Abstract
The frequent occurrence of oil spills in recent years has led to serious contamination of water resources, and materials with superhydrophobic surface properties have attracted much attention for crude oil recovery and water contamination remediation. However, the fragile robustness of superhydrophobic materials greatly hinders their practical applications. Herein, we prepared the robust, photocatalytic superhydrophobic material of SH-ZnO-PDMS@fabric by a simple two-step immersion method. Zinc oxide nanoparticles (ZnO NPs) provided the rough surface structure, and fluorine-free dodecyltrimethoxysilane (DTMS) provided the low surface energy. Polydimethylsiloxane (PDMS) was introduced as a binder to strengthen the force between the nanoparticles and the fabric. The cotton fabric showed excellent superhydrophobicity with a water contact angle (WCA) range of 146.9–156.6°. The methylene blue (MB) in water was basically degraded after 12 h of exposure to UV lamp, manifesting that the cotton fabric had excellent photocatalytic property. The cotton fabric also showed excellent self-cleaning and antifouling properties. Importantly, SH-ZnO-PDMS@fabric maintained superhydrophobic properties after mechanical abrasion, ultrasonic washing, UV irradiation, and acid/alkali immersion. The prepared superhydrophobic materials can be repeatedly used to separate various oil–water mixtures due to their superhydrophobic and recyclable properties. This versatile, efficient, and simple strategy has good application prospects in water pollution remediation and oily wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Petroleum remains a vital source of energy for the needs of modern society,1 and with it comes the inevitable problem of oil spills.2 Petroleum or organic pollutants leaking into the environment can cause huge economic losses and environmental hazards.3,4,5 Therefore, appropriate methods are needed to treat spilled oil and organic pollutants and to separate them from contaminated water.6,7,8 So far, several methods such as physical adsorption,9,10 chemical dispersion,11,12 vacuum degradation,13 and in situ combustion have been applied to water purification.14,15 However, most of these methods have some limitations in practical application due to the complexity of the process, high cost, low efficiency, or secondary pollution.16,17,18 Fortunately, special wettable materials have reportedly been developed that promise to solve the problem of oil spills.19,20,21
Superhydrophobic materials have developed rapidly in recent decades and have received much attention for selective filtration or absorption of oil in oil–water mixtures.22,23,24 A large body of previous work has demonstrated that the preparation of superhydrophobic materials requires the co-regulation of low surface energy and micro- and nanogeometry.25 For example, the cotton fabric with superhydrophobic properties was prepared by using the nanocomposite coating modified by fluorocarbon silane oligomers.26 The viscous fluorophene cotton was prepared by means of ultrasonic impregnation and autonomous loading.27 The hydrophobicity of cotton fabric was improved by grafting polymerization (MA-POSS)-PFDT coating.28 However, the above superhydrophobic materials were prone to contamination in practical applications, leading to a drastic decrease in their oil–water separation efficiency and lifetime, and they also use fluorine-containing reagents, which might cause the irreversible damage to human health and the natural environment.
The most promising nonfluorinated long chain silane coupling agents can reduce the environmental harm of superhydrophobic materials. It can enhance the hydrophobicity and durability of composites due to its hydrophobic soft long chains and easily hydrolysable crosslinked siloxane groups.29,30 But the use of single silane coupling agents alone will not enable the material to reach superhydrophobic levels. Therefore, there is an urgent need to composite the hydrophobic modifiers to diminish the surface energy.31,32 PDMS has attracted wide attention due to its low surface energy, cheapness, nonfluorine, good biocompatibility, good adhesion, and chemical stability.33,34,35 Ma et al. impregnated electrostatically spun silk films in PDMS solution to prepare good superhydrophobic membranes.36 Zhou et al. enhanced the bonding between metal composites and fabrics by PDMS, resulting in superhydrophobic fabrics with excellent durability.37 However, this superhydrophobic material can only separate oil and water, but cannot address contaminated water, so there is a need to find a way to address the contaminants from the water.
Semiconductor photocatalytic technology has gained widespread attention for its energy and environmental applications and is expected to be a solution for organic pollutants in water.38,39,40 In particular, ZnO nanoparticles have many advantages due to their good dispersion, photocatalytic ability, excellent stability, and biocompatibility, and the introduction of ZnO can not only endow the materials with excellent photocatalytic properties but also improve the surface roughness and durability of the materials.41,42,43 For example, a hydrogel with good photocatalytic effect was prepared by using cellulose nanofibers and ZnO.44 Previous work had reported the preparation of a durable superhydrophobic material by immobilizing zinc oxide on the surface of fibrous clay, which also has a good photocatalytic effect.45 Thus, by using the good hydrophobicity and excellent adhesion of PDMS, ZnO can be fixed on the fabric, and a superhydrophobic photocatalytic material could be obtained. Thus, this multifunctional superhydrophobic material is expected to treat oil–water mixtures and degrade organic pollutants in water.
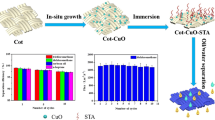
In this study, robust, superhydrophobic cotton fabrics with self-cleaning, photocatalytic, and oil/water separation were prepared by a simple two-step immersion method. ZnO provided the rough structure, DTMS provided the low surface, and PDMS was used as an adhesive to strengthen the force between ZnO and the fabric. Surface morphology, chemical composition, wettability, mechanical durability, UV stability, and corrosion protection, self-cleaning, photocatalytic, and oil/water separation properties were described for all samples. These results showed that the prepared cotton fabrics had an extensive range of applications as functional and durable fabrics.
