Abstract
Biofouling is a major issue for many industries including shipping, oil, and gas and can lead to accelerated corrosion, particularly for structures and components in and around salt water. Many efforts are undertaken to lessen its impact and financial losses. One of the promising methodologies is application of antibiofouling coatings to minimize biofouling, and the best results were observed with a superhydrophobic coating. Ample literature reviews on superhydrophobic coating have shown biofouling inhibition on the surface due to high wetting angles similar to the phenomenon on a lotus leaf. The hydrophobic coating can be deposited using multiple techniques such as electroless plating, chemical vapor deposition (CVD), sol–gel, and electrodeposition. In this review, an effort has been made to encompass such experimental work under a single domain and compare the effectiveness of each coating. In addition, mechanical properties and surface characteristics such as wetting angle, surface energy, and morphology were also discussed for various types of polymeric coating. Similarly, the application of nanoparticles such as ZnO, SiO2, TiO2, and CeO2 was found to improve the substrate's mechanical properties, the durability of coatings, improvement in wear properties, adhesion, interlaminar cohesion, and increased wetting angle and above all, improve superhydrophobicity. These improvements are compared for various nanoparticle integrated coatings. In addition, other novel approaches to prevent marine biofouling by various polymer-based coatings such as superhydrophobic, foul-release, and foul-resistant coatings are discussed in this paper.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biofouling is a natural mechanism of formation of undesirable bacteria, algae, and mussels on any structures and components of machines such as shipyard structures, underwater pipelines, underwater cables, and submarine hulls. Biofouling can accelerate corrosion, degrade structures, and reduce marine system efficiency, which can result in increased fuel consumption and CO2 emissions. The earliest case of biofouling was reported in papyrus plants in 412 BC, and a mixture of arsenic and sulfur was used to minimize its effect.1In the present scenario, biofouling has emerged as a primary issue for structures and equipment used for military or commercial ships, onshore industries, and other marine engineering applications.2,3,4 The undesirable adhesion of the marine species (flora and fauna) and its colonization over the substrate leads to the coining of the term called marine fouling. Microorganisms like bacteria, algae, diatoms, sponges, etc., and macro-organisms like mussels, balanus, barnacles, hydroid, etc., are two broad categories of water organisms responsible for marine fouling.5,6,7,8,9,10,11 The shipping industry is considered one of the leading sources of the nation’s income, contributing up to 90% in some countries.12 Biofouling in ships causes tremendous damage to its components and thus promotes losses in the annual economy of the shipping industry. Marine fouling deteriorates the performance of the ship by affecting the ship vessel's surface, increasing the vessel's weight, reducing speed, causing poor ship mileage, and contributing to environmental pollution by emission of SO2, NOx, CO2, and other harmful gases.13,14,15,16,17 A schematic diagram of marine fouling is shown in Fig. 1. Considering the above parameters, there are two key routes that describe marine biofouling’s growth. Initially, when the substrate is immersed in the seawater and gets wet, organic carbon residue (OCR) will immediately adsorb on the substrate's wet surface, generating a conditioned film in a few minutes. The composition of these OCRs solely depends upon the fulvic acids, humic acids, glycoproteins, and ions accessible in the liquid form. After a few hours under electrostatic and Van der Waals force, microbes get adsorbed on the conditioned film to form a biofilm. The adherence of microbes on the conditioned film is promoted by sedimentation, water flow, convective and Brownian motion.18,19,20,21 Finally, after a week of activity, some single-celled algal spores, protists, and marine biological larvae adhere over the biofilm surface to provide nutrition to increase their population to create a vast biological fouling community.22 The attached organism colonizes onto the ship's hull surface thereafter increasing the ship's drag, reducing the ship's speed, and increasing the hydrodynamic weight of the vessel.23 For more than 2000 years, humans have been fighting with marine fouling. Early generations used biocidal compounds which are capable of killing fouling organisms to prevent colonization. The developed biocidal compounds vary from simple Cu to Pb sheets on wooden boats for antimicrobial coatings containing arsenic, mercury, and copper on marine hulls. Copper was a popular and effective biocide, but it was only effective for two years. The researchers started to work on antifoul technology to attenuate the harsh effect of biofouling. Spirit-based vanish paint was initially applied to the bottom of the vessels in 1908 to prevent biofouling. Ships were painted with mercuric oxide disseminated in turpentine and alcohol-creating paint, with a nine-month durability rating. TBT (tributyltin)-based polishing and coating technique was developed in the twentieth century, composed of TBT acrylate ester, which acts as an antifouling agent.24,25 Unfortunately, it was observed that the toxic nature of TBT causes severe damage to aquatic life. After that, many antifouling paints and wax replacing TBT were developed, which inhibit biofouling growth on the substrate.26,27 The academic and industrial researchers started their research to find the most economical, ecological, and nontoxic coating that can replace the traditional method. The coating techniques were designed based on two prime objectives. First, it is necessary to develop an antifouling strategy to prevent marine organism attacks and secondly, to design a mechanism to create a fouling release strategy to lessen the adhesion force after an attack.28 Superhydrophobic coating is one of the durable and efficient methodologies recently developed for such purpose.29 Superhydrophobic surfaces have recently attracted a lot of attention due to their efficacy in the fields of self-cleaning, antifouling, water repellence, and antisticking properties.29,30,31,32,33,34 The term superhydrophobicity has been known since the 1940s,35 but the concept of superhydrophobicity inspired by nature has been utilized in the past decade. This nature-inspired surface consists of a micro–nano-structure and nano-enclosed pores that can repel the adhesion of oil, protein, and bacteria to prevent the surface from biofouling.36,37,38 The bio-mimicry of this natural phenomenon helps to suppress biofouling.39,40 Fouling bacteria or organisms may migrate with ships and could lead to bio-invasion when it reaches different sea water without natural opponents.41 In the Black Sea, fouling from the jelly comb Mnemiopsis arrived in the 1980s that endangered the existence of native anchovies. Hence, the International Organization of Maritime (IOM) circulated the resolution to diminish the impact of biofouling bio-invasion. The corrosion of surfaces can also be exacerbated by biofouling. The accumulation of fouling bacteria on surfaces accelerates regional variations in these types and deliberations of pH values, oxygen levels, and ions which can cause coatings to degrade, increase the conductivity of the liquid, and encourage electrochemical and chemical processes.42,43 This type of corrosion is known as biocorrosion or microbial-influenced corrosion (MIC). MIC accounts for 20% of the corrosion in aqueous systems.44 Marine corrosion can cause minute surface fissures, much like the proliferation of cancer cells. Over time, this can culminate in large-area corrosion, which weakens the surface and poses major security risks. Every year, the "cancer of ships" causes tremendous economic and financial damages that create global problems. According to the National Association of Corrosion Engineers (NACE) survey, the annual cost of corrosion around the world is 3.4% ($2.5 trillion) of global GDP.45 Thus, reducing the effects of marine biofouling and corrosion is crucial for the growth of the worldwide maritime sector. The previous research articles have suggested that biofouling and corrosive behavior under different field testing like underwater, seawater, river water, and chemical solution have affected the performance of coated materials. Moreover, surface coating methods have recently demonstrated promise as affordable and effective ways to shield subsea surfaces to prevent corrosion and biofouling. Although conventional coatings often serve just one purpose, such as antifouling or anticorrosion. Therefore, merging anticorrosion and antifouling properties into hybridized coating has excellent potential to mitigate fouling.
This review paper compares various environmentally friendly, efficient, and long-lasting antifouling methods for marine applications. Fouling-resistant polymer coatings, superhydrophobic coatings, and fouling-release polymeric coatings are the three main coating methods that have been discussed briefly. Further, the study also envisages the impact of various metal oxide nanoparticles, such as ZnO, SiO2, TiO2, nanorod, and others, on the design of superhydrophobic surfaces. Wenzel, Cassie–Baxter, and Young's wetting models are a few analytical models used to determine the appropriate sliding angle and water angle of contact for the superhydrophobic surface. Overall, this review will provide a brief insight to the reader about biofouling's effect and prevention methods.
