Abstract
Advanced multifunctional surfaces are widely used due to their unique surface properties and widespread applications. Developing a multifunctional fabric with a low cost, fluorine-free, and easily controllable method is a great challenge. This paper reports a multifunctional fabric with conductive, UV blocking, superhydrophobic and photosensing properties via an electrodeposition method. ZnO nano-/microarchitectures have been electrodeposited on polyester fabric with a carbon black screen-printed conductive layer. The deposition was carried out in various operating parameters. The optimized conditions for the ZnO electrodeposition are at − 1.0 V for 30 min deposition time in 5 mM Zn(NO3)2 in 0.1 M KNO3 at room temperature. The developed fabric showed 100% UV radiation blocking and a water contact angle (WCA) of 156° after self-assembly of stearic acid on the ZnO layer. The modified fabric showed fast photoresponse as a photosensor, which indicates that this can be used as flexible wearable photosensors in practical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multifunctional smart fabrics have gained significant attention over conventional fabrics due to their valuable properties and widespread applications.1 Some multifunctional textiles have advanced properties such as superhydrophobicity,2 omniphobicity (which can repel multiple liquids),3 radiation blocking properties,4 electrical conductivity,5 antibacterial activity,1 antistatic properties,6 photosensitivity,7 stain-resistant properties,8 and dust repelling properties.9 These multifunctional smart textiles can be fabricated using multiple methods, including hydrothermal methods,10 pad-dry-cure methods,11 dip coating,4 electroless deposition,12 chemical bath precipitation13 and wet chemical routes14 combining several materials such as metals (Ag, Cu),15 metal oxides (MgO, CuO),16,17 polymers (polyaniline, fluorinated acrylic copolymers)3,18,19 and semiconductor oxides (ZnO, TiO2).2,9 Among these materials, ZnO is a widely used semiconductor material as it is less expensive, easily synthesized, and a bio-safe material for human beings and animals.12
ZnO is an inorganic semiconductor material with three different crystal structures, including wurtzite, zinc blende, and rock salt, where wurtzite is the most stable form. ZnO has a wide bandgap of 3.3 eV.20 ZnO applied textiles have various properties such as being antibacterial,1 UV shielding,2 and piezoelectric.21 Several techniques like pad dry cure method, hydrothermal synthesis, sol–gel processing, dip coating, and spray coating can be used to deposit ZnO on textile substrates.1,3,10,21 However, these methods have several drawbacks, such as poor particle size and shape controllability, complex and time-consuming procedures, and requiring more precursors.22
Furthermore, recent research into preparation of nanomaterial-incorporated fabric materials has spread across a wide range of methods. For example, Zhang et al. prepared high tensile strength carbon fibers by UV-thermoxidative stabilization of dry-spun lignin fibers. The prepared fibers showed one of the highest recorded tensile strengths, which is 900 MPa.23 Gashti et al. prepared heat resistant wool fabric by colloidal dispersion of clay nanoparticles on the fabric material. However, the modified fabric appeared to have a drawback of adsorbing moisture.24 In addition, cotton fabrics with efficient bacteriostatic and bactericidal properties were prepared by assembling silver nanoparticles on cotton fabrics by using iota-carrageenan as a carbohydrate polymer under ultrasonic waves. The incorporation of silver nanoparticles on the fabric resulted in reduced thermal stability of the fabric.25 Another example is the preparation of polyvinylpyrrolidone/carbon nanotube (PVP/CNT) nanocomposite applied on cotton fabrics using pad-dry-cure method. This PVP/CNT coating is shown to increase the thermal stability of the cotton substrate.26 Another successful attempt in multifunctional fabric synthesis is through pad-dry-cure method to incorporate silica nanoparticles on the polyester fabrics. This incorporation improves both the mechanical properties and functional properties of the polyester fabric. Such mechanical properties include tensile, tearing, and crease recovery angle, as well as other functions like antimicrobial properties, self-cleaning, electrical surface resistivity, and dyeing behavior.27,28
Currently, researchers are trying to develop more convenient methods to deposit inorganic nanoparticles on textile surfaces.1,12,17 One of the methods is the electrochemical synthesis of inorganic nanoparticles. As mentioned earlier, compared to the other existing methods, electrochemical deposition presents various advantages such as less time and chemical consumption, a low-temperature process, better particle controllability, flexibility for large-scale industrial applications, low cost, and simplicity. However, despite the significant advantages of electrochemical deposition of inorganic material on textiles, there is one major limitation—the electrochemical method requires a conductive fabric to deposit inorganic material.
