. The control contained 60 μL (3 %) of a 5 min preboiled XR in place of native XR. At the end of the incubation period, all samples were boiled for 5 min and then frozen at −20 °C. The reaction samples were analyzed by HPLC for xylitol produced from xylose.
Analytical Methods
Xylose, glucose, acetic acid, arabinose, furfural, hydroxymethylfurfural (HMF), and xylitol concentrations were determined by HPLC, and lignin degradation products (LDPs) were estimated spectrophotometrically as described earlier by the authors [5, 21]. The total protein in the enzyme samples was determined following the Lowry method [22] using bovine serum albumin as a standard, and the absorbance was recorded at 750 nm.
Results and Discussion
Composition of MWSHH
The optimum conditions to obtain a xylose-rich hemicellulosic hydrolysate from hydrolysis of MWS were 124 °C, 3.26 % H2SO4, 80 min, and 1:8 (w/w) solid/liquid ratio [6]. Table 1 shows the composition of MWSHH. The MWSHH contained 18.8 g/L xylose, together with a low content of glucose, acetic acid, arabinose, LDPs, furfural, and HMF.
Growth Profile of Adapted C. tropicalis
A typical growth curve of adapted C. tropicalis was constructed to obtain cells from the exponential growth phase. Sampaio et al. [23] pointed out that yeast cells contain the highest protein content at its log growth phase. To determine growth curve of adapted yeast in terms of absorbance (A 600) and CDW as a function of time, cells were cultivated continuously at 30 °C and 150 rpm. The growth profile of adapted C. tropicalis cultivated on HGM is shown in Fig. 1. It was revealed that the curves for A 600 and CDW were of the same pattern (Fig. 1). It was also revealed that the growth curve of the adapted yeast strain in the HGM showed the standard pattern of lag, exponential, stationary, and death phases. The lag phase defined as the time of inoculation of the yeast strain in the culture medium with a negligible increase in cell density. During this time, C. tropicalis cells were adapted with their new environment (e.g., temperature, pH, and nutrients). A period of 2 h was required for acclimation of the strains with an initial CDW and A 600 values of 0.42 ± 0.05 g/L and 0.60 ± 0.06, respectively, and further cultivation led to an exponential growth phase (Fig. 1). Cells became highly active after 2 h, and they fully utilized the available nutrients including xylose. This phase is called exponential or log phase because during this period of time, the strains were growing and dividing at a maximum possible rate provided by their genetic potential, the nature of the medium, and the cultivation conditions. During this phase, the cells were divided and doubled in number at regular intervals. It has been reported that the yeast population demonstrates uniform chemical and physiological properties at its log phase [23].
The exponential phase lasted between 3 and 20 h, and the values of CDW and A 600 were found to be 4.87 ± 0.39 g/L and 11.98 ± 0.89, respectively, at 20 h of incubation. The exponential period is followed by the stationary phase during which the values of CDW and A 600 were fixed, and the growth curves become horizontal. In this phase, the total number of viable cells remained static. This resulted from a balance between cell division and death or simply due to cessation of proliferation though remaining metabolically active. The stationary phase was observed from 21 to 24 h with the constant values of CDW and A 600 and (4.88 ± 0.39 g/L and 11.99 ± 0.88, respectively). Death phase is the last phase of microbial growth profile. This phase began after 24 h, and the CDW and A 600 values reduced with incubation time. During this phase, the overall number of cells decreases, so the death rate is greater than the birth rate. The study on growth profile was carried out to see if there is any change in their growth pattern during adaptation of the yeast strain to hemicellulosic hydrolysate-based medium. The relationship among CDW and A 600 is CDW (g/L) = 0.421 (A 600) + 0.132. An A 600 value of 1 resulted 0.55 g/L CDW (i.e., 1 A 600nm = 0.55 g/L CDW) after linear correlation between CDW and A 600. It was observed that the xylose consumption was 71 % (13.35 g/L) of its initial content (18.8 g/L) at 20 h of incubation (the residual xylose in the medium was 29 %) and gave 4.87 g/L CDW (Fig. 1). Hence, the cell dry biomass production per gram of xylose was calculated to be 0.36 g/g. It is highlighted that C. tropicalis yeast was able to grow on medium containing crude MWSHH as carbon source, and thus, HGM was used as the cell biomass production medium.