Methodologies for preventing biofouling
Nature-inspired biomimetic coatings
A methodical replication of biological features on a surface is percieved to give a proficient, efficient, and eco-friendly method to prevent biofouling. Researchers have created and used this nature-inspired concept for engineering applications.46,47 Biofouling is primarily influenced by surface characteristics such as wettability and microtexture.48,49,50,51 Wettability impacts fouler colonization, ranging from water-loving hydrophilic to water-repelling superhydrophobic. Surface roughness and surface energy are the two characteristics that govern wettability. Siloxanes, stearic acid, and fluorocarbons are the most frequent materials used to reduce surface energy. The concept of superhydrophobicity was inspired by nature, with a WCA of more than 150° and a sliding angle below 10° to reduce the slippery and sticky properties of the surface against water.52,53,54,55,56 Superhydrophobic surfaces, such as lotus leaves,57,58 butterfly wings,59,60 peanut leaf,61 red rose petal,62,63 water strider legs,64 and fish scale,65,66 have low surface energy to resist wetting. Tables 1 and 2 provide various examples of nature-inspired flora and fauna, as well as their mechanisms which help to understand the superhydrophobic concept from nature and inspire engineers to use concepts of superhydrophobicity to prevent biofouling for engineering applications.
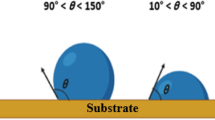
Table 2 presents an example of nature-inspired fauna such as shark fish, dog fish, stone fish, butterflies, blue mussels, dragonflies, pilot whales, and doves. They are mainly found in nature with superhydrophobic characteristics, low surface free energy, and ridge microtexture, which helps them swim on the water's surface. This nature-inspired fauna concept may be helpful for researchers to apply superhydrophobic concepts in engineering materials to prevent biofouling on the ship’s hull. The most frequent fouling organisms found at the test sites of the samples are bryozoans, barnacles, calcareous algae, bivalves, tubeworms, and hydroids. The surface can be classified as hydrophilic, hydrophobic, or superhydrophobic based on the constructed contact angle. The varying contact angles of wetting properties are depicted in Fig. 2. Bacterial attachment to ship hulls is reduced due to the low surface energy. It was created to minimize interactions with biomolecules by removing strong polar interactions (like hydrogen or ionic bonding). Due to uneven rough surfaces, the superhydrophobic surface can be beneficial. Air bubbles are trapped on the surface of hills and valleys, consisting of micro–nano-structures and nano-enclosed pores, increasing the water contact angle. Based on the Cassie–Wenzel hypothesis, researchers investigated the chemistry of nonstick and antiadhesion coatings.83,84,85,86 The Wenzel model is applicable for homogenous surfaces having the equation:
where θw = Wenzel angle of contact, θ = Young's angle of contact, r = Surface roughness factor.
The contact angle of wetting properties67
For heterogeneous surface Cassie and Baxter's model is applicable by the equation:
where θ = Baxter and Cassie angle of contact, f1 = contact area ratio where solid is in contact with liquid, f2 = contact area ratio with air packets that confine the inner side of surface cavities
Xie et al.87 fabricated a ZnO/Acrylic superhydrophobic surface on an aluminum substrate via spraying and co-curing to prevent marine biofouling. They obtained a rolling angle of 1.8° and a WCA of 171°, indicating a superhydrophobic surface after coating. SEM images of the sample shown in Fig. 3 confirmed the results of the antibiofouling test after 150 days in a chlorella-inoculated growth. In Figs. 3a and c, a few chlorellae were adsorbed on zinc oxide pure acrylic polyurethane superhydrophobic coating, indicating it suppresses biofouling. There was a considerable amount of chlorella with a diameter of 5 µm, as indicated by the red circle in Figs. 3b and d, adsorbed on the pure acrylic polyurethane (PUA) coating.
SEM images of samples after 150 days: (a and c) indicates zinc oxide pure acrylic polyurethane while (b and d) indicates pure acrylic polyurethane coating87
Sun et al.88 generated an SHS on a stainless steel substrate using a picosecond laser texturing approach. After five weeks of immersion in seawater, a specimen with SHS found considerable antibiofouling impact on a substrate compared to bare stainless steel plates. Figure 4a and b shows a microgroove and micropit array on the surface indicated by the red rectangle that suppresses biofouling.
(a) SEM image of microgroove and (b) Micropit pattern of SHS after 5 weeks88
Selim et al.89 developed a superhydrophobic PDMS/SiC nanowire composite for marine applications. It was observed that there was an excellent antibiofouling property due to micro/nanoscale roughness on their surfaces. The SiC nanowire with (0.5 wt%) showed excellent hydrophobicity with a WCA of 153° and a low surface free energy of 11.25 mN/m after three months in seawater. Selim et al.90 created superhydrophobic Si/MnO2 nanorod composite coating on sample and submerged them in seawater for 90 days in the same year to attenuate marine fouling, as illustrated in Fig. 5. It is noticed that uniformly scattered nanorods (0.5 wt%) have a superhydrophobic nature. The nanorod has a WCA of 158° and surface free energy of 12.65 mN/m. This results in an excellent eco-friendly foul-release coating for ship hulls.
In situ synthesis of PDMS/β-MnO2 nanorod composite coating90
Selim et al.91 considered an in situ approach to produce (PDMS)/Al2O3 nanorod composites for marine antibiofouling and found that uniform distribution of Al2O3 nanorods inside the PDMS resin gives improved fouling repellence with WCA of 169° and surface free energy of 10.03 mN/m. Similarly, in another study, He et al.92 used a spin-coating approach to create a superhydrophobic surface using PDMS precursor and silicon dioxide. It is reported that the addition of SiO2 nanoparticles increased the WCA from hydrophobic (106.8 ± 1.2°) to superhydrophobic (165.2 ± 2.3°) property. This is owing to the presence of micro–nano-scale features of SiO2 which aids to the increase in superhydrophobicity resulting in slipping of organisms from the substrate surface. An et al.93 used PDMS coated with fluoroalkyl silane and cerium dioxide (CeO2) nanoparticles to form a superhydrophobic composite coating with a rough texture. The results showed good superhydrophobicity with a water contact angle of (161 + 2°) and a sliding inclination of (4 + 1°). CeO2 nanoparticles also provided adequate corrosion protection for metal substrates even in adverse conditions due to their air cushion and corrosion hindrance.
Fouling release coating
Fouling release (FR) coatings are considered environmentally acceptable antifouling coating methods since they have low interface energy and elastic modulus. Low elastic modulus detaches hard foulants like barnacles, and low interface energy reduces relative adhesion.94,95,96,97 The low surface elasticity makes the coated surface flexible or dynamic and creates difficulty for microorganisms to settle on it. It can be described further by the dynamic surface antifouling (DSA) mechanism.98,99,100,101,102 FR does not get dissolved or decompose in seawater and does not release any hazardous chemicals or gases. They weaken the bond between the substrate and the fouling organism, therefore causing easy removal of the microorganisms with a simple cleaning approach.103,104 All fouling organisms will fall off automatically when a ship's hull is coated with fouling release coatings, allowing the ship to travel at a speed of 10 to 20 knots in usual conditions.105 Fluoropolymers and polysiloxanes are now widely utilized fouling-release coating materials to prevent biofouling. Fluoropolymers are nonpolar, with a low surface tension of 10–20 mN m−1 to confer the hydrophobic nature of the surface.106 However, fluoropolymer cannot cover large parts of a ship's hull effectively because it has various defects, including limited structural mobility, which limits fluorine stiffness and rotations in the entire polymer matrix, making fluorine monomers expensive.107 Polysiloxanes are cheaper than fluoropolymers and have superior physicochemical and mechanical qualities. Polysiloxanes are used in a variety of applications ranging from space to marine, with a market share of 17.2 billion dollars in 2017.108,109
Milne was the first to propose using polysiloxanes as antifouling agents.110 Polysiloxanes have a smooth surface and stable chemical characteristics and are resistant to corrosion. However, siloxane chains have low bonding strength as well as poor mechanical qualities, and also they get easily torn and detached from the substrate when subjected to a dynamic environment.111 Polysiloxanes exhibit poor antifouling performance in static conditions. PDMS (polydimethylsiloxane) with low surface energy and small Young's modulus is more efficient than polysiloxanes. PDMS is employed as a marine fouling release because it fits in the fouling release zone criteria of the Baier curve. In the late l960s, Baier gave the correlation between polymer surface properties and relative adhesion of fouling organisms, as shown in Fig. 5. According to the curve, relative adhesion is lowest when surface energy is 20–30 mN m−1, often known as the fouling release zone. Moreover, PDMS meets these conditions as it reduces microfouling settlements on the coated surface.112,113,114 Nontoxicity, cost-effectiveness, good heat resistance, nonleaching property, and long-term durability against ultraviolet irradiation are all advantages of PDMS coatings.115 However, its main drawback is poor mechanical potential, which can be enhanced by adding nanofillers (Fig. 6).