There are various methods to prepare a conductive fabric, such as incorporating metal wires and metal nanoparticles or applying coatings of conductive materials like conductive polymers and carbon black. Commercially available conductive fabrics are prepared by incorporating metal wires and metal nanoparticles. These commercially available fabrics have been used to deposit ZnO nanoarchitectures electrochemically.7,29 Ko et al.29 prepared ZnO nanorod arrays on a conductive fabric by electrochemical synthesis using a commercially available conductive textile, woven Ni plated polyester, for ZnO-based optoelectronic and electrochemical devices. Kim et al.7 prepared a flexible organic–inorganic hybrid material by loading ZnO nanostructures on a woven fabric of polyethylene terephthalate (PET). The PET fabric has been made conductive by depositing three layers of Ni and Cu in Ni-Cu-Ni by electroless deposition. The ZnO electrodeposited fabric was tested as a flexible photosensor.
However, due to the requirement of metal wires and nanoparticles, these conductive textiles are expensive and prone to corrosion.7 Therefore, applying coatings of conductive materials such as conductive polymers and carbon black has gained significant attention toward the preparation of conductive fabrics. Conductive textiles can also be prepared using conductive polymer coatings such as polyaniline, polythiophene, and polypyrrole.30,31,32 Usually, conductive polymer-coated conductive fabrics have higher resistivity than metal wire and metal nanoparticle-incorporated fabrics, making it challenging to use them in electrochemical applications.33,34,35,36,37
Another method to prepare a conductive fabric is coating with carbon black5 and advanced carbon materials such as graphene oxide33 on the fabric surface.5,15,16,17,29,30,31,32,33 Carbon black-coated fabrics show lower resistance than conductive polymer-coated fabrics, enabling them to be used in electrochemical applications. Furthermore, a quick and straightforward screen-printing method can be used to make the fabric conductive.5 Compared to metal nanoparticle/wire-incorporated fabrics, carbon black screen-printed fabric has no corrosion problems, which is essential for fabric in frequent contact with water.5,16,22,34
In this work, for the first time, the ZnO nanoparticle layer was grown on C-black-deposited conductive polyester fabric electrochemically. Commercially available polyurethane and polyacrylic-coated polyester were used as the fabric material. The fabric was made conductive by screen printing a layer of carbon black according to a method reported by Pahalagedara et al.5 On the surface of the prepared conductive fabric, ZnO nano-/microstructures were deposited electrochemically using an easy, quick, and single-step method. Even though there are studies on electrochemical deposition of ZnO nano-/microstructures on conductive fabrics as mentioned above, in all those instances, the fabrics have been made conductive by incorporating metal wires and metal nanoparticles. Here, the novelty of this study states the growth of ZnO nano-/microstructures electrochemically on screen-printed carbon black conductive polyester fabric, which is inexpensive and corrosion-resistant. The ZnO electrodeposited fabric has various functionalities such as UV blocking, superhydrophobic properties (by self-assembling the stearic acid layer on the ZnO nano-/microstructures), and photosensing properties.
Materials and methods
Materials
All the chemical reagents used in this study were of analytical grade (Sigma-Aldrich). They were used without further purification: carbon black nanopowder (99.5% purity), sodium dodecyl sulfate (CH3(CH2)11OSO3Na) (Purity 99%), zinc nitrate hexahydrate (Zn(NO3)2.6H2O) (99% purity), potassium nitrate (KNO3) (Purity 99%), stearic acid (CH3(CH2)16COOH) (99% purity). Polyurethane and polyacrylic-coated polyester fabrics were obtained from Rainco (Pvt), a leading umbrella manufacturer in Sri Lanka.