XR Activity Determination
The XR was biochemically characterized based on enzyme activity, stability, and kinetic parameters. It was observed that the isolated XR was completely specific to NADPH coenzyme for its activity, and the activity of XR for NADH was not detectable. These findings are in agreement with the previous reports that the NADPH-dependent XRs exhibited no activity with NADH in different yeasts under Candida genus [4, 12]. The activity of NADPH-dependent crude XR determined was 11.16 U/mL with the corresponding specific activity of 0.91 U/mg. It was pointed out that XR in the ethanol-producing yeasts (e.g., Pichia stipitis and Candida shehatae) is specific for both NADPH and NADH and XR in the xylitol-producing yeasts (e.g., C. tropicalis and Kluyveromyces fragilis) is specific for NADPH [11, 16]. It was also pointed out that the xylose-assimilating yeast Candida intermedia produces two isoforms of XR: one is NADPH-dependent and another preferred NADH about 4-folds over NADPH [10]. It is important to pay much attention on the maintenance of catalytic function of XR including its activity and stability. The results obtained from the analyses of XR activity and stability, and kinetic parameters, are described below.
Effect of Temperature on XR Activity and Stability
The outcomes of XR activity and stability at various temperatures are depicted in Fig. 2. It was observed that the optimum temperature for XR was 25 °C with xylose as substrate. A number of studies have been carried out on XR from C. intermedia [9], and Candida guilliermondii [4, 24] also discovered 25 °C as the suitable temperature, similar with XR isolated from adapted C. tropicalis. The XR from P. stipitis possessed a higher optimum temperature at 38 °C [11]. The effect of temperature on XR stability was further investigated by monitoring the activity after treatment at different temperatures for 1 h. It was also observed that the XR was fully active and stable up to 40 °C at pH 7 (Fig. 2). However, the enzyme lost 34.45 % of its original activity at 50 °C (retained 65.55 % relative activity) and retained only 18.26 % of its activity at 60 °C. The activity of XR was fully lost at 80 °C probably be due to the thermal denaturation of the enzyme.
XR activity and stability as affected by several temperatures (20–80 °C) at pH 7. The residual XR activity was measured at 25 °C, and the relative activity was calculated by comparing the activity with that of without thermal treatment. The XR activity, 11.16 U/mL, obtained at 25 °C and pH 7 was taken as 100 %. The error bars indicate the standard deviations of the mean values
Effect of pH on XR Activity and Stability
Optimum pH was determined by monitoring XR activities following the standard assay procedure within the pH range from 4 to 10 at 25 °C. The optimum pH and pH stability of XR are illustrated in Fig. 3. The adapted C. tropicalis XR exhibited optimum activity against xylose at pH 7 that is in accordance with XRs isolated from Candida parapsilosis [1] and C. intermedia [10]. It was found that XR exhibited negligible activity at pH 4 (6.12 % of the original activity) and 9 (only 4.34 %). This result reflected that the XR needs a near-neutral pH range to catalyze its reactions, and a higher pH was not favorable for the enzyme to conduct reduction–oxidation reactions. It is reported that most of the XRs showed optimum pH ranging from 5 to 7 [2]. At extreme pH (>7) condition, XR activity gradually declined, and at pH 8, the activity was 24.11 % of the original value (11.16 U/mL) (Fig. 3). The decrease in activity is assumed to be due to the limited ionization of groups involved in substrate binding and catalysis or in enzyme–coenzyme complex formation [10]. Moreover, extreme pH may cause partial denaturation of the enzyme. The enzyme completely lost its activity at pH 10 (100 % of original activity) perhaps due to the enzyme inactivation. The activity, 11.16 U/mL, obtained at 25 °C and pH 7 was taken as 100 %. The stability of enzyme affected by pH is important as it leads the enzyme to undergo reversible or irreversible structural changes under severe pH conditions. The crude XR remained stable within pH 6–7 at 25 °C and lost most of the activity when incubated at pH 4 and 8, retaining only 3.1 and 8.32 % (Fig. 3), respectively. It was seen that XR became fully inactive while incubated at pH 9–10.