Baier Curve112
Cavas et al.116 designed a PDMS-based FR coating with MWCTs and graphene oxide (GO) reinforcement. The antibiofouling performance and mechanical properties of such nanocomposite coating were improved with 0.5 wt% reinforcing elements. The characteristics of PDMS with varying wt% of multiwall carbon nanotubes (MWCNT) were investigated by Koumoulos et al.117 They discovered a steady diffusion of carbon nanotubes in the PDMS matrix which improved the composite's surface and mechanical properties. As the wt% of (CNT) gets increased, the surface's mechanical capabilities get reduced due to agglomeration of CNT in the polymer matrix, whereas the hydrophobicity of the material gets enhanced. Carl et al.118 investigated the incorporation of TiO2 and carbon nanotubes (CNTs) in PDMS to ameliorate (FR) characteristics against the mussel. Nanofillers make larvae difficult to adhere onto the surface and reduce adhesive bonding. As per the investigation, the dispersion of TiO2 and CNTs also improved the mechanical characteristics of the coatings. Roy et al.119 used octa-methyl-cyclo-tetra-siloxane to make a PDMS-sepiolite nanocomposite. The findings revealed that the consistent interaction of PDMS-sepiolite with the polymer matrix improves the polymer's thermal, dynamic, and mechanical properties while reduces the relative adhesion of fouling communities.
In another study, Irani et al.120 produced a PDMS coating with MWCNTs varying wt% of 0.05, 0.1, and 0.2. As nanotubes were applied in small amounts, the surface chemistry of the coating gets changed, therefore significantly improving fouling release properties and lowering adhesion strength by 67% compared to the uncoated sample. Yang et al.121 developed FR coating by reinforcing alumina in PDMS containing phenyl methyl silicone oil. Upon testing it was found that inclusion of alumina significantly improved the coating elastic modulus and shore hardness but sacrificed the antifouling performance. Zhao et al.122 used an epoxy silicone prepolymer combined with PDMS to make epoxy-modified silicone. The coating's adherence and mechanical properties are improved by changing epoxy to silicone. Ba et al.123 used phenylmethyl silicone oil to create an FR coating on PDMS (PSO). The addition of PSO improved the coating's antifouling capabilities by increasing its hydrophobicity and reducing its elastic modulus. Padmavathi et al.124 used a wet chemical precipitation process to make CuO nanoparticles, which were placed into a PDMS FR surface to improve micro–macro-fouling. The findings suggested that incorporating nanofillers into a PDMS matrix could be a feasible antifouling method to prevent algal fouling and larval colonization by imparting toxicity at the surface through metal ion release. Seo et al.125 proposed an environmentally friendly oleamide-PDMS copolymer (OPC)-based coating for antifouling and drag reduction. It is reported that algae spores and mussels could not adhere to the developed OPC surface due to its slippery characteristics. The suggested OPC surface coating can be used in various commercial products, such as biomedical equipment, marine vehicles, and water management.
Fouling-resistant coatings
Algae, bacteria, and protein discharge from marine species can be prevented or inhibited by fouling-resistant coatings.126,127,128 It is used mainly to modify the surface of marine components to prevent unwanted adherence. It involves high interfacial hydrated surfaces with a strong water network that inhibits foulants from adhering and is usually formed by hydrophilic polymers like polyethylene glycol (PEG) and zwitterions.129 However, they swell in maritime environments, resulting in poor mechanical qualities that limit their usage.
Polyethylene glycol (PEG)-based polymers
PEG is a nontoxic, extremely hydrophilic, and neutrally charged compound. It is used for coating because of its antifouling solid potential to minimize unwanted protein adsorption and cell adhesion. By forming hydrogen bonds with water, PEG lowered interfacial energy and inhibits the adhesion of larvae and spores.130,131 PEG decomposes on the coated surface due to autooxidation in the presence of oxygen and transition metal ions.132
Perrin et al.133 produced an antifouling coating by using a hydrosilylation procedure to covalently graft PEG chains onto silazane polymers, as illustrated in Fig. 7. The PEG-grafted coatings are very resistant to Gram-negative Neisseria sp. and Gram-positive Clostridium sp. bacteria compared to the pristine polysiloxane surface. Similarly, Leckband et al.134 described two methods for creating PEG layers on silicone surfaces, focusing on protein adsorption with the macroscopic surface and adjusting the molecular interaction between adsorbing proteins and the surface. The modified surface shows antifouling properties, which can resist massive protein molecules. Kingshott et al.135 found that PEG layer grafting reduces Gram-negative bacteria adhesion and prevents protein adsorption and consequent biofouling. Yang et al.136 used the Michael addition reaction to make PEG-based hydrogels by incorporating polycarbonate. These hydrogels were grafted on silicon rubber to evaluate S. aureus antibacterial activity for a day. The results demonstrated the presence of different S. aureus on the rubber surface without coating. After coating, nothing was found on the surface, indicating that coating prevents surface fouling. Zhou et al.137 combined PEG of various molecular weights with alkyl alcohol to create amphiphilic surfactants. The results obtained show an amphiphilic-coated surface can be employed for antifouling and foul-release coating, potentially paving the way for a fluorine-free, ecologically acceptable foul-release polymeric coating. Due to ether linkages, PEG is chemically unstable and prone to degradation. In contrast, zwitterionic polymers are chemically stable and most effective for fouling applications to attach water molecules through ionic interactions.
PEG grafted network for marine biofouling133
Zwitterionic polymers
PEG can be replaced with zwitterionic polymers. Zwitterionic, unlike amphiphilic PEG, is a neutral molecule with both positive and negative charges in its structure, making it very hydrophilic.138,139 It has been identified as a promising antifouling material due to its ability to build a hydration shell via electrostatic interactions, stronger than hydrogen bonds.140,141 Nonspecific adsorption at the solid/liquid interface can be reduced thin or thick coating of zwitterionic compound films.139 These materials are chemically stable and inexpensive. Due to its remarkable antifouling properties, it has been used to develop antifouling surfaces for biosensors, medical devices, and marine coatings applications.142,143
Dai et al.101 created antifouling copolymers incorporating hydrolysis-induced zwitterionic monomer tertiary carboxybetaine triisopropylsilyl ester acrylate (TCBSA) copolymerized with methyl methacrylate (MMA). It was noticed that the surface is resistant to marine bacteria, algae, and diatoms in terms of antifouling and protein resistance. Zhang et al.144 employed atom transfer radical polymerization (ATRP) to graft zwitterionic sulfobetaine methacrylate (SBMA) on glass surfaces to create a polymer layer. Zwitterionic polymers completely prevent spores from settling and reduce adhesion as well as form biofilm in bacteria. Zwitterionic polymers have weak mechanical properties and high water absorption and are not directly employed as homopolymers. Wu et al.145 developed copolymers with zwitterionic side chains attached to the glass substrate via a reverse addition-fragmentation chain transfer (RAFT) polymerization technique to solve this problem. The study has outlined a simple but effective process to produce nonfouling antifouling surfaces. Niu et al.146 designed copolymers with adhesion and antifouling properties for surface modification by combining the dopamine methacrylamide (DMA) and the zwitterionic monomer 2- methacryloyloxyethylphosphocholine (MPC). It was found that copolymer-modified substrate has outstanding antifouling performance in resisting protein adsorption and repelling oil attachments.