Preparation of the conductive fabric (CF)
A conductive fabric was prepared by screen-printing a carbon black (CB) dispersion on the fabric according to a method reported elsewhere.5 The CB dispersion was prepared by stirring a mixture of CB (20% w/w) and sodium dodecyl sulfate (SDS) (10% w/w) in water for 1 h at room temperature (25°C). Then, the dispersion was sonicated in a bath sonicator for 2 h at a frequency range of 50–60 kHz. A (4 cm × 5 cm) sized piece of the fabric was screen-printed using a nylon mesh frame with filament spacing of approximately 0.3 mm. The screen was placed on the fabric, and SDS-stabilized CB dispersion was screen-printed. This process was repeated three times in order to ensure the smooth surface coverage of CB.
Deposition of ZnO nanostructures
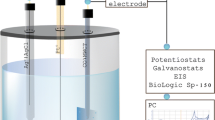
A three-electrode system was used to deposit a ZnO layer on the conductive fabric (supplementary document Fig. S1). Conductive fabric (2 cm × 2 cm) was used as the working electrode (WE), and Pt sheet and Ag/AgCl (3 M KCl) were used as the counter and reference electrodes, respectively. Electrodes were dipped in 50 mL of 0.5 M Zn(NO3)2 solution, and cyclic voltammetry (CV) was carried out in the N2 purged solution at 10 mV/s scan rate in the potential range of 0 to − 1.5 V. CV was carried out in order to understand the electrochemical process and to determine the suitable ZnO deposition potential. To optimize the conditions, the deposition was carried out under different concentrations (5 mM Zn(NO3)2 in 0.1 M KNO3, 0.5 M Zn(NO3)2), deposition potentials (0.0, − 0.5, − 1.0, − 1.5, − 2.0 V), deposition times (15, 30, 45, 60 min), deposition temperatures (28°C, 50°C, 60°C, 70°C) with N2 purged solution. Energy-dispersive X-ray spectroscopy (EDX) characterization of treated fabrics was carried out to analyze ZnO deposition. Each deposition was performed by a chronoamperometry experiment using Metrohm Autolab PGSTAT 302N potentiostat.
Depositing superhydrophobic layer
The ZnO-deposited fabric was dipped in a stearic acid solution with varying stearic acid concentrations and dipping time to prepare the superhydrophobic fabric. According to the study, the optimum stearic acid dipping time and concentration to achieve superhydrophobicity were 1 g dm−3 and 1 h, respectively (supplementary document Fig. S2).
Characterization techniques
A scanning electron microscope (SEM, HITACHI SU 600) was used to determine the morphological features of the fabrics with an operating voltage of 10 kV; a layer of gold was deposited before the samples were analyzed. Energy-dispersive X-ray analysis (EDX) (OXFORD Instruments) coupled with SEM and X-ray fluorescence (XRF, Horiba XGT 5200) were used for the elemental analysis. X-ray photoelectron spectroscopy (XPS) was performed using an EXCALABXi+ hemispherical electron energy analyzer (Thermo Fisher Scientific, United States). Monochromatic Al Kα X-rays (1486.6 eV) were used with a spot size of 900 μm. Survey spectra were recorded with a pass energy of 150 eV and a resolution of 1.0 eV, whereas narrow scans were recorded with a pass energy of 20 eV and 0.05 eV resolution. All XPS measurements were performed in Constant Analyzer Energy (CAE) mode. Narrow spectra were resolved by curve fitting (combination Gaussian/Lorentzian function after subtracting the background) using Avantage (5.892) software with a correction for charge compensation effects relatively to adventitious C1s spectrum at 284.8 eV.
UV–Vis spectrometery (Shimadzu, UV-3600) was used to analyze the UV transmittance of the fabrics in the wavelength range of 200 nm to 800 nm. Water contact angle (WCA) measurements were taken of the fabric using a KRUSS drop shape analyzer (DSA25). Fourier-transform infrared (FTIR) spectra of the fabric were recorded using Bruker VERTEX 80 with a spectral range of 4000 to 400 cm−1 with 32 scans per measurement and 0.4 cm−1 resolution. The deposited nanoparticles were separated and analyzed using Bruker Focus D8 Powder X-ray diffractometer (Cu Kα radiation of wavelength λ = 0.154 and scan rate of 5° min−1).
To analyze the photosensitivity, a treated fabric (2 cm × 3 cm) was placed under a sun simulator (US 900) and connected to the potentiostat. Open circuit potential versus time was measured in the presence and absence of the illumination with 30 s time intervals.