XR activity and stability as affected by various pH values ranging from 4 to 10 at 25 °C. The residual XR activity was measured at pH 7, and the relative activity was calculated by comparing the activity with that of without pH treatment. The XR activity, 11.16 U/mL, obtained at 25 °C and pH 7 was taken as 100 %. The error bars indicate the standard deviations of the mean values
Effect of Incubation Period and Storage Time on XR Activity and Stability
The impacts of incubation period and storage time on XR activity and stability were explored, and the results are presented in Fig. 4. The effect of incubation period on XR stability was evaluated by measuring the residual activity at various intervals (at 25 °C and pH 7) and expressed as relative activity after incubation at different periods of time (1–48 h). The XR did not lose any activity up to 24 h of incubation, and its activity gradually decreased with further increase in incubation period (>24 h) and reduced drastically at 48 h of incubation, retaining only 19.33 % relative activity (Fig. 4a). This decline in enzyme activity and stability during incubation might be attributed to protein denaturation or to degradation by proteases present in the crude XR extract. The storagability of XR was analyzed up to 150 days (5 months), and its remaining activity was measured at 30 day intervals. The XR remained stable at −80 °C for 90 days, and there was a slight decrease in activity (4.75 % of the original activity) after 120 days of storage (Fig. 4b). This might be due to the chilling effect, a usual phenomenon for protein.
XR activity and stability as affected by different a incubation periods at 25 °C and pH 7 [the residual activity was measured at 25 °C and pH, and the relative activity was calculated by comparing the activity with that of without incubation treatment], b storage time [the remaining XR activity was measured at 25 °C and pH 7, and the relative activity was defined by (remaining activity) / (original activity) × 100]. The XR activity, 11.16 U/mL, obtained at 25 °C and pH 7 was taken as 100 %
Kinetic Parameters of XR
The values of kinetic parameters K m and V max were determined via the L-B plot at defined reaction conditions (at 12.25 h, 35 °C, pH 6.5, 100 rpm, and 3 % XR) for both xylose and NADPH. The kinetic parameters obtained from the L-B plot are presented in Fig. 5. The obtained velocities yielded straight converging lines that intersected to the left of the ordinate for the double-reciprocal plot of initial velocity against varied substrate concentration (Fig. 5a, b). These findings implied that the M-M constant (K m) for one substrate is dependent on the concentration of the other substrate. The K m values of XR for xylose and NADPH were 81.78 mM and 7.29 μM with the corresponding V max for xylose and NADPH of 178.57 and 12.5 μM/min, respectively. The K m for xylose and NADPH obtained in this work are consistent with other researchers [11, 13] who reported those ranging from 14 to 72 mM and 4.8–21 μM, respectively. It is noticeable that higher V max and lower K m of XR for xylose are associated with the greater xylitol production. The higher value of V max proved that this reaction would produce a higher concentration of xylitol. Furthermore, the lower K m value of xylose reflects that XR has a strong affinity to its substrate xylose.
The values K m and V max for a xylose and b NADPH calculated by Lineweaver-Burk plot. V X and V N are the initial velocities of XR for xylose and NADPH, respectively. Kinetic experiments were run at 35 °C, 12.25 h, and 100 rpm in 0.1 M phosphate buffer (pH 6.5) using 3 % XR. The error bars indicate the standard deviations of the mean values
This is the first report on the biochemical characterization of XR from adapted yeast. XR is one of the key enzymes for xylitol production; thus, understanding the mechanisms that regulate its activity could help in establishing optimum conditions for biomanufacturing xylitol. The activity and stability studies showed that XR was stable at temperature of 25 °C for 24 h in the pH range from 6 to 7 and retained above 95 % of its original activity after 4 months of storage at −80 °C. The activity and stability of XR obtained in this study seem to be promising for the efficient production of xylitol using MWSHH as a source of xylose in the enzymatic conversion. XR exhibited M-M kinetics with respect to its substrates xylose and NADPH. The enzymatic reaction between XR and xylose is highly productive because of the high V max and low K m values. Hence, detail knowledge of the xylitol production kinetics in multi-substrate enzymatic reaction could help in the design of processes for large-scale production of xylitol from LCB. The utilization of cheap MWSHH as an alternative to more commonly utilized high-priced medium would result in a reasonable reduction in costs of XR enzyme preparation. Moreover, the interest in XR preparation could be increased if the required xylose can be obtained from LCBs.
Conclusion
In the present study, MWSHH proved to be a promising alternative xylose source for yeast cultivation and for XR preparation. The adapted C. tropicalis XR was specific to NADPH as coenzyme with the activity of 11.16 U/mL, which corresponds to a specific activity of 0.91 U/mg. The values of K m for xylose and NADPH were 81.78 mM and 7.29 μM with a corresponding V max for xylose and NADPH 178.57 and 12.5 μM/min, respectively. The lower K m value for xylose indicated that XR has a strong binding affinity for its substrate xylose. The XR can be used as biocatalyst for biomanufacturing a variety of high-value specialty chemicals, mainly xylitol. The use of MWSHH as carbon source will not only decline the application of commercial xylose but also prepare an industrially important enzyme XR from hydrolysate of sawmill’s waste. In addition, the economic interest in utilizing xylose as carbon source for XR production from microbes would be enhanced if the growth media could be made from hydrolysate of LCBs instead of using high-priced commercial xylose.