Role of inorganic nano-fillers in preventing biofouling
Incorporating inorganic nano-fillers is often advantageous to the coating mechanical properties such as wear and scratch resistance and has more potential to prevent biofouling. It increases cost savings while improving mechanical, self-cleaning, and lotus effect properties. 147,148 Self-cleaning nano-surfaces could achieve fouling prevention on ship hulls with the lotus effect, superhydrophobic surfaces with greater WCA, and low surface-free energy parameters.149 Selim et al.150 used an in situ approach to create a polymethyl/ZnO nanorod composite for a superhydrophobic surface and foul release (FR) antifouling coating surface. The rough topography inhibits water from pervading the surface. Including 0.5 wt% ZnO nano-fillers in the silicone matrix promotes nonwettability and surface roughness and reduces surface-free energy. More significantly, nano-filler concentrations (up to 5 wt%) result in low water and fouling repellence due to the clustering of nano-fillers, which generates gaps, pinholes, and crevices on the matrix surface. It affects coating adherence and performance against marine organisms. The study developed a cost-effective and environmentally friendly alternative for marine antifouling coatings using nanofillers at a concentration of 0.5 wt%. They offer favorable FR characteristics with a maximum WCA of 158° and minimum free energy of 11.25 mN/m. The bare silicone and dispersed ZnO NR composites (0.5–5 wt%) were tested in sea water for six months. The results are presented in Fig. 8 which shows high fouling prevention at 0.5 wt% due to low free surface energy and homogenous filler dispersion.
(A, A1, A2, and A3) show foul release coating of bare PDMS; while (B, B1, B2, and B3) show (0.5 wt% NRs) for silicone/ZnO nanocomposites and (C, C1, C2, and C3) show (5 wt% NRs) silicone/ZnO composite coating immersion in seawater for six months150
Arukalam et al.151 coated Q235 steel with a perfluoro-decyl trichlorosilane (FDTS)-based hydrophobic polydimethylsiloxane (PDMS)-ZnO nanocomposite coating to achieve antifouling and anticorrosive properties. Due to their antibacterial characteristics and hydrophobic nature, ZnO nanoparticles provide antifouling and minimize hydrophobicity. The surface energy of the coating was modified via FDTS, which reduced the contact angle while enhancing antibiofouling and anticorrosive efficacy. Because the trichloro group of FDTS grows and forms a hydroxyl bond on the steel surface, the adhesion strength of Q235 steel improves. Zhang et al.152 successfully used a simple dipping approach to fabricate superhydrophobic TiO2 nanowire coating. The coated surface has antifouling properties for organic solvents with a low boiling point. The TiO2 nanowire allows damaged coating surfaces to be easily regenerated and repaired. Using fluorinated silica (F-SiO2) particles as raw material, Liu et al.153 designed an inorganic/organic composite coating with good mechanical features. The addition of F-SiO2 to composites results in a 27.7% drag reduction. The application of graphene for preventing marine biofouling is reviewed by Jin et al.,154 who observed that graphene incorporation on coated surfaces improved mechanical strength and antifouling characteristics, and created superhydrophobic surfaces that are environmentally friendly.
Anticorrosive paints to prevent biofouling and corrosion
The depletion and failure of structures and components are caused by physical, chemical, and certain biological phenomena known as wear, erosion, and corrosion. Several intricate elements frequently contribute to marine corrosion. For instance, rapid liquid flow and sand impact on surfaces cause erosion and corrosion, and high-speed propeller rotation causes rusting due to cavitation. In sea water, different salts, such as magnesium chloride, potassium sulfate, magnesium sulfate, and sodium chloride, are present that significantly increase corrosive behavior due to the increment of the electrical conductivity of seawater. This phenomenon has been observed because the dissolved salt conducts more ions in seawater, releasing positively charged cations and negatively charged anions. Furthermore, under the influence of waves and seawater scouring, the surface seawater is sufficiently rich in CO2 and O2 to touch the metal surface thoroughly, enhancing the chemical and electrochemical processes between the metal and seawater.155
Anticorrosive paint on superhydrophobic surfaces is another superficial method to prevent corrosive behavior and avoid fouling. Anticorrosive paint is a system developed in which different layers are coated using various coatings processes to influence the corrosive properties.156 These individual coatings can be metallic, inorganic, or organic, consisting of primer, topcoat, and one or several intermediate coats, preventing high material corrosion in marine environments. Nurani et al.157 coated (20 × 25 × 0.3 cm3) mild steel specimen with anticorrosion and antifouling paint. Before coating, the mild steel specimen was sandblasted. The test specimens were immersed in splash and tidal zone (0, 1, and 3 m from sea level) for a month, considering various seawater parameters. It was found that after antifouling coating, no fouling and corrosion took place, whereas the bare mild steel sample got corroded by 17 MPY. Similarly, in other studies, proactive water grooming was used to improve the performance of ship hull antifouling coating. Before coating, the surface was groomed (cleaned) to free it from fouling. Furthermore, an ablative copper antifouling coating, silicone fouling release coating, epoxy coating, and coating with PTFE solid sheer upon sample underwent exposure to static seawater immersion situated at East West of Florida for 120 days and grooming intervals of 3, 6, 12, and 24 days. It was found that grooming reduces the biofilm development on ablative copper-coated samples.158 Various laboratory tests can be performed on the samples to analyze the corrosion behavior on coated samples, such as the physical, chemical, and acid–alkali tests. The physical examinations determine the degree of adhesion, hardness, and flexibility. The chemical tests are based on the presumption that the pace at which paint pigments dissolve in artificial aging solutions in the laboratory accelerates at a rate similar to that of seawater. The acid–alkali test depends on hydrochloric acid's leaching rate and pH value.
Conclusions
Marine biofouling is an issue that has received special attention from scientists, engineers, biologists, and other experts in diverse sectors. Collaborative work with numerous advancements has led to the development of antifouling technology based on various surface treatments. Inspired by nature (like flora and fauna), a researcher discovered antifouling technology and applied it to remove biofouling. In this review, various novel methods of advanced coating technology to prevent biofouling are discussed, and the following conclusions were drawn:
-
1.
Biologically inspired superhydrophobic surface can curtail biofouling by forming nonadhesive micro–nano-grooves and nano-enclosed pores on the surface.
-
2.
Several foul-release coating materials such as fluoropolymers, polysiloxanes, and PDMS can prevent biofouling, but PDMS is found to be efficient with low surface energy and small Young's modulus, and hence, it is highly effective in preventing biofouling.
-
3.
The addition of nanofiller to PDMS surfaces resulted in a broad-spectrum antibacterial and antifouling property, which improved biofouling prevention in the marine environment.
-
4.
PEG has been found to be effective as a surface coating to minimize unwanted protein adsorption and cell adhesion. In contrast, zwitterionic polymer is slowly replacing PEG polymers because of its better antifouling properties.
-
5.
Inorganic nanoparticles are effectively used in coatings which act as different foul release agents and increase the coating mechanical properties, wetting angle, and surface energy reduction.
-
6.
Finally, widespread application on the basis of the coating's nature and level of toxicity is still a major setback since the residues of these coatings are entering the marine environment and directly/indirectly may harm the ecosystems. Hence, additional research is recommended to determine the best eco-friendly strategy that will benefit humans and ecosystems.
-
7.
It is also observed that corrosion in a laboratory environment is limited to a small number of tests, such as electrochemical, algae related and salt bath tests. However, field test has a wide variety of corrosion, such as sea water temperature, dissolved oxygen, chloride concentration, and water flow velocity.
Nevertheless, the present review has shown that coatings can be tailored to improve the biofouling ability and other mechanical properties. Further, a wide range of nanoparticles are available that have shown specific improvement in mechanical properties, and structural integrity of composite nanocoatings. Further, the composite coatings have shown remarkable improvement in preventing biofouling, increase in adhesion, superhydrophobicity, and adhesion property. This review will also help the present researchers working in this field and coating industries to explore the advantages of composite layers and consider the critical results mentioned in this paper.