Experimental section
Materials
Zinc oxide (ZnO, 20–40 nm) was purchased from Aladdin (China). Polydimethylsiloxane (PDMS) and hardener were provided by Dow Corning (America). Dodecyltrimethoxysilane (DTMS), absolute ethanol, ethyl acetate, petroleum ether, n-hexane, dichloromethane, hydrochloric acid (HCl), methylene blue (MB), and Sudan (III) were purchased from Sinopharm Group Chemical Reagent Co. Ltd. (China). Sodium hydroxide (NaOH), sodium chloride (NaCl), and oleic acid were bought from Hubei University Chemical Plant (China). Cotton fabrics were bought from a local store.
Characterizations
The microscopic morphology of samples before and after modification was observed by using field emission scanning electron microscopy (FESEM, JSM7100F, Japan). The element mapping of the samples was analyzed by energy-dispersive X-ray spectrometer (EDS, Escalab 250Xi, Japan). The crystal structure of the samples was determined by X-ray diffractometer (XRD, D8A25, Germany). Fourier transform infrared spectrometer was used to scan the infrared spectrum of the sample and collect the FTIR diagram of the samples (FTIR, Spectrum One, America). The water contact angle was measured by a contact angle measuring instrument (JC2000D, China).
Preparation of superhydrophobic fabrics
The fabrics (35% cotton, 65% polyester) were, respectively, immersed in acetone, ethanol, and deionized water for 10 sonication to remove the stains on the fabric surface. Then, 0.6 g of DTMS was dissolved in 25 mL of deionized ethanol and stirred for 30 min at room temperature for prehydrolysis. Different masses of ZnO (0, 1, 2, 3, 4 g) were added into the above dispersion and stirred for 1 h. The washed fabrics were immersed in the above dispersion and ultrasonicated for 20 min, then the fabrics were taken out and dried in an oven at 120 °C for 1 h. In order to strengthen the binding force between SH-ZnO and fabrics, the fabrics were immersed in an ethyl acetate solution with 1 wt% PDMS (the mass ratio of PDMS and curing agent was 10: 1) for 30 min. After sonication in ethanol for 60 s to remove the weakly adsorbed hydrophobic ZnO, the samples were dried at 80 °C. The obtained samples were named SH-ZnOx-PDMS@fabric, where x was the amount of ZnO.
Oil/water separation
An oil–water mixture (40 mL, 1:1, v/v) was prepared by mixing oil (n-hexane, petroleum ether, xylene, and cooking oil, Sudan III dyed) and water. The SH-ZnO-PDMS@fabric was wetted well with water and fixed in the separation device, and the oil–water mixture was poured slowly into the separation device. The separation efficiency was calculated by measuring the mass of water before and after separation, as shown in equation (1).
where m0 and m were the mass of water before and after separation, respectively.
Oil–water separation flux was calculated as equation (2).
where V was the volume of water after separation, S was the effective test area of the membrane, and T was the time required for oil and water to completely separate.
Antiultraviolet radiation experiments
The SH-ZnO-PDMS@fabric was exposed to UV light (30 W/m2) for 24 h. The WCA of the SH-ZnO-PDMS@fabric was measured every 3 h.
Mechanical durability testing
The mechanical durability of the superhydrophobic fabric was evaluated by sandpaper abrasion and ultrasonic washing. For the sandpaper abrasion test, the samples of SH-ZnOx-PDMS@fabric were placed on sandpaper (600 mesh), with a 100 g of weight as the downward pressure, and the samples were pulled to right at a constant speed for 10 cm at room temperature as one sanding. The surface wettability of SH-ZnOx-PDMS@fabric was tested by measuring the contact angle after every ten scratch cycles. Likewise, for the ultrasonic washing test, the samples were immersed in ethanol and sonicated for 120 min. The surface wettability of SH-ZnOx-PDMS@fabric was tested by measuring the contact angle after every ten scratch cycles. The surface wettability of SH-ZnOx-PDMS@fabric was tested after every 15 min by measuring the contact angle.
Photocatalytic activity
Methylene blue (MB) was used as a water-soluble contaminant to assess the photocatalytic properties of the samples. The samples were placed in 10 mL of an aqueous solution of MB at a concentration of approximately 10 ppm. The sample-impregnated solution was placed under a UV lamp (9 W) for 12 h. In order to monitor the photocatalytic efficiency of the samples in real time, the test solution was analyzed every 3 h with a UV spectrophotometer and the optical pictures of the solution were recorded. In addition, in order to test the cyclic catalytic degradation performance of SH-ZnOx-PDMS@fabric, the fabrics were first soaked in MB solution, dried, and placed in deionized water. Next, the optical photographs of the samples were taken before and after exposure to UV light (9 W) at room temperature for 12 h. The samples were wetted with oleic acid (OA) and placed under an external lamp for 12 h. The contact angles of the samples before and after contamination with OA and before and after UV irradiation were measured to characterize their photocatalytic degradation performance.
