Abstract
In this work, a novel bio-based phosphorus-containing reagent was synthesized and characterized. To obtain a flame retardant agent with acid functionality, 9, 10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) and bio-based itaconic acid were first reacted. The synthesized acid then was reacted with glycidyl methacrylate to form a difunctional phosphorus-containing methacrylate flame retardant monomer. The flame retardant monomer was added to a reactive monomer matrix with a photoinitiator to prepare UV-curable coatings. The molecular structure of the flame retardant was confirmed by Fourier-transform infrared-attenuated total reflection spectroscopy and proton nuclear magnetic resonance (1H NMR) spectroscopy. The flame retardant behavior of coatings was determined using limiting oxygen index. Thermal behaviors of the films were studied by thermal gravimetric analysis. Furthermore, scanning electron microscopy/energy-dispersive spectroscopy was used to determine the elemental composition and to observe the morphology of the coatings. The properties (water adsorption, gel content, etc.) of the coatings were also studied. It was found that the addition of DOPO resulted in a significant improvement in the flame retardant and thermal properties of the coatings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to their lightweight and very useful properties, polymers are increasingly used instead of metal and ceramics in so many different applications such as, automotive industry, construction, and paints. However, polymers are more flammable than metals and ceramics because they have organic chemical structures such as, carbon and hydrogen.1,2,3 While the cost of the damages caused by the combustion of polymers corresponds to 1% or 2% of the country's budgets on average, the death of people and the damage to the environment are large.4,5,6
In this context, the use of flame-retardants, which have been used since the 1970s, has increased due to the increase in polymer consumption, while the use of many flame retardants has been prohibited due to the harm they cause to human health and the environment.7,8 Because of toxic gases and smoke they emit during combustion, the use of healthy and more environmentally friendly halogen-free flame-retardants is increasing instead of halogen-containing chemicals.9,10,11,12,13
One of the flame-retardants, DOPO (9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide) has been used commercially due to its non-toxicity and high fire retardant feature. It is especially used in many fields such as epoxy, polyurethane and so on. With the modification of the DOPO chemical, the application areas are also expanding.14,15,16,17,18,19,20,21
Due to the decrease in petroleum and petroleum derivatives, the prices of chemicals used in polymer production increase production costs. In addition, due to the negative effects of these chemicals on human health and the environment, research on the use of sustainable, nature-friendly and bio-based chemicals that do not harm human health is increasing.22,23,24,25,26
Itaconic acid is one of the most widely used bio-based chemicals, produced industrially mainly by fermentation with Aspergillus terreus. Due to its reactive carboxylic acid functionalities and α, β-unsaturated double bond, it is suitable for modification and reacting with other raw materials. This bond aids in crosslinking with the help of thermal or ultraviolet light.27,28,29,30
The use of UV curing technology has become widespread in recent years due to the fact that the reactions take place in a short time in the order of seconds, it requires less energy and is environmentally friendly because it does not contain solvents. This technology, which has become widespread with 3D printers, is very useful, especially for the production of plastic materials with its ease of application.31,32,33,34,35,36,37
In this present study, we report the synthesis and characterization of a novel phosphorous-containing difunctional flame retardant chemical agent, as a UV-curable monomer. The monomer was used for the preparation of flame-retardant UV-curable coatings. The flame retardant DOPO chemical was first reacted with itaconic acid to obtain a difunctional acid. Later, this acid was modified with glycidyl methacrylate to obtain a difunctional acrylic monomer suitable for UV curing. The molecular structure of the flame retardant has been performed by means of Fourier-transform infrared-attenuated total reflection (FTIR-ATR) spectroscopy and proton nuclear magnetic resonance (1H NMR) spectroscopy. The flame retardant monomer was put into a reactive monomer matrix with a photoinitiator to prepare UV-curable coatings. The flame retardant behavior of coatings has been conducted using limiting oxygen index (LOI). Scanning electron microscopy (SEM)/energy-dispersive spectroscopy (EDAX) was for determining the elemental composition and observing the morphology of the coatings. The physical properties (contact angle, gel content etc.) of the coatings were studied. It was found that the incorporation of DOPO led to a significant improvement in the flame retardant and thermal properties of the UV curable coatings.