References
He, Z, et al. “Antifouling Strategies Based on Super-Phobic Polymer Materials.” Prog. Org. Coat., 157 106285 (2021)
Fitridge, I, Dempster, T, Guenther, J, de Nys, R, “The Impact and Control of Biofouling in Marine Aquaculture: A Review.” Biofouling, 28 649–669 (2012)
Callow, JA, Callow, ME, “Trends in the Development of Environmentally Friendly Fouling-Resistant Marine Coatings.” Nat. Commun. 2 (2011)
Tesler, AB, et al, “Extremely Durable Biofouling-resistant Metallic Surfaces Based on Electrodeposited Nanoporous Tungstite Films on Steel.” Nat. Commun. 6 (2015)
Hellio, C, Yebra, D, Advances in Marine Antifouling Coatings and Technologies. Woodhead (2009)
Yebra, DM, Kiil, S, Dam-Johansen, K, “Antifouling Technology—Past, Present and Future Steps Towards Efficient and Environmentally Friendly Antifouling Coatings.” Prog. Org. Coat., 50 75–104 (2004)
Dürr, S, Thomason, J, Biofouling. Wiley, Blackwell (2010)
Bers, AV, Wahl, M, “The Influence of Natural Surface Microtopographies on Fouling.” Biofouling, 20 43–51 (2004)
Dobretsov, S, Dahms, HU, Qian, PY, “Inhibition of Biofouling by Marine Microorganisms and Their Metabolites.” Biofouling, 22 43–54 (2006)
Almeida, E, Diamantino, TC, de Sousa, O, “Marine Paints the Particular Case of Antifouling Paints.” Prog. Org. Coat., 59 2–20 (2007)
Zobell, CE, Allen, EC, The Significance of Marine Bacteria in the Fouling of Submerged Surfaces (2021)
Selim, MS, et al. “Recent Progress in Marine Foul-Release Polymeric Nanocomposite Coatings.” Prog. Mater. Sci., 87 1–32 (2017)
Lindholdt, A, Dam-Johansen, K, Olsen, SM, Yebra, DM, Kiil, S, “Effects of Biofouling Development on Drag Forces of Hull Coatings for Ocean-Going Ships: A Review.” J. Coat. Technol. Res., 12 415–444. https://doi.org/10.1007/s11998-014-9651-2 (2015)
Schultz, MP, “Effects of Coating Roughness and Biofouling on Ship Resistance and Powering.” Biofouling, 23 331–341 (2007)
Miller, AW, et al. “Evaluation of Wetted Surface Area of Commercial Ships as Biofouling Habitat Flux to the United States.” Biol. Invasions, 20 1977–1990 (2018)
Schultz, MP, Bendick, JA, Holm, ER, Hertel, WM, “Economic Impact of Biofouling on a Naval Surface Ship.” Biofouling, 27 87–98 (2011)
Silva, ER, et al. “Eco-friendly Non-biocide-Release Coatings for Marine Biofouling Prevention.” Sci. Total Environ., 650 2499–2511 (2019)
Tian, L, Yin, Y, Bing, W, Jin, E, “Antifouling Technology Trends in Marine Environmental Protection.” J. Bionic Eng., 18 239–263 (2021)
Chambers, LD, Stokes, KR, Walsh, FC, Wood, RJK, “Modern Approaches to Marine Antifouling Coatings.” Surf. Coat. Technol., 201 3642–3652 (2006)
Zhang, P, Lin, L, Zang, D, Guo, X, Liu, M, “Designing Bioinspired Anti-Biofouling Surfaces Based on a Superwettability Strategy.” Small 13 (2017)
Gule, NP, Begum, NM, Klumperman, B, “Advances in Biofouling Mitigation: A Review.” Crit. Rev. Environ. Sci. Technol., 46 535–555 (2016)
Pistone, A, Scolaro, C, Visco, A, “Mechanical Properties of Protective Coatings Against Marine Fouling: A Review.” Polymers, 13 1–19 (2021)
Ali, A. et al. “An Overview of Controlled-Biocide-Release Coating Based on Polymer Resin for Marine Antifouling Applications.” https://doi.org/10.1007/s10965-020-02054-z/Published
Kiil, S, Weinell, CE, Pedersen, MS, Dam-Johansen, K, “Analysis of Self-polishing Antifouling Paints Using Rotary Experiments and Mathematical Modeling.” Ind. Eng. Chem. Res., 40 3906–3920 (2001)
Champ, MA, “A Review of Organotin Regulatory Strategies, Pending Actions, Related Costs, and Benefits.” Sci. Total Environ. 258 (2000)
Ciriminna, R, Bright, FV, Pagliaro, M, “Ecofriendly Antifouling Marine Coatings.” ACS Sustain. Chem. Eng., 3 559–565 (2015)
Buskens, P, Wouters, M, Rentrop, C, Vroon, Z, “A Brief Review of Environmentally Benign Antifouling and Foul-Release Coatings for Marine Applications.” J. Coat. Technol. Res., 10 29–36 (2013)
Feng, XJ, Shi, YL, Wang, YS, Yue, GR, Yang, W, “Preparation of Superhydrophobic Silver Nanocoatings with Feather-like Structures by Electroless Galvanic Deposition.” Chin. Sci. Bull., 58 1887–1891 (2013)
Mumm, F, Van Helvoort, ATJ, Sikorski, P, “Easy Route to Superhydrophobic Copper-Based Wire-Guided Droplet Microfluidic Systems.” ACS Nano, 3 2647–2652 (2009)
Parkin, IP, Palgrave, RG, “Self-cleaning Coatings.” J. Mater. Chem., 15 1689–1695 (2005)
Sun, T, Feng, L, Gao, X, Jiang, L, “Bioinspired Surfaces with Special Wettability.” Acc. Chem. Res., 38 644–652 (2005)
Nakajima, A, Hashimoto, K, Watanabe, T, “Recent Studies on Super-Hydrophobic Films.” In: Molecular Materials and Functional Polymers, pp. 31–41.
Feng, L, et al. Super-Hydrophobic Surfaces: From Natural to Artificial.
Tuteja, A. et al. Designing Superoleophobic Surfaces.
Cassie, ABD, Baxter, S, “Wettability of Porous Surfaces.” Trans. Faraday Soc., 40 546–551 (1944)
Wang, D, Liu, H, Yang, J, Zhou, S, “Seawater-Induced Healable Underwater Superoleophobic Antifouling Coatings.” ACS Appl. Mater. Interfaces, 11 1353–1362 (2019)
Chen, K, Zhou, S, Wu, L, “Self-healing Underwater Superoleophobic and Anti Biofouling Coatings Based on the Assembly of Hierarchical Microgel Spheres.” ACS Nano, 10 1386–1394 (2016)
Su, X, Hao, D, Li, Z, Guo, X, Jiang, L, “Design of Hierarchical Comb Hydrophilic Polymer Brush (HCHPB) Surfaces Inspired by Fish Mucus for Anti-biofouling.” J. Mater. Chem. B, 7 1322–1332 (2019)
Wen, L, Weaver, JC, Lauder, GV, “Biomimetic Shark Skin: Design, Fabrication, and Hydrodynamic Function.” J. Exp. Biol., 217 1656–1666 (2014)
Liu, Y, Li, G, “A New Method for Producing ‘Lotus Effect’ on Biomimetic Shark Skin.” J. Colloid Interface Sci., 388 235–242 (2012)
Davidson, IC, Smith, G, Ashton, GV, Ruiz, GM, Scianni, C, “An Experimental Test of Stationary Lay-up Periods and Simulated Transit on Biofouling Accumulation and Transfer on Ships.” Biofouling, 36 455–466 (2020)
Videla, HA, “Prevention and Control of Biocorrosion.” Int. Biodeterior. Biodegrad., 49 259–270 (2002)
Little, BJ, et al. “Microbially Influenced Corrosion—Any Progress?” Corros. Sci., 170 108641 (2020)
Usher, KM, Kaksonen, AH, Cole, I, Marney, D, “Critical Review: Microbially Influenced Corrosion of Buried Carbon Steel Pipes.” Int. Biodeterior. Biodegrad., 93 84–106 (2014)
Saranya, J, et al. “Quinoxaline Derivatives as Anticorrosion Additives for Metals.” Corros. Rev., 39 79–92 (2021)
Bixler, GD, Theiss, A, Bhushan, B, Lee, SC, “Anti-fouling Properties of Microstructured Surfaces Bio-inspired by Rice Leaves and Butterfly Wings.” J. Colloid Interface Sci., 419 114–133 (2014)
Bixler, GD, Bhushan, B, “Bioinspired Rice Leaf and Butterfly Wing Surface Structures Combining Shark Skin and Lotus Effects.” Soft Matter, 8 11271–11284 (2012)
Fingerman, M, Nagabhushanam, R, Recent Advances in Marine Biotechnology.