Results and discussion
Electrochemical deposition of ZnO
ZnO thin films are generally fabricated using gas-phase techniques such as chemical/physical vapor deposition and sputtering and solution-phase techniques such as sol–gel, wet chemical, and electrodeposition. Solution phase techniques are promising for simple, low-cost, and large-area applications. In this work, the electrodeposition technique is used because, unlike other solution-phase techniques, electrodeposition has advantages such as better particle controllability, lower processing temperature, less time consumption, and lower cost.36
There are two main methods of electrodepositing ZnO; deposition in O2 saturated solution or deposition in NO3− containing solution.36,37 In the first method, O2 (g) is purged into the solution. This dissolved oxygen can contribute as the oxygen precursor for the ZnO deposition. Dissolved oxygen is reduced to form hydroxide ions, and this leads to eventual ZnO formation. However, this reaction is complex due to the existence of parallel reactions such as the formation of hydrogen peroxide and is not commercially feasible.37
The second method is electrodepositing ZnO using a NO3− containing solution. In this study, Zn(NO3)2 solution was used to deposit ZnO electrochemically. It is advantageous that Zn(NO3)2 is highly soluble in water to be used as the precursor solution. There are three species in a Zn(NO3)2 solution that can react in the presence of an applied potential. First, deposition of metallic Zn can occur at the cathode electrode.37 According to the previous studies, reducing Zn2+ to metallic Zn does not occur in the Zn(NO3)2 solution even when scanned down to a far negative potential (− 1.4 V).38,39
Deposition of metallic Zn can be avoided by selecting proper deposition potential according to the CV. The CV of a N2 purged 0.5 M Zn(NO3)2 solution at a scan rate of 10 mV/s is given in Fig. 1a. In CV, there is no electrochemical reaction observed between 0 and − 0.8 V, and from − 0.8 V to − 1.5 V, there is a gradual increase in the current. In the reverse scan, a similar observation can also be made. There is a gradual decrease in current from − 1.5 V to 0.8 V, no evidence of electrochemical reaction between − 0.8 V and 0 V and there is no evidence for metallic Zn deposition from CV between 0 V and − 1.5 V. Zn deposition is usually identified by the gradual increase in current or a peak in the forward scan to the cathodic direction and appearance of a peak in the reverse scan to the anodic direction. In CV, there is no such observation and deposition of Zn can be ruled out.
(a) Cyclic voltammogram of 0.5 M Zn(NO3)2 in N2 purged solutions from − 1.5 V to 0.0 V at a scan rate of 10 mV s−1, (b) chronoamperometry of 5 mM Zn(NO3)2 in a 0.1 M KNO3 solution at − 1.0 V for 30 min in a N2 purged solution for ZnO deposition. The measurements were made in a three-electrode setup using modified fabric, Pt sheet, and Ag/AgCl (3M KCl) as the working, counter, and reference electrodes
The increase in current in CV is attributed to a second reaction that can occur—nitrate reduction. Therefore, to avoid Zn deposition and to have a sufficient nitrate reduction, the suitable deposition potential was selected as − 1.0 V. This was further confirmed by conducting chronoamperometry experiments at different deposition potentials.
The third reaction that can take place is the reduction of oxygen. It is reported in the literature that the presence of Zn2+ hinders oxygen reduction while nitrate reduction is promoted.40 However, the experiment was conducted in a N2 (g) purged Zn(NO3)2 solution where the dissolved oxygen concentration is negligible. Therefore, nitrate reduction is the primary reaction promoted at the applied potential of − 1.0 V. The standard potential for reducing nitrate ions is − 0.240 V versus SCE.40 The working electrode here is a conductive fabric. It has a comparatively higher resistivity than other conductive surfaces such as metals, indium-doped tin oxide-coated glass (ITO), and fluorine-doped tin oxide (FTO) glasses, subsequently introducing increased potential.39
ZnO deposits on the working electrode (the conductive fabric surface) when the required potential is applied according to the below mechanism. Dissociated \({\text{NO}}_{3}^{ - }\) is reduced to form OH− (equations (1) and (2)) in the solution, consequently forming Zn(OH)2 (equation (3)). Zn(OH)2 is dissociated to ZnO and water, where ZnO is deposited on the WE (fabric) surface (equation (4)).20,41
Elemental analysis
The ZnO deposition can be further optimized by varying the operating parameters such as the deposition potential, deposition time, solution concentration, and temperature. EDX was carried out to analyze the elemental mapping on the fabric surface. Fabrics were subjected to electrodeposition under various conditions by chronoamperometry, as mentioned in the experimental section, to identify the optimum conditions for the electrodeposition process. EDX mapping was obtained for treated fabrics under each condition (supplementary document Figs. S4–S8). The optimum conditions were identified as 5 mM Zn(NO3)2 in 0.1 M KNO3 solution concentration, − 1.0 V deposition voltage, 30 min deposition time at room temperature (25°C). The deposition performed under these conditions achieved the highest weight percentage of Zn on the fabric surface.