Results and discussion
Preparation and characterization of SH-ZnO-PDMS@fabric
Oxide nanoparticles have attracted great interest due to their chemical stability, low cost, and nontoxicity. For example, Han et al. prepared superhydrophobic fabrics using PDMS-coated SiO2 nanoparticles,46 which have good durability in water and oil environments.47 The preparation process of SH-ZnO-PDMS@fabric is shown in Fig. 1a. We used a simple soaking method to prepare superhydrophobic fabrics. The original fabric was immersed in an ethanolic dispersion containing DTMS/ZnO. The DTMS in an ethanolic dispersion was crosslinked with the ZnO. The rich hydroxyl groups on the surface of the fabric formed hydrogen bonds with the crosslinked ZnO, thus causing it to coat the surface of the fabric. Finally, in order to enhance the bonding between fabric and SH-ZnO, we introduced PDMS, which not only improved the stability of the material but also further reduced the surface energy of the fabric.
Figure 1b reflects whether ZnO had been grafted onto DTMS. The characteristic absorption peaks of the original fabric after loading DTMS at 2924, 2857, 1463, and 1043 cm−1 were due to –CH2 stretching vibration,48 –CH3 stretching vibration,49 –C–H bending vibration, and Si-O-Si asymmetric stretching vibration.50 These results indicated that DTMS had been successfully grafted onto zinc oxide.
Figure 1c shows the XRD analyses of the original fabric and the sample. Pristine fabrics and SH-ZnO0-PDMS@ fabrics had the same crystalline shape, indicating that the addition of DTMS or PDMS did not affect the crystalline shape of the fabrics. The peaks at 2θ = 32.01°, 34.63°, 36.43°, 47.69°, 56.77°, 63.09°, 68.15°, and 69.25 correspond to the crystal planes of (100), (002), (101), (102), (110), (103), (112), and (201) of ZnO.51 The appearance of the above characteristic peaks demonstrated the successful deposition of ZnO nanoparticles on the fabric surface.
Surface microstructure analysis of SH-ZnO-PDMS@fabric
As shown in Figs. 2a–2f, we analyzed the surface microstructure of the samples at different ZnO contents. The original fabric was interwoven smoothly. As shown in Fig. 2b, the SH-ZnO0-PDMS@fabric surface has a thin film-like rough structure, indicating that DTMS and PDMS have successfully constructed some micro- and nanorough structures on the fabric surface and also reduced its surface energy. Subsequently, with the introduction of ZnO, the surface of modified fabrics had an increasingly visible micro- and nanostructure (Fig. 2c–f). Among them, the SH-ZnO3-PDMS@fabric surface had the most obvious coral-like rough structure (Fig. 2e), which together with PDMS and DTMS imparted superhydrophobicity to the material. It was worth noting that if too little or too much ZnO was introduced, it led to a decrease in roughness (Fig. 2c, d, f). In addition, the EDS images of SH-ZnO3-PDMS@fabric showed that the surface contained the four main elements of C, O, Si, and Zn, which demonstrated the successful attachment of DTMS, PDMS, and ZnO to the fabric (Fig. 2g, h).
Water contact angle tests of the superhydrophobic fabrics
It is well known that surface roughness is an important indicator of a material's hydrophobic properties.52 It has been confirmed that different contents of ZnO had the effect on the roughness of SH-ZnO-PDMS@fabric. Next, in order to explore the effect of ZnO content on fabric wettability, we tested the contact angle and rolling angle of the samples with different ZnO content. For the sample of SH-ZnO0-PDMS@fabric without ZnO, its water contact angle and rolling angle were 139° and 28.5°, respectively, which could not reach the superhydrophobic standard of materials. Although DTMS and PDMS provide low surface energy materials for the sample, due to the lack of micro-nanorough structure on the surface, there is a large contact area between the water droplets and the sample, and under the action of surface tension, the water droplets and the sample surface have a large adhesion effect. This wetting behavior can be explained by the Cassie–Baxter model. The Cassie–Baxter equation is cosθ ∗ = rfcosθ + f − 1. The large contact area between water droplets and the surface of SH-ZnO0-PDMS-fabric results in a contact angle of less than 150° for the fabric. With the addition of ZnO, micro- and nanogeometric structures are constructed on the fabric surface. The contact area between water droplets and the fabric surface decreases; thus, the contact angle of fabric was bigger than 150°. As shown in Fig. 3, with the increase of the amount of ZnO, the greater the contact angle, the smaller the rolling angle of the samples, and the better the hydrophobic performance presented in the samples, which was caused by the increase of surface roughness. However, the excessive addition ZnO into ZnOSH-ZnO4-PDMS@fabric caused a slightly lower hydrophobic performance than SH-ZnO3-PDMS@fabric, probably due to the excess accumulation of SH-ZnO on the fabric surface that reduced the surface roughness of the fabric. The results showed that SH-ZnO3-PDMS@fabric had the best wettability with a high contact angle of 156.5° and a rolling angle of 6.5°.