Telegdi, J, Trif, L, Roma, L, “Smart Anti-biofouling Composite Coatings for Naval Applications” (2016). https://doi.org/10.1016/B978-1-78242-283-9.00005-1
Lu, X, Peng, Y, Qiu, H, Liu, X, Ge, L, “Anti-fouling Membranes by Manipulating Surface Wettability and Their Anti-fouling Mechanism.” Desalination, 413 127–135 (2017)
Scardino, AJ, Fletcher, LE, Lewis, JA, “Fouling Control Using Air Bubble Curtains: Protection for Stationary Vessels.” J. Mar. Eng. Technol., 8 (1) 3–10 (2014)
Song, J, Liu, X, Lu, Y, Wu, L, Xu, W, “A Rapid Two-step Electroless Deposition Process to Fabricate Superhydrophobic Coatings on Steel Substrates.” J. Coat. Technol. Res., 9 643–650 (2012)
Bayer, IS, “Superhydrophobic Coatings from Ecofriendly Materials and Processes: A Review.” Adv. Mater. Interfaces, 7 (13) 2000095 (2020)
Shao, Y. et al. “Shape Memory Superhydrophobic Surface with the Switchable Transition Between ‘Lotus Effect’ to ‘Rose Petal Effect.’” Chem. Eng. J. 382 (2020)
Vazirinasab, E, Jafari, R, Momen, G, “Application of Superhydrophobic Coatings as a Corrosion Barrier: A Review.” Surf. Coat. Technol., 341 40–56 (2018)
Jiaqiang, E. et al. “Wetting Models and Working Mechanisms of Typical Surfaces Existing in Nature and Their Application on Superhydrophobic Surfaces: A Review.” Adv. Mater. Interfaces 5 (2018)
Samaha, MA, Tafreshi, HV, Gad-el-hak, M, “Comptes Rendus Mecanique Superhydrophobic Surfaces : From the Lotus Leaf to the Submarine.” Comptes Rendus Mec., 340 18–34 (2012)
Neinhuis, C, Barthlott, W, “Characterization and Distribution of Water-repellent, Self-cleaning Plant Surfaces.” Ann. Bot., 79 (1997)
Niu, S, et al. “Excellent Structure-Based Multifunction of Morpho Butterfly Wings: A Review.” J. Bionic Eng., 12 170–189 (2015)
Zheng, Y, Gao, X, Jiang, L, “Directional Adhesion of Superhydrophobic Butterfly Wings.” Soft Matter, 3 178–182 (2007)
Darmanin, T, Guittard, F, “Superhydrophobic and Superoleophobic Properties in Nature.” Mater. Today, 18 273–285 (2015)
Feng, L, et al. “Petal Effect: A Superhydrophobic State with High Adhesive Force.” Langmuir, 24 4114–4119 (2008)
Long, J, et al. “Superhydrophobic Surfaces Fabricated by Femtosecond Laser with Tunable Water Adhesion: From Lotus Leaf to Rose Petal.” ACS Appl. Mater. Interfaces, 7 9858–9865 (2015)
Su, Y, Ji, B, Huang, Y, Hwang, K, “Nature’s Design of Hierarchical Superhydrophobic Surfaces of a Water Strider for Low Adhesion and Low-Energy Dissipation.” Langmuir, 26 18926–18937 (2010)
Das, A, et al. “Synthesis of Fish Scale and Lotus Leaf Mimicking Stretchable and Durable Multilayers.” J. Mater. Chem. A, 6 15993–16002 (2018)
Zhang, J, Wu, L, Zhang, Y, Wang, A, “Mussel, and Fish Scale-Inspired Underwater Superoleophobic Kapok Membranes for Continuous and Simultaneous Removal of Insoluble Oils and Soluble Dyes in Water.” J. Mater. Chem. A, 3 18475–18482 (2015)
Comanns, P, “Passive Water Collection with the Integument: Mechanisms and their Biomimetic Potential.” J. Exp. Biol., 221 (2018)
Armstrong, RE, Drapeau, M, Loeb, CA, Valdes, JJ, Bio-inspired Innovation and National Security, pp. 1–374. (2010)
Koch, K, Bhushan, B, Barthlott, W, “Multifunctional Surface Structures of Plants: An Inspiration for Biomimetics.” Prog. Mater. Sci., 54 137–178 (2009)
Papazian, S, Parrot, D, Burýšková, B, Weinberger, F, Tasdemir, D, “Surface Chemical Defense of the Eelgrass Zostera marina Against Microbial Foulers.” Sci. Rep., 9 1–12 (2019)
Lee, HJ, Willis, CR, Stone, CA, “Modeling and Preparation of a Super-oleophobic Non-woven Fabric.” J. Mater. Sci., 46 3907–3913 (2011)
De Nys, R, Steinberg, PD, “Linking Marine Biology and Biotechnology.” Curr. Opin. Biotechnol., 13 244–248 (2002)
Scardino, AJ “Surface Modification Approaches to Control Marine Biofouling.” In: Advances in Marine Antifouling Coatings and Technologies, pp 664–692. Elsevier Ltd., (2009). https://doi.org/10.1533/9781845696313.4.664
Rao, D, Webb, JS, Kjelleberg, S, “Competitive Interactions in Mixed-species Biofilms Containing the Marine Bacterium Pseudoalteromonas tunicata.” Appl. Environ. Microbiol., 71 1729–1736 (2005)
Bhushan, B, “Biomimetics Inspired Surfaces for Drag Reduction and Oleophobicity/Philicity.” Belstein J. Nanotechnol., 2 66–84 (2011). https://doi.org/10.3762/bjnano.2.9
Martin, S, Bhushan, B, “Modeling and Optimization of Shark-inspired Riblet Geometries for Low Drag Applications.” J. Colloid Interface Sci., 474 206–215 (2016)
Leblanc, N, Davidson, J, Tremblay, R, Mcniven, M, Landry, T, “The Effect of Anti-fouling Treatments for the Clubbed Tunicate on the Blue Mussel, Mytilus edulis.” Aquaculture, 264 205–213 (2007)
Feng, X, Jiang, L, “Design and Creation of Super Wetting/Anti Wetting Surfaces.” Adv. Mater., 18 3063–3078 (2006)
Bar-Cohen, Y, Biomimetics Series.