Experimental
Materials
9, 10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) was obtained from MCT CHEM. Itaconic acid (IA) (≥ 99%), N-vinyl-2-pyrrolidone (NVP) (97%) and trimethylolpropane triacrylate) (TMPTA, ≥ 95.0%) were purchased from Sigma-Aldrich. Glycidyl methacrylate (GMA) (≥ 97%) was bought from Mitsubishi Gas Chemical Company, Japan. Chloroform (CHCl3) (99.9%), sodium hydroxide (NaOH) and acetone were obtained from Merck. The aliphatic polyester acrylate (LAROMER LR8992) was provided from BASF. 1-Hydroxy cyclohexyl phenyl ketone (Irgacure 184) was obtained from Ciba Specialty Chemicals and used as a radical photoinitiator. All other materials were used without any further purification.
Synthesis
Synthesis of DOPO-ITA
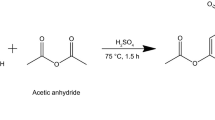
DOPO-ITA synthesis was accomplished according to an adapted procedure (Fig. 1).38 DOPO (0.1 mol, 216.7 g), itaconic acid (0.1 mol, 130.1 g) and xylene (500 mL) were put into a 5-necked round-bottomed flasks with a volume of 1000 mL, fitted with a condenser, nitrogen inlet, an overhead mechanical stirrer and a Dean and Stark trap. The reaction was performed about 5–6 h at 130°C. The reaction was stopped, and heat was removed. The crude DOPO-ITA was washed with acetone three times and then the white solid (DOPO-ITA) was obtained. The purified DOPO-ITA as a solid was obtained after the drying at 80°C. After holding 24 h in a vacuum oven, a white solid was obtained with a yield of 78%.
Synthesis of DOPO dimethacrylate (DOPO-DMA)
First, the solution was prepared with 5.32 g (15.8 mmol) DOPO-ITA in 20 mL distilled water or 1.2 g sodium hydroxide in 15 mL distilled water and then mixed in a flask equipped with a magnetic stirrer (Fig. 2).39 Next, the prepared solution was stirred by adding GMA (8.0 g, 56.3 mmol) at 70°C for an additional 2 h. The reaction mixture was then extracted with chloroform (3 times) and the solvent phase was separated to isolate the product. The remaining solvent and water were evaporated on a rotary evaporator. A yellowish liquid was obtained in 65% yield.
Preparation of UV curable compositions
The compositions of DOPO-DMA containing photocurable formulations are given in Table 1. Firstly, an aliphatic polyester acrylate (Laromer LR 8992), a diluent (TMPTA), a reactive solvent (NVP) and a photoinitiator (Irgacure 184) were mixed to prepare a blank UV-curable mixture (F0). Different formulations were prepared by decreasing the amount of the polyester and by adding DOPO-DMA instead. Each formulation contains 0%, 5%, 10% and 20% DOPO-DMA, separately. UV curing process is schematically shown in Fig. 3.
The film was obtained by pouring a UV curable viscous formulation into a Teflon™ coated mold (10 mm × 50 mm × 1 mm). After irradiation for 180 s under a UV lamp, a photocurable coating having a thickness of about 1 mm was obtained. The hybrid coating was washed with excess distilled water for 24 h to remove unreacted monomers and initiator residues. Then the films were dried in a vacuum oven at 30°C for 5 h. Formulations of UV-curable coatings are given in Table 1.