The EDS mapping of the ZnO electrodeposited fabric and weight percentages for the conditions mentioned above are shown in Figs. 2a–2h. In Fig. 2b, it is clearly shown that ZnO has been deposited on top of the C layer (O 35.6%, Zn 28.2%). In addition to Zn, C, and O, a small amount of K (Fig. 2f) and N (Fig. 2g) are shown because KNO3 was used as the electrolyte in the deposition solution. The color dispersion of Zn (Fig. 3c) and O (Fig. 2d) shows approximately similar patterns in the figures. However, the weight percentage of O is higher than the Zn percentage. The reason for this is likely K and N present in the form of KNO3.
Further elemental and chemical characteristics of the surface were analyzed by performing XPS analysis, seen in Fig. 3. The wide scan XPS analysis shows clear peaks of C1s, Zn2p, and O1s (Fig. 3a). The high-resolution analysis of the C spectra shows the prominent peak at 285 eV, which is attributed to C–C binding energy, and two other peaks at 286 eV and 288.5 eV, which correspond to C–O–C and O–C=O binding energies, respectively.15 This indicates the bond characteristics in the polyester fabric and treated C black on the surface. The high-resolution spectra of O=1s are an asymmetric spectrum indicating peaks at 531 eV and 533 eV (Fig. 3c). The O=1s peak positioned at a lower binding energy of 531 eV can be assigned to O2− ions in the ZnO bonding.
Furthermore, the peak located at 533 eV can be associated with C–O bonding. Figure 3d shows the high-resolution spectra of Zn2p. The peaks observed at binding energies of 1023 eV and 1046 eV correspond to the spin-orbit of Zn2p3/2 and Zn2p1/2, respectively, which are attributed to ZnO.16,21 XRF further confirmed elemental analysis (supplementary document Fig. S3) that Zn and K exist as ZnO and K2O. The chronoamperometry graph for the ZnO deposition at − 1.0 V for 30 min is shown in Fig. 2b. The thickness of the deposited ZnO layer was 0.005 mm as measured using a microgauge (supplementary document Figure S1)
Further characterizations were performed using XRD and FTIR to analyze the deposited particles and the coatings (Fig. 4). According to the XRD spectra (Fig. 4a), the peaks can be indexed to Zincite (PDF 04-003-2106), with major peaks observed at 37.0°, 40.2°, 42.4°, 55.8°, 66.8°, 74.5°, 78.9°, 80.9°, and 118.2°. The sharp and strong peaks confirm that deposited ZnO nanostructures are crystalline. The coated fabrics were further analyzed using FTIR (Fig. 4b). The FTIR spectrum of the pure fabric has high-intensity peaks that are related to the commercial polyester fabric used. In the FTIR spectrum of the nontreated fabric, asymmetric C–H stretching can be seen between 3000 and 2800 cm−1.
In addition to that, broad –OH stretching can be observed around 3300 cm−1. Furthermore, C=O stretch and C–H bending can be seen at 1490 cm−1 and 750 cm−1, respectively. A significant reduction in peak intensity can be observed in the spectra of the coated samples. The FTIR spectra were recorded using ATR-FTIR; hence, the film thickness increment could affect the evanescence wave, leading to a lower intensity. Moreover, the attachment of C black to the functional groups of the fabric could lead to the reduction in peak intensity.40
Evaluation of superhydrophobic property
The morphology of the deposited ZnO can be analyzed using SEM. Figures 5a–5d displays the surface morphology of the regular polyester fabric, carbon black screen-printed fabric (CB fabric), ZnO electrochemically deposited fabric (CB-ZO fabric), and stearic acid self-assembled fabric on the ZnO deposit (CB-ZO-SA fabric). The carbon black layer has been uniformly coated on the polyester fabric (Fig. 5b). ZnO layer has been deposited on the C black conductive layer. Figure 5c shows ZnO structures grown on C black screen-printed fabric as 2D plate-like structures, and the thickness of the deposited ZnO layer is in the nanometer range (200–300 nm) (Fig. S1).