Stability study of SH-ZnO 3 -PDMS@fabric
Further, the static/dynamic wettability properties of the superhydrophobic cotton fabrics were used to analyze the surface wettability. As displayed in Fig. 4a and b, droplets of pure water show spherical shapes on the SH-ZnO3-PDMS@fabric, and droplets of protein, brine, water stained by methylene blue, milk, etc., show spherical shapes, and droplets of pH 1 and 14 also show spherical shapes on the SH-ZnO3-PDMS@fabric. These results all show that the SH-ZnO3-PDMS@fabric has good hydrophobicity not only for pure water but also for various other water-containing droplets, which proves that superhydrophobic fabrics have good hydrophobicity. This demonstrates the general hydrophobicity of superhydrophobic fabrics for all kinds of droplets. Interestingly, a distinct silver mirror phenomenon was observed after immersing the SH-ZnO3-PDMS@ fabric into water (Fig. 4c). This was because when the superhydrophobic fabric was pressed into water by an external force, the air was carried into the water by the voids in the micro- and nanorough structure of the fabric surface to form an air film between the fabric surface and the water, which was refracted under light illumination. In addition, we used a syringe to control the contact of water droplets with the SH-ZnO3-PDMS@ fabric surface to evaluate the strength of the adhesion between them (Fig. 4d). Obviously, water droplets squeezed onto the fabric surface could be completely absorbed by the syringe, indicating weak adhesion of SH-ZnO3-PDMS@ fabric to the water droplets, attributed to the low surface energy and high roughness of the superhydrophobic fabric surface. What is more, Fig. 4e shows that the water stream bounced away to form a certain angle after being shot to the surface of SH-ZnO3-PDMS@fabric, which proved that SH-ZnO3-PDMS@fabric had an ejection property to the water stream.
Photographs of (a) pure water and (b) various droplets (salt water, albumin, methylene blue dyed water, milk, solvent droplets with different pH values placed on the surface of SH-ZnO3-PDMS@fabric; photographs of SH-ZnO3-PDMS@fabric; (c) immersed in water with silver-like mirror phenomenon; (d) with low adhesion to water droplets and (e) water ejection
Self-cleaning performance of SH-ZnO 3 -PDMS@fabric
Self-cleaning performance is an important inherent ability of superhydrophobic materials, which confers the property that superhydrophobic materials are not easily contaminated by dust and water bodies during use. Therefore, we used carbon ash as a simulated contaminant to verify the self-cleaning performance of superhydrophobic SH-ZnO3-PDMS@fabric (Fig. 5). First, the carbon ash was sprinkled on the tilted sliding plate attached with the original fabric and SH-ZnO3-PDMS@ fabric closely so that a small amount of charcoal ash was randomly distributed on the surface of the fabric, and then water was slowly dripped onto the surface of the fabrics. As shown in Fig. 5a, the original fabric was gradually wetted by the water droplets, and most of the carbon ash was adsorbed on the fabric surface and contaminated the original fabric. On the contrary, water droplets formed spheres on the surface of SH-ZnO3-PDMS@fabric and rolled down, adsorbing and carrying charcoal ash, allowing the SH-ZnO3-PDMS@ fabric to return to a clean state (Fig. 5b). A diagram of self-cleaning is shown in the diagram (Fig. 5c). In addition, to verify whether the superhydrophobic SH-ZnO3-PDMS@fabric maintains self-cleaning in complex water environment, the original fabric and superhydrophobic SH-ZnO3-PDMS@fabric were completely immersed in methylene blue (MB) aqueous solution, and then removed to observe whether the fabric was contaminated. As shown in Fig. 5d, the initial fabric was rapidly soaked and contaminated by methylene blue solution. By contrast, SH-ZnO3-PDMS@ fabric was not only not wetted by the dyeing solution but also not contaminated after removal due to its excellent hydrophobic property (Fig. 5e). The above experimental results confirmed that the superhydrophobic SH-ZnO3-PDMS@fabric had good hydrophobic property, which played a crucial role in the antifouling behavior.
Durability evaluation of SH-ZnO 3 -PDMS@fabric
In practical applications, superhydrophobic materials often face harsh usage environments. To characterize the stability and robustness of superhydrophobic materials, the robustness of superhydrophobic materials was investigated using harsh conditions such as mechanical abrasion, physical ultrasonics, chemical corrosion, and UV irradiation. As shown in Fig. 6a, a 30 mm × 30 mm square of SH-ZnO3-PDMS@fabric was moved in one direction on 600 grit sandpaper at a pressure of 100 g. The movement of 10 cm was recorded was one cycle. It is noteworthy that the WCA of the fabric surface remained at around 147° after 100 cycles (Fig. 6b). This showed that the SH-ZnO3-PDMS@fabric has good abrasion resistance. Furthermore, we verified that PDMS enhanced the adhesion between the fabric and SH-ZnO, which, as shown in Fig. 6b, had lost its superhydrophobic properties after 10 cycles of abrasion. Since SH-ZnO was mostly physically adsorbed to the fabric, PDMS has good adhesive ability, thus enhancing the adhesion of SH-ZnO to the fabric.
(a) Photographs of sandpaper abrasion experiments; (b) Water contact angle of SH-ZnO3-PDMS@fabric, SH-ZnO3@fabric at different friction times; (c) Water contact angles of SH-ZnO3-PDMS@fabric and SH-ZnO3@fabric at different sonication times; Water contact angle and rolling angle of superhydrophobic SH-ZnO3-PDMS@fabric after (d) immersion in different pH solutions and (e) different times of UV irradiation
Moreover, the SH-ZnO3-PDMS@fabric still exhibited good superhydrophobicity after 120 min of sonication treatment, while the modified fabrics without PDMS quickly lost their superhydrophobicity, again indicating that PDMS enhanced the stability of the modified fabrics (Fig. 6c). Figure 6d records the changes in WCA and SA of the modified fabrics after 24 h soaking at different pHs, showing that the modified fabrics still exhibit good hydrophobicity in both acid and alkaline solutions (WCA > 146.9°, SA < 16.7°). In addition, the WCA remained above 150° and the roll angle below 16° after the modified fabrics were exposed to UV light for 24 h (Fig. 6e). Thus, this superhydrophobic fabric has good stability and durability, which can significantly extend its use in harsh conditions.