Results
FTIR spectra of the synthesized compounds and the prepared films were determined with JASCO FT/IR-4200 with ATR (JASCO Corp., Tokyo, Japan), which can make measurements in a wavelength 4000–700 cm−1 at a resolution of 4 cm−1 with 16 scans (Spectra Manager II software, JASCO Corp., Tokyo, Japan). ATR-FTIR spectra of DOPO-ITA and its raw materials are shown in Fig. 4. In the spectrum of ITA there is a peak at 1625 cm−1 related to (C=C) double bonds. The other peak at 1680 cm−1 is related to acid functional groups. After reacting with DOPO, the peak at 2385 cm−1 (P–H) was disappeared with the peak at 1625 cm−1 that shows us all the double bonds is reacted with the P–H in the DOPO structure. The acid peak is shifted toward the upper region to a peak at 1703 cm−1, providing evidence that the acid peak is bonded to a bulky aromatic structure. The other peaks between the regions of 1580–1610 cm−1 are sourced from the aromatic rings coming from the DOPO structure. The peaks at 1242 cm−1 (P=O) and 910 cm−1 (P-O-phenyl) are also evidence of that the DOPO successfully reacted with itaconic acid.
For further analysis of molecular structure, 1H-NMR spectra were recorded on Mercury-VX 400 MHz Model NMR spectrometer and are shown in Fig. 5. Deuterated chloroform (CDCl3) was used to dissolve the samples. In the DOPO spectrum, the signals between 6.50 and 8.92 ppm can be attributed to the phenyl protons. In the ITA spectrum, the chemical shift at 12.25 ppm is related to carboxyl protons indicating the presence of acid groups. In the DOPO-ITA spectrum, there are several chemical shifts (2.40, 2.70, 3.25, 7.20–8.3 and 12.5 ppm) showing that primary C-H, phenyl-H and carboxylic acid protons are in the chemical structure, respectively. These results prove that the reaction was achieved between DOPO and ITA successfully.
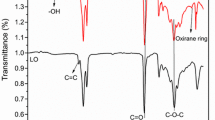
In the ATR-FTIR spectra of GMA, DOPO-ITA and DOPO-DMA given in Fig. 6, C=O stretching vibrations are observed at 1715, 1704 and 1716 cm−1, respectively. The vibration of –C=C– vinyl carbons is observed at 1635 cm−1. The absorption peaks in the GMA spectrum at 1257, 1111, and 996 cm−1 are attributed to the epoxy group of GMA.
In the ATR-FTIR spectrum of DOPO-DMA, these peaks are not seen due to the reaction of the epoxide group of GMA with the carboxylic acid groups of DOPO-ITA. Also, the new broad peak is observed at 3413 cm−1. This is attributed to –OH groups of DOPO-DMA that is sourced from the opening of the epoxide groups of GMA while reacting with carboxylic acids.
In Fig. 7, thermal gravimetric analysis (TGA) results show that the addition of DOPO-DMA improves the thermal properties. In the blank film, the polymer undergoes thermal degradation beginning at nearly 350°C. In the case of the films containing 10% and 20% DOPO-DMA, similar behaviors were observed and the results showed a significant inhibition of thermal degradation beginning at 425°C. It was also observed that the addition of DOPO-DMA increased the char yield. Thermal properties of UV curing formulations were given in Table 2.
The gel content (insoluble fraction) of the UV-cured films is the indication of the degree of crosslinking. It was determined by measuring the weight of the insoluble parts of the films after 6 h of Soxlet extraction with acetone at the boiling point. The values were obtained between 95% and 99% as shown in Table 3. The increase in the amount of the DOPO-DMA in UV formulations resulted in higher gel content showing an increase in crosslinking degree.
The mechanical properties of the UV-curable films were determined by standard tensile elongation tests to measure modulus of elasticity (E), tensile strength (σ), and elongation at break (ε). The tests were performed at room temperature using a Devotrans DVT tensile tester with a crosshead speed of 5 mm/min. The measurements represent the average of at least 5 runs. The Young’s modulus, ultimate tensile strength and elongation values of the films showed that the increase of the amount of DOPO-DMA in UV-curable formulations led to higher hardness values due to the increased crosslinking degree. Elongation and strength of the films were decreased disproportionally with crosslinking degree. All test results were shown in Table 4.