In Fig. 5d, it can be seen that stearic acid is self-assembled on the ZnO layer with a flower-like structure. The polar carboxylic group of the stearic acid attaches to the ZnO, while the nonpolar hydrocarbon chain is directed out of the surface.
To achieve superhydrophobicity, a ZnO electrodeposited fabric was used to attach the stearic acid hydrophobic layer. There are two main requirements to achieve superhydrophobicity: (1) surface roughness and (2) low surface free energy.2 The deposited ZnO layer introduces the surface roughness, and stearic acid provides low surface free energy.2 Furthermore, ZnO remains an intermediate layer to attach stearic acid to the fabric (Fig. 5d).2
Figure 6 represents the WCA of an untreated regular fabric and of a CB-ZO-SA fabric. The standard fabric shows 132° WCA because the polyester fabric was coated with polyacrylic and polyurethane (Fig. 6a). The modified fabric shows 156° WCA, which is considered superhydrophobic (Fig. 6b). The WCA of C black screen-printed and ZnO electrodeposited fabrics shows 140° and 135°, respectively (supplementary document Fig. S9), which confirm the stearic acid layer self-assembled fabric is superhydrophobic. A reduction in WCA of 5° can be seen in the ZnO-deposited fabric, which can be attributed to the presence of hydroxyl groups on the surface of ZnO.
Evaluation of the UV blocking property
The UV blocking ability of the treated and nontreated fabrics was tested by analyzing the UV-transmittance. Figure 7 displays the UV transmittance of the normal and the treated fabrics. As seen in the spectra, the regular fabric transmits more radiation in the UV and the visible regions. However, the CB, CB-ZO, and CB-ZO-SA fabrics transmit 0% UV and visible radiation compared to the untreated fabric. The ZnO and the C black nanoparticles have UV blocking properties as C black and ZnO can absorb UV radiation.2,4,42,43,44
Evaluation of the photosensing property
Photosensors are used in many devices, which convert light radiation into electrical signals. Photosensors are usually prepared using rigid substrates.45,46 On the other hand, photosensitive fabrics can be beneficial in wearable applications as they are flexible. We envisaged that the presence of ZnO, which is reported to be photosensitive, would render the fabric with photosensing ability.7 This hypothesis was tested by vertically illuminating solar light from a sun simulator, which periodically turned on/off in 30 s intervals on the modified fabric, and recording the corresponding photoresponsive behavior.
Figure 8 shows the open circuit potential versus time characteristics of CB fabric and CB-ZO fabric. As shown in Fig. 8, there is a significant change (80%) in the potential response of the ZnO electrodeposited sample when exposed to light. Photoresponse was not observed in the CB fabric. According to Fig. 8, photovoltage increases with solar radiation exposure.
Conclusions
Herein, we have successfully demonstrated the design and development of a multifunctional polyester fabric for the first time, by screen printing a C black conductive layer and electrodepositing a ZnO layer, with superhydrophobicity, UV blocking property, and photosensitivity. The screen-printing of C black to make fabrics conductive is an inexpensive and single-step method. Various parameters have been tested to identify the optimal conditions for the ZnO electrodeposition process. The optimized deposition conditions were 5 mM Zn(NO3)2 in a 0.1 M KNO3 concentration at − 1.0 V for 30 min at room temperature (25°C).
The electrochemical deposition of ZnO offers many advantages, including less time and chemical consumption, a low-temperature process, flexibility to be scaled up to industrial applications, low cost, and a well-controlled fabrication process. The superhydrophobicity was achieved by self-assembling stearic acid on the ZnO-deposited fabric, and the measured WCA was 156°. The CB fabric, CB-ZO fabric, and CB-ZO-SA fabric showed 100% UV radiation blocking properties. The photosensing ability of the ZnO-deposited fabric was tested. There is a significant change in the potential during the on and off states of the solar radiation, which indicates that the fabric modification we developed can be used in flexible photosensing applications.