Application of SH-ZnO 3 -PDMS@fabric in oil–water separation
The SH-ZnO3-PDMS@fabric is superhydrophobic and is expected to achieve good oil–water separation efficiency. Figure 7a shows the selective adsorption of oil by the fabric. When the fabric comes into contact with the oil, the oil is rapidly absorbed and the oil–water mixture becomes clean and transparent. Furthermore, Fig. 7b shows the gravity oil–water separation process of SH-ZnO3-PDMS@fabric. When the oil–water mixture was poured into the oil–water separation unit, the water (methylene blue dyeing) was completely retained in the upper container, while the oil (Sudan III dye) was rapidly collected by the lower container through the superhydrophobic fabric, thus achieving an effective oil–water separation.
(a) Selective absorption of Sudan III-stained dichloromethane in the aqueous phase by SH-ZnO3-PDMS@fabric. (b) Gravity oil–water separation process for water and dichloromethane. Water was stained with methylene blue and dichloromethane was stained with methyl red for clear observation; Separation efficiency and flux of superhydrophobic SH-ZnO3-PDMS@fabric for (c) different oil/water mixtures and (d) dichloromethane/water after 10 consecutive separation cycles
The separation of petroleum ether–water and n-hexane–water was tested using a similar apparatus. It was worth noting that both petroleum ether and n-hexane were less dense oils than water, and the oil–water mixture should be poured slowly during the oil–water separation process to ensure that the oil first contacts the superhydrophobic fabric, collected by the lower device. The oil–water separation efficiency and separation flux of SH-ZnO3-PDMS@fabric are shown in Fig. 7c. Although the superhydrophobic fabric absorbs part of the oil and the oil is volatile, making the mass of oil decrease during the weighing process, the separation efficiency of superhydrophobic materials still exceeded 95.5%. These results demonstrated that SH-ZnO3-PDMS@fabric has high separation efficiency. SH-ZnO3-PDMS@fabric showed different fluxes for the three oils due to the different densities and viscosities of the different oils. Among them, the methylene chloride flux was as high as 20000 L·m−2·h−1. Next, 10 consecutive oil–water separation cycles were tested for methylene chloride/water mixture (Fig. 7d). After 10 separation cycles, the separation efficiency of SH-ZnO3-PDMS dropped from 96.1% to 91.5%, due to residual oil stained on the fabric. Additionally, the separation efficiency for the 10 consecutive separation cycles was maintained above 90%, indicating that the modified fabric has good separation cycling performance. Therefore, the prepared superhydrophobic SH-ZnO3-PDMS@fabric had excellent performance and could be used as oil–water separation membrane material.
Photocatalytic performance of SH-ZnO 3 -PDMS@fabric
Rapid industrial development has led to huge effluent discharges, and the treatment of this polluted water has become imminent. Figure 8a shows the degradation of the MB solution by SH-ZnO3-PDMS@fabric. Aqueous methylene blue (MB) solutions were chosen to simulate contaminated water sources to evaluate the photocatalytic performance of the superhydrophobic SH-ZnO3-PDMS@fabric. SH-ZnO3-PDMS@fabric wetted with ethanol was immersed in MB solution (5 ppm) and irradiated under UV. The absorption intensity of MB solution was measured by UV spectrophotometry, and the results are shown in Fig. 8a. With the prolongation of light exposure time, the UV absorption peak of methyl bromide gradually decreased, and the color of MB aqueous solution gradually faded, which means that MB was degraded under light. It showed that SH-ZnO3-PDMS@fabric had a good degradation effect.
The outstanding photocatalytic activity of SH-ZnO3-PDMS@fabric is related to the deposition of the indicated ZnO. ZnO is highly oxidizing and promotes the transfer of electrons from the valence band (VB) to the conduction band (CB) and the generation of electron holes, the photogenerated electrons on CB which react with oxygen to form superoxide radicals, and the photogenerated electrons on VB which react with water to form hydroxyl radicals. Due to the super oxidation of superoxide radicals and hydroxyl radicals, the most organic can be oxidized to the end products CO2 and H2O. Therefore, nano-ZnO has good photocatalytic properties and can be used to degrade organic pollutants.53
The prepared superhydrophobic SH-ZnO3-PDMS@fabric was self-cleaning and can clean the surface dust under water rinsing, and keep itself clean in MB solution. However, SH-ZnO3-PDMS@fabric was easy to absorb oil and be polluted by oil. When contaminated by easily volatile oils, such as methylene chloride and hexane, the method of heating can make the oil volatilize at high-temperature to realize the cleanliness of the fabric and restore the superhydrophobicity of the fabrics. However, it is difficult to achieve the same effect with high-temperature treatment for oil that is difficult to volatilize. We expect that the adsorbed oil would be degraded by photocatalysis, thus restoring the material's self-cleaning ability.