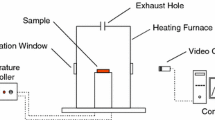
The LOI values of the prepared films were measured by using a LOI (Devotrans) type instrument, on the test specimen bar of 50 × 10 × 1 mm3 according to ASTM D2863-08. The LOI values of the DOPO-DMA films were increased as the amount of DOPO-DMA was increased in the UV-curable formulations. The LOI values of the films were graphed in Fig. 8. An increase in DOPO content in the formulations led to an increase in LOI values from 18% to 21.2%.
As seen in Fig. 9, pictures were taken while performing the LOI tests to determine the burning states of the films, F0 (not containing phosphorous) and F4 (containing maximum phosphorous). After 42 s from the ignition, F4 was self-extinguished at the LOI value of 21.2% but F0 continued to burn at a low value of 18. This may be attributed to the effect of the flame retardant in F4 film. The flame retardant DOPO led to a tightly bonded intumescent char that inhibited the spread of flame by acting as a thermal barrier preventing heat and mass transfer. In addition, phosphorous containing low molecular weight species may have reacted with existing ·H and ·OH radicals while burning, inhibiting exothermic oxidation reaction by reducing the energy feedback with radical scavenging.40,41,42
Hydrophilic or hydrophobic nature of the films was determined by contact angle analyses of the coating surfaces with water using Kruss Easy Drop DSA2. For this, 3–5 µL drops of distilled water were put on the surfaces for measurements. At least three measurements were conducted for each film. The contact angle values were increased with an increase for DOPO-DMA in the UV formulations as shown in Fig. 10. This may be due to the hydrophobic benzene rings in the DOPO-DMA structure.
SEM imaging of coatings was performed on Philips XL30 ESEM-FEG/EDAX. Film samples were prepared by freeze fracturing in liquid N2 and applying a gold coating of approximately 300 Å. The morphology of the prepared films were analyzed by SEM-EDAX mictograms as shown in Fig. 11. It was observed that the control film (F0) has a smooth structure. When the modified-DOPO loadings increase from 0 to 20 wt%, the impact fracture surface becomes increasingly rough. These results are similar to other studies in the literature.43 In other words, the increase in the roughness of the prepared films also explains the increase in the contact angle value. It has been stated in other academic studies that the contact angle increases as the roughness increases.44 As seen in the elemental compositions of the control film without DOPO-DMA, there is no phosphorous in its structure. The elemental compositions of the other films show that there is a phosphorous element in each formulation and the intensity of the phosphorous peak increases with increasing of the DOPO-DMA in each formulation as expected.
Discussion
In conclusion, a reactive difunctional phosphorous containing DOPO-DMA acrylic monomer was successfully synthesized from the reaction between GMA and DOPO-ITA. DOPO-DMA was added in UV-curable formulations, and the films were obtained. The mechanical and chemical properties of the films were characterized. The films containing DOPO-DMA showed higher thermal degradations. According to all values obtained, the LOI, hydrophobicity, hardness and crosslinking degree of UV curable films were increased with an increase in the amount of the DOPO-DMA in UV curing formulations. An increase in DOPO content in the formulations led to an increase in LOI values from 18% to 21.2%. Tensile modulus of F1 and F3 formulation was 301 and 336 MPa, respectively. Tensile modulus of prepared films increased with an increase in the amount of the DOPO-DMA in UV curing formulations.