Conflict of interest
The authors declare that they have no conflict of interest.
Data availability
The datasets supporting this article have been uploaded as part of the electronic supplementary material, and any data can be provided upon request by the corresponding author.
References
Pandimurugan, R, Thambidurai, S, “UV Protection and Antibacterial Properties of Seaweed Capped ZnO Nanoparticles Coated Cotton Fabrics.” Int. J. Biol. Macromol., 105 788–795 (2017)
Ekanayake, UGM, Rathuwadu, N, Mantilaka, MMMGPG, Rajapakse, RMG, “Fabrication of ZnO Nanoarchitectured Fluorine-Free Robust Superhydrophobic and UV Shielding Polyester Fabrics for Umbrella Canopies.” RSC Adv., 8 (55) 31406–31413 (2018)
Marco, CD, Oldani, V, Bianchi, C, Levi, M, Turri, S, “A Biomimetic Surface Treatment to Obtain Durable Omniphobic Textiles.” J. Appl. Polym. Sci., 132 (32) 42404 (2015). https://doi.org/10.1002/app.42404
Zhu, T, Li, S, Huang, J, Mihailiasa, M, Lai, Y, “Rational Design of Multi-layered Superhydrophobic Coating on Cotton Fabrics for UV Shielding, Self-Cleaning and Oil-Water Separation.” Mater. Des., 134 342–351 (2017)
Pahalagedara, LR, Siriwardane, IW, Tissera, ND, Wijesena, RN, de Silva, KMN, “Carbon Black Functionalized Stretchable Conductive Fabrics for Wearable Heating Applications.” RSC Adv., 7 (31) 19174–19180 (2017)
Textor, T, Mahltig, B, “A Sol–Gel Based Surface Treatment for Preparation of Water Repellent Antistatic Textiles.” Appl. Surf. Sci., 256 (6) 1668–1674 (2010)
Kim, YK, Hwang, S-H, Kim, S, Park, H, Lim, SK, “ZnO Nanostructure Electrodeposited on Flexible Conductive Fabric: A Flexible Photo-Sensor.” Sens. Actuators B Chem., 240 1106–1113 (2017)
Stoppa, M, Chiolerio, A, “Wearable Electronics and Smart Textiles: A Critical Review.” Sensors (Basel), 14 (7) 11957–11992 (2014)
Ahmad, D, van den Boogaert, I, Miller, J, Presswell, R, Jouhara, H, “Hydrophilic and Hydrophobic Materials and Their Applications.” Energy Sources Part A Recovery Util. Environ. Effects, 40 (22) 2686–2725 (2018)
Ashraf, M, Campagne, C, Perwuelz, A, Champagne, P, Leriche, A, Courtois, C, “Development of Superhydrophilic and Superhydrophobic Polyester Fabric by Growing Zinc Oxide Nanorods.” J. Colloid Interface Sci., 394 545–553 (2013)
Richard, E, Lakshmi, RV, Aruna, ST, Basu, BJ, “A Simple Cost-Effective and Eco-friendly Wet Chemical Process for the Fabrication of Superhydrophobic Cotton Fabrics.” Appl. Surf. Sci., 277 302–309 (2013)
Preda, N, Enculescu, M, Zgura, I, Socol, M, Matei, E, Vasilache, V, Enculescu, I, “Superhydrophobic Properties of Cotton Fabrics Functionalized with ZnO by Electroless Deposition.” Mater. Chem. Phys., 138 (1) 253–261 (2013)
Velayi, E, Norouzbeigi, R, “Synthesis of Hierarchical Superhydrophobic Zinc Oxide Nano-structures for Oil/Water Separation.” Ceram. Int., 44 (12) 14202–14208 (2018)
Xu, B, Cai, Z, “Fabrication of a Superhydrophobic ZnO Nanorod Array Film on Cotton Fabrics via a Wet Chemical Route and Hydrophobic Modification.” Appl. Surf. Sci., 254 (18) 5899–5904 (2008)