The superhydrophobic SH-ZnO3-PDMS@fabric contaminated with oleic acid (OA) was selected for simulation experiments to measure the water contact angle before and after contamination or UV irradiation (Fig. 8b). The water contact angle of the uncontaminated fabric was 155°, indicating that the modified fabric was superhydrophobic. When impregnated with oleic acid, the contact angle decreased to 113° and lost the superhydrophobic property because the micro-nanostructure and hydrophobic groups on the fabric surface were covered by oleic acid. We placed the oil-contaminated fabric under UV irradiation for 24 h, and the fabric recovered the superhydrophobic property with a contact angle of 154°. The superhydrophobic SH-ZnO3-PDMS@fabric could degrade the oleic acid on its surface under UV light induction, thus re-exposing the micro-nanostructure and hydrophobic groups and exhibiting superhydrophobicity again. The superhydrophobic SH-ZnO3-PDMS@fabric showed good photocatalytic self-cleaning properties.
Conclusions
In summary, we prepared a robust, photocatalytic superhydrophobic material of SH-ZnO3-PDMS@fabric by a simple two-step immersion method. The SH-ZnO3-PDMS@fabric had good superhydrophobic properties (contact angle up to 156.5° and roll angle of 6.5°), good environmental stability, excellent self-cleaning properties, and the ability to keep itself clean in polluted water and clean itself under the rinsing of water droplets solid contaminants on the surface. In addition, SH-ZnO3-PDMS@fabric displayed excellent oil–water separation performance (separation efficiency up to 95.5%), good photocatalytic performance (methylene blue in water is basically degraded completely under UV irradiation for 12 h), and superior photocatalytic self-restoring property. SH-ZnO3-PDMS@fabric provided a feasible method for constructing superhydrophobic materials with environmental durability, photocatalytic property, and self-cleaning property.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Zhang, W, Liu, N, Cao, Y, Lin, X, Liu, Y, “Superwetting porous materials for wastewater treatment: from immiscible oil/water mixture to emulsion separation.” Adv. Mater. Interfaces, 4 (10) 1600029 (2017)
Ge, J, Zhao, H-Y, Zhu, H-W, Huang, J, Shi, L-A, “Advanced sorbents for oil-spill cleanup: recent advances and future perspectives.” Adv. Mater., 28 (47) 10459–10490 (2016)
Kong, D, Yang, H, He, X, “Impact of wind on in-situ burning behavior of spilled oil on open water.” J. Loss. Prevent Proc., 65 104147 (2020)
Li, F, Miao, G, Gao, Z, Xu, T, Zhu, X, Miao, X, Li, X, “A versatile hydrogel platform for oil/water separation, dye adsorption, and wastewater purification.” Cellulose, 29 (8) 4427–4438 (2022)
Kujawinski, EB, Kido Soule, MC, Valentine, DL, Boysen, AK, Longnecker, K, Redmond, MC, “Fate of dispersants associated with the Deepwater Horizon oil spill.” Environ. Sci. Technol., 45 (4) 1298–1306 (2011)
Lu, J, Li, F, Miao, G, Miao, X, Ren, G, Wang, B, Zhu, X, “Superhydrophilic/superoleophobic shell powder coating as a versatile platform for both oil/water and oil/oil separation.” J. Membrane Sci., 637 119624 (2021)
Wang, Z, Yao, D, He, Z, Liu, Y, Wang, H, “Fabrication of durable, chemically stable, self-healing superhydrophobic fabrics utilizing gellable fluorinated block copolymer for multifunctional applications.” ACS. Appl. Mater. Interfaces, 14 (42) 48106–48122 (2022)
Sathya, RA, Ponraj, C, “Superhydrophobic route of fabricating antireflective, self-cleaning, and durable coatings for solar cell applications.” J. Coat. Technol. Res., 21 1–30 (2023)
Hou, J, Yang, Y, Yu, D-G, Chen, Z, Wang, K, Liu, Y, “Multifunctional fabrics finished using electrosprayed hybrid Janus particles containing nanocatalysts.” Chem. Eng. J., 411 128474 (2021)
Yin, L, Wang, P, Wen, T, Shujun, Y, Wang, X, Hayat, T, Alsaedi, A, Wang, X, “Synthesis of layered titanate nanowires at low temperature and their application in efficient removal of U(VI).” Environ. Pollut., 226 125–134 (2017)
Zhang, X, Li, Z, Liu, K, Jiang, L, “Bioinspired multifunctional foam with self-cleaning and oil/water separation.” Adv. Funct. Mater., 23 (22) 2881–2886 (2013)
Gupta, P, Kandasubramanian, B, “Directional fluid gating by Janus membranes with heterogeneous wetting properties for selective oil–water separation.” ACS Appl. Mater. Interfaces, 9 (22) 19102–19113 (2017)
Al-Sabagh, AM, Azzam, EMS, El-Din, MN, “Synthesis and evaluation of ethoxylated alkyl sulfosuccinates as oil spill dispersants.” J. Disper. Sci. Technol., 29 (6) 866–872 (2008)