References
Shi, Y, Yu, B, Wang, X, Yuen, ACY, “Editorial: Flame-Retardant Polymeric Materials and Polymer Composites.” Front. Mater., https://doi.org/10.3389/fmats.2021.703123 (2021)
Vahidi, G, Bajwa, D, Bajwa, S, Shojaeiarani, J, Stark, N, Darabi, A, “Advancements in Traditional and Nanosized Flame Retardants for Polymers—A Review.” J. Appl. Polym. Sci., 138 e50050. https://doi.org/10.1002/app.50050 (2020)
Xu, Y, “Introductory Chapter: Flame Retardant and Thermally Insulating Polymers.” (2021). https://doi.org/10.5772/intechopen.99111
Kim, Y, Lee, S, Yoon, H, “Fire-Safe Polymer Composites: Flame-Retardant Effect of Nanofillers.” Polymers, 13 540. https://doi.org/10.3390/polym13040540 (2021)
Van Krevelen, DW, Te Nijenhuis, K, Properties of Polymers: Their Correlation with Chemical Structure: Their Numerical Estimation and Prediction from Additive Group Contributions. Elsevier, New York (2009)
Doerr, SH, Santín, C, “Global Trends in Wildfire and Its Impacts: Perceptions Versus Realities in a Changing World.” Philos. Trans. R. Soc. B Biol. Sci., 371 20150345. https://doi.org/10.1098/rstb.2015.0345 (2016)
Babrauskas, V, Fuoco, R, Blum, A, “Flame Retardant Additives in Polymers.” In: When Do the Fire Safety Benefits Outweigh the Toxicity Risks?. https://doi.org/10.1016/B978-0-444-53808-6.00003-2 (2014)
Vahabi, H, Laoutid, F, Saeb, MR, Dubois, P, Mehrpouya, M, “Flame Retardant Polymer Materials: An Update and the Future for 3D Printing Developments.” Mater. Sci. Eng. R Rep., 144 100604. https://doi.org/10.1016/j.mser.2020.100604 (2021)
Shaw, SD, Blum, A, Weber, R, Kannan, K, Rich, D, Lucas, D, Koshland, CP, Dobraca, D, Hanson, S, Birnbaum, LS, “Halogenated Flame-Retardants: Do the Fire Safety Benefits Justify the Risks?” Rev. Environ. Health, 25 (4) 261–305 (2010)
Morgan, AB, Gilman, JW, “An Overview of Flame Retardancy of Polymeric Materials: Application, Technology, and Future Directions.” Fire Mater., 37 259–279 (2013)
Hu, Y, Yu, B, Song, L, “3—Novel Fire-Retardant Coatings.” In: Wang, D-Y (ed.) Novel Fire Retardant Polymers and Composite Materials, pp. 53–91. Woodhead Publishing, New York (2017)
Morgan, AB, “The Future of Flame Retardant Polymers—Unmet Needs and Likely New Approaches.” Polym. Rev., 59 (1) 25–54 (2019)
Kundu, C, Li, Z, Song, L, Hu, Y, “An Overview of Fire Retardant Treatments for Synthetic Textiles: From Traditional Approaches to Recent Applications.” Eur. Polym. J., 137 109911. https://doi.org/10.1016/j.eurpolymj.2020.109911 (2020)
Perret, B, Schartel, B, Stöß, K, Ciesielski, M, Diederichs, J, Döring, M, Krämer, J, Altstädt, V, “Novel DOPO-based Flame Retardants in High-performance Carbon Fibre Epoxy Composites for Aviation.” Eur. Polym. J., 47 1081–1089 (2011)
Salmeia, K, Gaan, S, “An Overview of Some Recent Advances in DOPO-derivatives: Chemistry and Flame Retardant Applications.” Polym. Degrad. Stab., https://doi.org/10.1016/j.polymdegradstab.2014.12.014 (2014)
Gu, L, Qiu, C, Qiu, J, Yao, Y, Sakai, E, Yang, L, “Preparation and Characterization of DOPO-Functionalized MWCNT and Its High Flame-Retardant Performance in Epoxy Nanocomposites.” Polymers, 12 613. https://doi.org/10.3390/polym12030613 (2020)