References
Lee, J. K., Koo, B. S., & Kim, S. Y. (2003). Applied and Environmental Microbiology, 69(10), 6179–6188.
Ronzon, Y. C., Zaldo, M. Z., Lozano, M. L. C., & Uscanga, M. G. A. (2012). Advances in Chemical Engineering and Science, 2, 9–14.
Rawat, U. B., & Rao, M. B. (1996). Biochemica et Biophysica Acta, 1293, 222–230.
Tomotani, E. J., Arruda, P. V., Vitolo, M., & Felipe, M. G. A. (2009). Brazilian Journal of Microbiology, 40, 631–635.
Rafiqul, I. S. M., & Sakinah, A. M. M. (2012). Chemical Engineering Science, 71, 431–437.
Rafiqul, I. S. M., Sakinah, A. M. M., & Karim, M. R. (2014). Applied Biochemistry and Biotechnology, in press. doi:10.1007/s12010-014-1059-z.
Rafiqul, I. S. M., & Sakinah, A. M. M. (2013). Food Reviews International, 29(2), 127–156.
Natah, S. S., Hussien, K. R., Tuominen, J. A., & Koivisto, V. A. (1997). American Journal of Clinical Nutrition, 65, 947–950.
Mayr, P., Brüggler, K., Kulbe, K. D., & Nidetzky, B. (2000). Journal of Chromatography B, 737, 195–202.
Nidetzky, B., Brüggler, K., Kratzer, R., & Mayr, P. (2003). Journal of Agricultural and Food Chemistry, 51(27), 7930–7935.
Verduyn, C., Kleef, R. V., Jzn, J. F., Schreuer, H., Dijken, J. P. V., & Scheffers, W. A. (1985). Biochemical Journal, 226, 669–677.
Yokoyama, S., Suzuki, T., Kawai, K., Horitsu, H., & Takamizawa, K. (1995). Journal of Fermentation and Bioengineering, 79(3), 217–223.
Neuhauser, W., Haltrich, D., Kulbe, K. D., & Nidetzky, B. (1997). Biochemical Journal, 326, 683–692.
Bengtsson, O., Hägerdal, B. H., & Grauslund, M. F. G. (2009). Biotechnology for Biofuels, 2(9), 1–10.
Horitsu, H., Yahashi, Y., Takamizawa, K., Kawai, K., Suzuki, T., & Watanabe, N. (1992). Biotechnology and Bioengineering, 40, 1085–1091.
Granström, T., Aristidou, A. A., & Leisola, M. (2002). Metabolic Engineering, 4, 248–256.
Rao, R. S., Jyothi, C. P., Prakasham, R. S., Sarma, P. N., & Rao, L. V. (2006). Bioresource Technology, 97, 1974–1978.
Nidetzky, B., Neuhauser, W., Haltrich, D., & Kulbe, K. D. (1996). Biotechnology and Bioengineering, 52(3), 387–396.
Lineweaver, H., & Burk, D. (1934). Journal of the American Chemical Society, 56(3), 658–666.
Fernandes, S., Tuohy, M. G., & Murray, P. G. (2009). Journal of Biosciences, 34(6), 881–890.
Rafiqul, I. S. M., & Sakinah, A. M. M. (2012). Chemical Engineering Research and Design, 90, 1307–1312.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Journal of Biological Chemistry, 193, 265–275.
Sampaio, F. C., Faria, J. T., Coimbra, J. S. R., Passos, F. M. L., Converti, A., & Minin, L. A. (2009). Bioprocess and Biosystems Engineering, 32, 747–754.
Rodrigues, R. C. L. B., Sene, L., Matos, G. S., Roberto, I. C., Pessoa, A., Jr., & Felipe, M. G. A. (2006). Current Microbiology, 53, 53–59.
Acknowledgments
The financial support for this research work from the Universiti Malaysia Pahang (UMP) and Ministry of Higher Education, Malaysia (MTUN-COE Research Grant No. RDU 121205) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rafiqul, I.S.M., Sakinah, A.M.M. Biochemical Properties of Xylose Reductase Prepared from Adapted Strain of Candida tropicalis . Appl Biochem Biotechnol 175, 387–399 (2015). https://doi.org/10.1007/s12010-014-1269-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1269-4