Shi, X, Ranellone, RT, Sezer, H, Lamie, N, Zabilansky, L, Stone, K, Rangwala, AS, “Influence of ullage to cavity size ratio on in-situ burning of oil spills in ice-infested water.” Cold. Reg. Sci. Technol., 140 5–13 (2017)
Liu, Y, Kong, F, Chen, Z, Xu, Y, Liu, Y, Newton, MAA, “Fabrication of poly(vinylidene fluoride)/graphite heterogeneous porous carbon nanofiber composite mat by electrospraying method for efficient oil–water separation.” J. Coat. Technol. Res., 21 1–10 (2023)
Li, J, Li, D, Yang, Y, Li, J, Zha, F, Lei, Z, “A prewetting induced underwater superoleophobic or underoil (super) hydrophobic waste potato residue-coated mesh for selective efficient oil/water separation.” Green Chem., 18 (2) 541–549 (2016)
Yin, K, Chu, D, Dong, X, Wang, C, Duan, J-A, He, J, “Femtosecond laser induced robust periodic nanoripple structured mesh for highly efficient oil–water separation.” Nanoscale, 9 (37) 14229–14235 (2017)
Wang, B, Liang, W, Guo, Z, Liu, W, “Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: a new strategy beyond nature.” Chem. Soc. Rev., 44 (1) 336–361 (2015)
Lu, J, Gao, Z, Xu, T, Zhu, X, Miao, X, Song, Y, Li, X, “Robust hydrogel coating with oil-repellent property in air, water, and oil surroundings.” ACS Appl. Mater. Interfaces, 12 (43) 49138–49145 (2020)
Xiang, B, Gong, J, Sun, Y, Li, J, “Robust PVA/GO@ MOF membrane with fast photothermal self-cleaning property for oily wastewater purification.” J. Hazard. Mater., 462 132803 (2024)
Wu, Y, Xu, G, Wang, T, Liu, K, Lu, J, Wang, D, “Preparation of biomimetic hair-like composite coatings with water-collecting and superamphiphobic properties.” Prog. Org. Coat., 158 106372 (2021)
Zhu, X, Gao, Z, Li, F, Miao, G, Xu, T, Miao, X, Ren, G, “Superlyophobic graphene oxide/polydopamine coating under liquid system for liquid/liquid separation, dye removal, and anti-corrosion.” Carbon, 190 329–336 (2022)
Xiang, B, Gong, J, Sun, Y, Yan, W, Li, J, “High permeability PEG/MXene@ MOF membrane with stable interlayer spacing and efficient fouling resistance for continuous oily wastewater purification.” J. Membrane Sci., 691 122247 (2024)
Gong, J, Xiang, B, Sun, Y, Li, J, “Janus smart materials with asymmetrical wettability for on-demand oil/water separation: a comprehensive review.” J. Mater. Chem. A, 11 25093–25114 (2023)
Chu, Z, Feng, Y, Seeger, S, “Oil/water separation with selective superantiwetting/superwetting surface materials.” Angew Chem. Int. Ed., 54 (8) 2328–2338 (2015)
Yamashita, K, Yasukawa, A, Sawada, H, “Fabrication of cotton fabric with superoleophilic/superhydrophobic characteristic on the modified surface by using fluoroalkylated oligomeric silica/triazine derivative nanocomposites.” Coatings, 10 (2) 174 (2020)
Zhang, G, Chen, Q, Yang, F, Chen, G, Fu, J, “Gecko foot-inspired reduced graphene oxide surface with multi-resistant, nonpolar/polar separation and reliable adhesion utility.” J. Mater. Sci., 56 7372–7385 (2021)
Li, H, Tang, S, Zhou, Q, Chen, W, Yang, X, Xing, T, Chen, G, “Durable superhydrophobic cotton fabrics prepared by surface-initiated electrochemically mediated ATRP of polyhedral vinylsilsesquioxane and subsequent fluorination via thiol-Michael addition reaction.” J. Colloid Interf. Sci., 593 79–88 (2021)
Lin, Y, Qian, Q, Chen, Z, Tuan, P-D, Feng, D, “Fabrication of high specific surface area TiO2 nanopowders by anodization of porous titanium.” Electrochem Commun., 136 107234 (2022)
Pakdel, E, Zhao, H, Wang, J, Tang, B, Varley, RJ, Wang, X, “Superhydrophobic and photocatalytic self-cleaning cotton fabric using flower-like N-doped TiO2/PDMS coating.” Cellulose, 28 8807–8820 (2021)
Rahimipour, S, Rafiei, B, Salahinejad, E, “Organosilane-functionalized hydrothermal-derived coatings on titanium alloys for hydrophobization and corrosion protection.” Prog. Org. Coat., 142 105594 (2021)
Stevens, N, Tedeschi, S, Powers, K, Moudgil, B, El-Shall, H, “Controlling unconfined yield strength in a humid environment through surface modification of powders.” Powder Technol., 191 (1–2) 170–175 (2009)
Ge, M, Cao, C, Liang, F, Liu, R, Zhang, Y, Zhang, W, Lai, Y, “A ‘PDMS-in-water’ emulsion enables mechanochemically robust superhydrophobic surfaces with self-healing nature.” Nanoscale Horiz., 5 (1) 65–73 (2020)
Zhang, C, Li, Y, Sun, S, Kalulu, M, Wang, Y, Zhou, X, Jiang, Y, “Novel magnetic and flame-retardant superhydrophobic sponge for solar-assisted high-viscosity oil/water separation.” Prog. Org. Coat., 139 105369 (2020)