Wu, Q, Cassia, R, Valle, R, Valle, J, Bezerra, F, Meng, X, Lis, M, Arias, L, “Recent Progress of DOPO-Containing Compounds as Flame Retardants for Versatile Polymeric Materials: Review.” World J. Text. Eng. Technol., 6 89–103 (2020)
Serbezeanu, D, Vlad-Bubulac, T, Hamciuc, E, Corneliu, H, Lisa, G, Anghel, I, Sofran, I-E, Preda, D-M, “Study on Thermal and Flame Retardant Properties of Phosphorus-containing Polyimides.” Revista de Chimie, 72 13–21 (2020)
Fang, M, Qian, J, Wang, X, Chen, Z, Guo, R, Shi, Y, “Synthesis of a Novel Flame Retardant Containing Phosphorus, Nitrogen, and Silicon and Its Application in Epoxy Resin.” ACS Omega, 6 7094–7105 (2021)
Zhang, J, Mi, X, Chen, S, Xu, Z, Zhang, D, Miao, M, Wang, J, “A Bio-Based Hyperbranched Flame Retardant for Epoxy Resins.” Chem. Eng. J., 381 122719. https://doi.org/10.1016/j.cej.2019.122719 (2019)
Wang, H, Wang, S, Du, X, Wang, H, Cheng, X, Du, Z, “Synthesis of a Novel Flame Retardant Based on DOPO Derivatives and Its Application in Waterborne Polyurethane.” RSC Adv., 9 7411–7419 (2019)
Kumar, S, Samal, S, Mohanty, S, Nayak, S, “Recent Development of Bio-Based Epoxy Resins: A Review.” Polym. Plast. Technol. Eng., 57 133–155 (2016)
Liu, J, Zhang, L, Shun, W, Dai, J, Peng, Y, Liu, X, “Recent Development on Bio-Based Thermosetting Resins.” J. Polym. Sci., 59 1474–1490 (2021)
Paluvai, NR, Mohanty, S, Nayak, SK, “Synthesis and Modifications of Epoxy Resins and Their Composites: A Review.” Polym. Plast. Technol. Eng., 53 (16) 1723–1758 (2014)
Seidi, F, Movahedifar, E, Naderi, G, Akbari, V, Ducos, F, Shamsi, R, Vahabi, H, Paran, SMR, “Flame Retardant Polypropylenes: A Review.” Polymers, 12 1701 (2020)
Tylkowski, B, Marturano, V, Cerruti, P, Ambrogi, V, “Polymer Additives.” Phys. Sci. Rev., 2 26 (2017)
Robert, T, Friebel, S, “Itaconic Acid-A Versatile Building Block for Renewable Polyesters with Enhanced Functionality.” Green Chem., 18 2922–2934 (2016)
Bernadette-Emoke, T, Vodnar, D, “Biomass-Derived Production of Itaconic Acid as a Building Block in Specialty Polymers.” Polymers, 11 1035. https://doi.org/10.3390/polym11061035 (2019)
Dai, Z, Yang, Z, Chen, Z, Zhao, Z, Lou, Y, Zhang, Y, Liu, T, Fu, F, Fu, Y, Liu, X, “Fully Bio-based Composites of an Itaconic Acid Derived Unsaturated Polyester Reinforced with Cotton Fabrics.” ACS Sustain. Chem. Eng., 6 15056–15063 (2018)
Kumar, S, Krishnan, S, Samal, S, Mohanty, S, Nayak, S, “Itaconic Acid Used as a Versatile Building Block for the Synthesis of Renewable Resource Based Resins and Polyesters for Future Prospective: A Review.” Polym. Int., 66 1349–1363 (2017)
Huang, J, Zhang, J, Zhu, G, Yu, X, Hu, Y, Shang, Q, Chen, J, Hu, L, Zhou, Y, Liu, C, “Self-Healing, High-Performance, and High-Biobased-Content UV-Curable Coatings Derived from Rubber Seed Oil and Itaconic Acid.” Prog. Org. Coat., 159 106391 (2021)
Liang, B, Chen, J, Guo, X, Yang, Z, Yuan, T, “Bio-Based Organic-Inorganic Hybrid UV-Curable Hydrophobic Coating Prepared from Epoxidized Vegetable Oils.” Ind. Crops Prod., 163 113331. https://doi.org/10.1016/j.indcrop.2021.113331 (2021)