Baum, C, et al. “Surface Properties of the Skin of the Pilot Whale Globicephala Melas.” Biofouling, 19 181–186 (2003)
Meyer, W, Seegers, U, “A Preliminary Approach to Epidermal Antimicrobial Defense in the Delphinidae.” Mar. Biol., 144 841–844 (2004)
Bhushan, B, “Biomimetics: Lessons from Nature—An Overview.” Philos. Trans. R. Soc. A. Math. Phys. Eng. Sci., 367 1445–1486 (2009)
Barati Darband, G, Aliofkhazraei, M, Khorsand, S, Sokhanvar, S, Kaboli, A, “Science and Engineering of Superhydrophobic Surfaces: Review of Corrosion Resistance, Chemical and Mechanical Stability.” Arabian J. Chem., 13 1763–1802 (2020)
Shadmani, S, Khodaei, M, Chen, X, Li, H, Superhydrophobicity Through Coatings Prepared by Chemical Methods (2020)
Nguyen, HH, et al. “Surface Characteristics and Wettability of Superhydrophobic Silanized Inorganic Glass Coating Surfaces Textured with a Picosecond Laser.” Appl. Surf. Sci., 537 (2021)
Samanta, A, Wang, Q, Shaw, SK, Ding, H, “Roles of Chemistry Modification for Laser Textured Metal Alloys to Achieve Extreme Surface Wetting Behaviors.” Mater. Des., 192 (2020)
Xie, C. et al. “ZnO/Acrylic Polyurethane Nanocomposite Superhydrophobic Coating on Aluminum Substrate Obtained via Spraying and Co-Curing for the Control of Marine Biofouling.” Surf. Interfaces, 22 (2021)
Sun, K, et al. “Anti-biofouling Superhydrophobic Surface Fabricated by Picosecond Laser Texturing Stainless Steel.” Appl. Surf. Sci., 436 263–267 (2018)
Selim, MS, et al. “Superhydrophobic Silicone/SiC Nanowire Composite as a Fouling Release Coating Material.” J. Coat. Technol. Res., 16 1165–1180 (2019)
Selim, MS, et al. “Superhydrophobic Coating of Silicone/β–MnO2 Nanorod Composite for Marine Antifouling.” Colloids Surf. A Physicochem. Eng. Asp., 570 518–530 (2019)
Selim, MS, Shenashen, MA, Fatthallah, NA, Elmarakbi, A, El-Safty, SA, “In Situ Fabrication of One-Dimensional-Based Lotus-Like Silicone/γ–Al2O3 Nanocomposites for Marine Fouling Release Coatings.” ChemistrySelect, 2 9691–9700 (2017)
He, Z, et al. “Fabrication of Superhydrophobic Coating via a Facile and Versatile Method Based on Nanoparticle Aggregates.” Appl. Surf. Sci., 258 2544–2550 (2012)
An, K. et al. “Large-scale Preparation of Superhydrophobic Cerium Dioxide Nanocomposite Coating with UV Resistance, Mechanical Robustness, and Anticorrosion Properties.” Surf. Coatings Technol., 384 (2020)
Ober, C, “Fifty Years of the Baier Curve: Progress in Understanding Antifouling Coatings.” Green Mater., 5 1–3 (2017)
Magin, CM, et al. “Antifouling Performance of Crosslinked Hydrogels: Refinement of an Attachment Model.” Biomacromolecules, 12 915–922 (2011)
Kim, J, Chisholm, BJ, Bahr, J, “Adhesion Study of Silicone Coatings: The Interaction of Thickness, Modulus and Shear Rate on Adhesion Force.” Biofouling, 23 113–120 (2007)
Chaudhury, MK, Finlay, JA, Jun, YC, Callow, ME, Callow, JA, “The Influence of Elastic Modulus and Thickness on the Release of the Soft-Fouling Green Alga Ulva linza (syn. Enteromorpha linza) from Poly(dimethylsiloxane) (PDMS) Model Networks.” Biofouling, 21 41–48 (2005)
Pocivavsek, L, et al. “Topography-driven Surface Renewal.” Nat. Phys., 14 948–953 (2018)
Dai, G, Xie, Q, Ma, C, Zhang, G, “Biodegradable Poly(ester- co-acrylate) with Antifoulant Pendant Groups for Marine Anti-Biofouling.” ACS Appl. Mater. Interfaces, 11 11947–11953 (2019)
Dai, G, Xie, Q, Chen, S, Ma, C, Zhang, G, “Biodegradable Poly(ester)-Poly(methyl methacrylate) Copolymer for Marine Anti-biofouling.” Prog. Org. Coat., 124 55–60 (2018)
Dai, G, Xie, Q, Ai, X, Ma, C, Zhang, G, “Self-Generating and Self-Renewing Zwitterionic Polymer Surfaces for Marine Anti-Biofouling.” ACS Appl. Mater. Interfaces, 11 41750–41757 (2019)
Xie, Q, Pan, J, Ma, C, Zhang, G, “Dynamic Surface Antifouling: Mechanism and Systems.” Soft Matter, 15 1087–1107 (2019)
Bressy, C, Lejars, M, “Marine Fouling: An Overview.” J. Ocean Technol., 9 (4) 19 (2014)
Ayyavoo, J, Nguyen, TPN, Jun, BM, Kim, IC, Kwon, YN, “Protection of Polymeric Membranes with Antifouling Surfacing via Surface Modifications.” Colloids Surf. A Physicochem. Eng. Asp, 506 190–201 (2016)
Liu, C, Ma, C, Xie, Q, Zhang, G, “Self-repairing Silicone Coatings for Marine Anti-biofouling.” J. Mater. Chem. A, 5 15855–15861 (2017)
Lejars, M, Margaillan, A, Bressy, C, “Fouling Release Coatings: A Nontoxic Alternative to Biocidal Antifouling Coatings.” Chem. Rev., 112 4347–4390 (2012)
Selim, MS, El-Safty, SA, Shenashen, MA, Higazy, SA, Elmarakbi, A, “Progress in Biomimetic Leverages for Marine Antifouling Using Nanocomposite Coatings.” J. Mater. Chem. B, 8 3701–3732 (2020)
Kozakiewicz, J, Ofat, I, Trzaskowska, J, “Silicone-containing Aqueous Polymer Dispersions with Hybrid Particle Structure.” Adv. Colloid Interface Sci., 223 1–39 (2015)
Truby, K. et al. “Evaluation of the Performance Enhancement of Silicone Biofouling-release Coatings by Oil Incorporation.” Biofouling, 15 141–150 (2000)
Chaudhari, C, “Adhesion of Fouling Organisms and its Prevention Technique.” Int. J. Adv. Res. Ideas Innov. Technol., 3 427–439 (2017)
Yan, F, Zhang, X, Liu, F, Li, X, Zhang, Z, “Adjusting the Properties of Silicone Rubber Filled with Nano Silica by Changing the Surface Organic Groups of Nano Silica.” Compos. Part B Eng., 75 47–52 (2015)
Magin, CM, Cooper, SP, Brennan, AB, “Non-toxic Antifouling Strategies.” Mater. Today, 13 36–44 (2010)
Brady, RF, Properties Which Influence Marine Fouling Resistance in Polymers Containing Silicon and Fluorine.