Guo, Z, Long, B, Gao, S, Luo, J, Wang, L, Huang, X, Gao, J, “Carbon nanofiber based superhydrophobic foam composite for high performance oil/water separation.” J. Hazard. Mater., 402 123838 (2021)
Ma, W, Ding, Y, Zhang, M, Gao, S, Li, Y, Huang, C, Fu, G, “Nature-inspired chemistry toward hierarchical superhydrophobic, antibacterial and biocompatible nanofibrous membranes for effective UV-shielding, self-cleaning and oil-water separation.” J. Hazard. Mater., 384 121476 (2020)
Zhou, C, Chen, Z, Yang, H, Hou, K, Zeng, X, Zheng, Y, Chen, J, “Nature-inspired strategy toward superhydrophobic fabrics for versatile oil/water separation.” ACS Appl. Mater. Interfaces, 9 (10) 9184–9194 (2017)
Yan, S, Li, Y, Xie, F, Wu, J, Jia, X, Yang, J, Zhang, Z, “Environmentally safe and porous MS@ TiO2@PPy monoliths with superior visible-light photocatalytic properties for rapid oil–water separation and water purification.” ACS Sustain. Chem. Eng., 8 (13) 5347–5359 (2020)
Wang, Q, Gao, Q, Al-Enizi, AM, Nafady, A, Ma, S, “Recent advances in MOF-based photocatalysis: environmental remediation under visible light.” Inorg. Chem. Front., 7 (2) 300–339 (2020)
Miao, G, Li, F, Gao, Z, Xu, T, Miao, X, Ren, G, Zhu, X, “Ag/polydopamine-coated textile for enhanced liquid/liquid mixtures separation and dye removal.” iScience, 25 (5) 104213 (2022)
Toh-ae, P, Paradee, N, Saramolee, P, Kalkornsurapranee, E, Johns, J, Nakaramontri, Y, “Nano-titania doped NR foams: influence on photocatalysis and physical properties.” Polym. Degrad. Stabil., 190 109640 (2021)
Pino-Ramos, VH, Bucio, E, Diaz, D, “Fast photocatalytic polypropylene degradation by nanostructured bismuth catalysts.” Polym. Degrad. Stabil., 190 109648 (2021)
Yu, F, Tian, F, Zou, H, Ye, Z, Peng, C, Huang, J, Gao, B, “ZnO/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue.” J. Hazard. Mater., 415 125511 (2021)
Zhang, X, Peng, J, Qi, X, Huang, Y, Qiao, J, Guo, Y, Wu, Y, “Nanocellulose/carbon dots hydrogel as superior intensifier of ZnO/AgBr nanocomposite with adsorption and photocatalysis synergy for Cr (VI) removal.” Int. J. Biol. Macromol., 233 123566 (2023)
Shahzad, K, Hussain, S, Nazir, MA, Jamshaid, M, Rehman, A, Alkorbi, AS, Alhemiary, NA, “Versatile Ag2O and ZnO nanomaterials fabricated via annealed Ag-PMOS and ZnO-PMOS: an efficient photocatalysis tool for azo dyes.” J. Mol. Liq., 356 119036 (2022)
Han, SW, Park, EJ, Jeong, MG, Kim, IH, Seo, HO, Kim, JH, Kim, YD, “Fabrication of recyclable superhydrophobic cotton fabrics.” Appl. Surf. Sci., 400 405–412 (2017)
Zhou, Q, Yan, B, Xing, T, Chen, G, “Fabrication of superhydrophobic caffeic acid/Fe@ cotton fabric and its oil-water separation performance.” Carbohyd. Polym., 203 1–9 (2019)
Ren, J, Tao, F, Liu, L, Wang, X, Cui, Y, “A novel TiO2@ stearic acid/chitosan coating with reversible wettability for controllable oil/water and emulsions separation.” Carbohyd. Polym., 232 115807 (2020)
Nguyen-Tri, P, Altiparmak, F, Nguyen, N, Tuduri, L, Ouellet-Plamondon, CM, “Robust superhydrophobic cotton fibers prepared by simple dip-coating approach using chemical and plasma-etching pretreatments.” ACS Omega, 4 (4) 7829–7837 (2019)
Chauhan, P, Kumar, A, Bhushan, B, “Self-cleaning, stain-resistant and anti-bacterial superhydrophobic cotton fabric prepared by simple immersion technique.” J. Colloid. Interf. Sci., 535 66–74 (2019)
Agrawal, N, Tan, JSJ, Low, PS, Fong, EWM, Lai, Y, Chen, Z, “Green synthesis of robust superhydrophobic antibacterial and UV-blocking cotton fabrics by a dual-stage silanization approach.” Adv. Mater. Interfaces, 6 (11) 1900032 (2019)
Buzzetti, L, Crisenza, GE, Melchiorre, P, “Mechanistic studies in photocatalysis.” Angew. Chem. Int. Ed., 58 (12) 3730–3747 (2019)
Di Mauro, A, Cantarella, M, Nicotra, G, Privitera, V, Impellizzeri, G, “Low temperature atomic layer deposition of ZnO: applications in photocatalysis.” Appl. Catal. B, 196 68–76. https://doi.org/10.1016/j.apcatb.2016.05.015 (2016)
Acknowledgments
This work was supported by the open project funding of Ministry of Education Key Laboratory for the Synthesis and Application of Organic Functional Molecules (KLSAOFM2306), Hubei University, China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declared that we have no conflicts of interest to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, C., Chen, D., Mao, L. et al. Photocatalytic superhydrophobic SH-ZnO-PDMS-coated fabric for efficiency self-cleaning and oily water separation. J Coat Technol Res (2024). https://doi.org/10.1007/s11998-024-00966-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11998-024-00966-9