Chukwunwike, SA, Okafor, KJ, “A Review on Some Selected Bio-Based (Green) Flame Retardants.” Res. Rev. J. Eng. Technol., 8 38–43 (2019)
Qian, X, Song, L, Jiang, S, Tang, G, Xing, W, Wang, B, Yuen, RKK, “Novel Flame Retardants Containing 9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide and Unsaturated Bonds: Synthesis, Characterization, and Application in the Flame Retardancy of Epoxy Acrylates.” Ind. Eng. Chem. Res., 52 (22) 7307–7315 (2013)
Phalak, G, Patil, D, Patil, A, Mhaske, ST, “Synthesis of Acrylated Cardanol Diphenyl Phosphate for UV Curable Flame-Retardant Coating Application.” Eur. Polym. J., 121 109320 (2019)
Bier, F, Six, J-L, Durand, A, “DOPO-Based Phosphorus-Containing Methacrylic (Co) Polymers: Glass Transition Temperature Investigation.” Macromol. Mater. Eng., 304 1800645. https://doi.org/10.1002/mame.201800645 (2019)
Yao, C, Xing, W, Ma, C, Song, L, Hu, Y, Zhuang, Z, “Synthesis of Phytic Acid-Based Monomer for UV-Cured Coating to Improve Fire Safety of PMMA.” Prog. Org. Coat., 140 105497. https://doi.org/10.1016/j.porgcoat.2019.105497 (2020)
Ma, Y, Gong, X, Liao, C, Geng, X, Wang, C, Chu, F, “Preparation and Characterization of DOPO-ITA Modified Ethyl Cellulose and Its Application in Phenolic Foams.” Polymers, 10 1049. https://doi.org/10.3390/polym10101049 (2018)
Naderi, P, Kabiri, K, Jahanmardi, R, Zohuriaan-Mehr, MJ, “Preparation of Itaconic Acid Bio-Based Cross-Linkers for Hydrogels.” J. Macromol. Sci. Part A, 58 (3) 165–174 (2021)
Zhang, T, Wang, C, Wang, Y, Qian, L, Han, Z, “Enhanced Flame Retardancy in Ethylene-Vinyl Acetate Copolymer/Magnesium Hydroxide/Polycarbosilane Blends.” Polymers, 14 36 (2021)
Liu, L, Lv, R, “Synthesis of a DOPO-Triazine Additive and Its Flame-Retardant Effect in Rigid Polyurethane Foam.” E-Polymers, 19 235–243 (2019)
Muriel, R, Sebastian, W, Manfred, D, “Recent Developments in Halogen Free Flame Retardants for Epoxy Resins for Electrical and Electronic Applications.” Materials, 3 4300–4327 (2010)
Tang, C, Yan, H, Li, M, Lv, Q, “A novel Phosphorus-Containing Polysiloxane for Fabricating High Performance Electronic Material with Excellent Dielectric and Thermal Properties.” J. Mater. Sci. Mater. Electron., https://doi.org/10.1007/s10854-017-7904-4 (2018)
Wang, J, Wu, Y, Cao, Y, Li, G, Liao, Y, “Influence of Surface Roughness on Contact Angle Hysteresis and Spreading Work.” Colloid Polym. Sci., 298 1107–1112 (2020)
Acknowledgments
The financial support for this work by İZEL KİMYA and The Scientific and Technological Research Council of Turkey (TUBITAK)-Technology and Innovation Funding Programs Directorate (TEYDEB) under the project 3192309 is gratefully acknowledged. We would like to thank Rasim AKKOCA for conducting analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ozman, E., Dizman, C., Birtane, H. et al. Novel bio-based phosphorous-containing UV-curable flame-retardant coatings. J Coat Technol Res 20, 1257–1268 (2023). https://doi.org/10.1007/s11998-022-00740-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-022-00740-9