Brady, RF, Singer, IL, “Mechanical Factors Favoring Release from Fouling Release Coatings.” Biofouling, 15 73–81 (2000)
Wanka, R, et al. “Sol-Gel-Based Hybrid Materials as Antifouling and Fouling-Release Coatings for Marine Applications.” ACS Appl. Mater. Interfaces, 12 53286–53296 (2020)
Cavas, L, Yildiz, PG, Mimigianni, P, Sapalidis, A, Nitodas, S, “Reinforcement Effects of Multiwall Carbon Nanotubes and Graphene Oxide on PDMS Marine Coatings.” J. Coat. Technol. Res., 15 105–120 (2018)
Koumoulos, EP, Parousis, T, Trompeta, AFA, Kartsonakis, IA, Charitidis, CA, “Investigation of MWCNT Addition into Poly-dimethylsiloxane-based Coatings.” Plast. Rubber Compos., 45 106–117 (2016)
Carl, C, et al. “Enhancing the Efficacy of Fouling-release Coatings Against Fouling by Mytilus galloprovincialis Using Nanofillers.” Biofouling, 28 1077–1091 (2012)
Roy, N, Bhowmick, AK, “Novel In Situ Polydimethylsiloxane-sepiolite Nanocomposites: Structure-Property Relationship.” Polymer (Guildf), 51 5172–5185 (2010)
Irani, F, Jannesari, A, Bastani, S, “Effect of Fluorination of Multiwalled Carbon Nanotubes (MWCNTs) on the Surface Properties of Fouling-Release Silicone/MWCNTs Coatings.” Prog. Org. Coat., 76 375–383 (2013)
Yang, Q, Zhang, Z, Qi, Y, Zhang, H, “The Antifouling and Drag‐reduction Performance of Alumina-reinforced Polydimethylsiloxane Coatings Containing Phenylmethyl Silicone Oil.” Polymers (Basel) 13 (2021)
Zhao, R, Zhang, Z, Qi, Y, “Influence of Epoxy Content on the Properties and Marine Bacterial Adhesion of Epoxy Modified Silicone Coatings.” Coatings 10 (2020)
Ba, M, Zhang, Z, Qi, Y, “Fouling Release Coatings Based on Polydimethylsiloxane with the Incorporation of Phenylmethyl Silicone Oil.” Coatings 8 (2018)
Ramanujam Padmavathi, A, Sriyutha Murthy, P, Das, A, Subba Rao, T, “Enhanced Antifouling Property of Polydimethylsiloxane-CuO Nanocomposite in the Marine Environment.” Mater. Lett., 301 130342 (2021)
Seo, E, et al. “Anti-Biofouling Features of Eco-Friendly Oleamide-PDMS Copolymers.” ACS Omega, 5 11515–11521 (2020)
Thérien-Aubin, H, Chen, L, Ober, CK, “Fouling-Resistant Polymer Brush Coatings.” Polymer (Guildf), 52 5419–5425 (2011)
Damodaran, VB, Bhatnagar, D, Murthy, NS, “Biomedical Polymers: An Overview.” In: SpringerBriefs in Applied Sciences and Technology, pp 1–22. Springer (2016). https://doi.org/10.1007/978-3-319-32053-3_1
Sakala, GP, Reches, M, “Peptide-Based Approaches to Fight Biofouling.” Adv. Mater. Interfaces 5 (2018)
Nurioglu, AG, Esteves, ACC, De With, G, “Non-toxic, Non-biocide-release Antifouling Coatings Based on Molecular Structure Design for Marine Applications.” J. Mater. Chem. B, 3 6547–6570 (2015)
Kingshott, P, Thissen, H, Griesser, HJ, “Effects of Cloud-Point Grafting, Chain Length, and Density of PEG Layers on Competitive Adsorption of Ocular Proteins.” Biomaterials 23 (2002)
Kingshott, P, Griesser, HJ, “Surfaces that Resist Bioadhesion.” Current Opinion in Solid State and Materials Science 4 (1999)
Lundberg, P, et al. “Poly(ethylene glycol)-Based Thiol-ene Hydrogel Coatings? Curing Chemistry, Aqueous Stability, and Potential Marine Antifouling Applications.” ACS Appl. Mater. Interfaces, 2 903–912 (2010)
Perrin, FX, Nguyen, TDH, Nguyen, DL, “Formation, Structure and Antibacterial Activities of Silazane Networks Grafted with Poly(ethylene glycol) Branches.” Prog. Org. Coat., 88 92–105 (2015)
Leckband, D, Sheth1, S, Halperin, A, “Grafted Poly(ethylene oxide) Brushes as Nonfouling Surface Coatings.” J. Biomater. Sci. Polym. Ed., 10 125 (2012)
Kingshott, P, Wei, J, Bagge-Ravn, D, Gadegaard, N, Gram, L, “Covalent Attachment of poly(ethylene glycol) to Surfaces, Critical for Reducing Bacterial Adhesion.” Langmuir, 19 6912–6921 (2003)
Yang, C, et al. “Brush-like Polycarbonates Containing Dopamine, Cations, and PEG Provide a Broad-Spectrum, Antibacterial, and Antifouling Surface via One-Step Coating.” Adv. Mater., 26 7346–7351 (2014)
Zhou, Z, et al. “Amphiphilic Triblock Copolymers with PEGylated Hydrocarbon Structures as Environmentally Friendly Marine Antifouling and Fouling-Release Coatings.” Biofouling, 30 589–604 (2014)
Laschewsky, A, “Structures and Synthesis of Zwitterionic Polymers.” Polymers, 6 1544–1601 (2014)
Lowe, AB, McCormick, CL, “Synthesis and Solution Properties of Zwitterionic Polymers.” Chem. Rev., 102 4177–4189 (2002)
Jiang, S, Cao, Z, “Ultralow-Fouling, Functionalize, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications.” Adv. Mater., 22 920–932 (2010)
Chen, S, Zheng, J, Li, L, Jiang, S, “Strong Resistance of Phosphorylcholine Self-assembled Monolayers to Protein Adsorption: Insights into Non-fouling Properties of Zwitterionic Materials.” J. Am. Chem. Soc., 127 14473–14478 (2005)
Xuan, F, Liu, J, “Preparation, Characterization and Application of Zwitterionic Polymers and Membranes: Current Developments and Perspective.” Polym. Int., 58 1350–1361 (2009)
Asha, AB, Chen, Y, Narain, R, “Bioinspired Dopamine and Zwitterionic Polymers for Non-fouling Surface Engineering.” Chem. Soc. Rev., 50 11668–11683 (2021)
Qing, W, et al. “Robust Superhydrophobic-Superoleophilic Polytetrafluoroethylene Nanofibrous Membrane for Oil/Water Separation.” J. Memb. Sci., 540 354–361 (2017)
Wu, C, Zhou, Y, Wang, H, Hu, J, “P4VP Modified Zwitterionic Polymer for the Preparation of Antifouling Functionalized Surfaces.” Nanomaterials 9 (2019)
Niu, J. et al. “Bio-inspired Zwitterionic Copolymers for Antifouling Surface and Oil-Water Separation.” Colloids Surf. A Physicochem. Eng. Asp. 626 (2021)
Wouters, M, Rentrop, C, Willemsen, P, “Surface Structuring and Coating Performance. Novel Biocide-Free Nanocomposite Coatings with Anti-fouling and Fouling-Release Properties.” Prog. Org. Coat., 68 4–11 (2010)
Gomathi Sankar, G, et al. “Polydimethyl Siloxane Nanocomposites: Their Antifouling Efficacy Invitro and in Marine Conditions.” Int. Biodeterior. Biodegrad., 104 307–314 (2015)
Liu, K, Tian, Y, Jiang, L, “Bio-inspired Superoleophobic and Smart Materials: Design, Fabrication, and Application.” Prog. Mater. Sci., 58 503–564 (2013)
Selim, MS, et al. “Silicone/ZnO Nanorod Composite Coating as a Marine Antifouling Surface.” Appl. Surf. Sci., 466 40–50 (2019)
Arukalam, IO, Oguzie, EE, Li, Y, “Fabrication of FDTS-Modified PDMS-ZnO Nanocomposite Hydrophobic Coating with Anti-fouling Capability for Corrosion Protection of Q235 Steel.” J. Colloid Interface Sci., 484 220–228 (2016)
Zhang, X, Guo, Y, Zhang, Z, Zhang, P, “Self-cleaning Superhydrophobic Surface Based on Titanium Dioxide Nanowires Combined with Polydimethylsiloxane.” Appl. Surf. Sci., 284 319–323 (2013)
Liu, Y, et al. “Robust Organic-Inorganic Composite Films with Multifunctional Properties of Superhydrophobicity, Self-Healing, and Drag Reduction.” Ind. Eng. Chem. Res., 58 4468–4478 (2019)
Jin, H, Tian, L, Bing, W, Zhao, J, Ren, L, “Toward the Application of Graphene for Combating Marine Biofouling.” Adv. Sustain. Syst., 5 (2021)
Jin, H, et al. “Recent Advances in Emerging Integrated Antifouling and Anticorrosion Coatings.” Mater. Des., 213 110307 (2022)
Sørensen, PA, Kiil, S, Dam-Johansen, K, Weinell, CE, “Anticorrosive Coatings: A Review.” J. Coat. Technol. Res., 6 135–176 (2009)
Nuraini, L, Prifiharni, S, Priyotomo, G, Sundjono H, “Evaluation of Anticorrosion and Antifouling Paint Performance After Exposure Under Seawater Surabaya-Madura (Suramadu) Bridge.” AIP Conf. Proc. 1823 (2017)
Tribou, M, Swain, G, “The Use of Proactive In-water Grooming to Improve the Performance of Ship Hull Antifouling Coatings.” Biofouling, 26 47–56 (2010)
Funding
The authors do not receive any financial support from any organization to carry out the present review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of financial interest for this study's investigation, authorship, and publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, A., Mishra, V., Negi, S. et al. A systematic review on polymer-based superhydrophobic coating for preventing biofouling menace. J Coat Technol Res 20, 1499–1512 (2023). https://doi.org/10.1007/s11998-023-00773-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-023-00773-8