Yu, D, Kang, G, Tian, W, Lin, L, Wang, W, “Preparation of Conductive Silk Fabric with Antibacterial Properties by Electroless Silver Plating.” Appl. Surf. Sci., 357 1157–1162 (2015)
Xue, C-H, Chen, J, Yin, W, Jia, S-T, Ma, J-Z, “Superhydrophobic Conductive Textiles with Antibacterial Property by Coating Fibers with Silver Nanoparticles.” Appl. Surf. Sci., 258 (7) 2468–2472 (2012)
El Nahhal, IM, Elmanama, AA, Amara, NM, in “Synthesis of Nanometal Oxide–Coated Cotton Composites.” Cotton Research, Chapter 13 (2016), pp 279–294
Gomes, EC, Oliveira, MAS, “Chemical Polymerization of Aniline in Hydrochloric Acid (HCl) and Formic Acid (HCOOH) Media. Differences Between the Two Synthesized Polyanilines.” Am. J. Polym. Sci., 2 (2) 5–13 (2012)
Hensel, R, Neinhuis, C, Werner, C, “The Springtail Cuticle as a Blueprint for Omniphobic Surfaces.” Chem. Soc. Rev., 45 (2) 323–341 (2016)
Kumar, M, Sasikumar, C, “Electrodeposition of Nanostructured ZnO Thin Film: A Review.” Am. J. Mater. Sci. Eng., 2 (2) 18–23 (2014)
Fortunato, M, Chandraiahgari, CR, De Bellis, G, Ballirano, P, Soltani, P, Kaciulis, S, Caneve, L, Sarto F, Sarto, MS, “Piezoelectric Thin Films of ZnO-Nanorods/Nanowalls Grown by Chemical Bath Deposition.” IEEE Trans. Nanotechnol., 17(2) 311–317 (2018)
Oliveira, FF, Proenca, MP, Araújo, JP, Ventura, J, “Electrodeposition of ZnO Thin Films on Conducting Flexible Substrates.” J. Mater. Sci., 51 (12) 5589–5597 (2016)
Zhang, M, Jin, J, Ogale, A, “Carbon Fibers from UV-Assisted Stabilization of Lignin-Based Precursors.” Fibers, 3 (4) 184–196 (2015)
Gashti, MP, Gashti, MP, “Effect of Colloidal Dispersion of Clay on Some Properties of Wool Fiber.” J. Dispers. Sci. Technol., 34 (6) 853–858 (2013)
Noralian, Z, Gashti, MP, Moghaddam, MR, Tayyeb, H, Erfanian, I, “Ultrasonically Developed Silver/Iota-Carrageenan/Cotton Bionanocomposite as an Efficient Material for Biomedical Applications.” Int. J. Biol. Macromol., 180 439–457 (2021)
Alimohammadi, F, Gashti, MP, Mozaffari, A, “Polyvinylpyrrolidone/Carbon Nanotube/Cotton Functional Nanocomposite: Preparation and Characterization of Properties.” Fibers Polym., 19 (9) 1940–1947 (2018)
Bhattacharya, SS, Chaudhari, S, “Study on Structural, Mechanical and Functional Properties of Polyester Silica Nanocomposite Fabric.” Int. J. Pure Appl. Sci. Technol., 21 (1) 43–52 (2014)
Gashti, MP, Eslami, S, “A Robust Method for Producing Electromagnetic Shielding Cellulose via Iron Oxide Pillared Clay Coating Under Ultraviolet Irradiation.” Funct. Mater. Lett., 8 (6) 1–4 (2015)
Ko, YH, Kim, MS, Park, W, Yu, JS, “Well-Integrated ZnO Nanorod Arrays on Conductive Textiles by Electrochemical Synthesis and Their Physical Properties.” Nanoscale Res. Lett., 8 (1) 28 (2013)
Abu-Thabit, NY, “Thermochemistry of Acrylamide Polymerization: An Illustration of Auto-acceleration and Gel Effect.” World J. Chem. Educ., 5 (3) 94–101 (2017)
David, NC, Anavi, D, Milanovich, M, Popowski, Y, Frid, L, Amir, E, “Preparation and Properties of Electro-conductive Fabrics Based on Polypyrrole: Covalent vs. Non-covalent Attachment.” IOP Conf. Ser.: Mater. Sci. Eng., 254 032002 (2017)
Molina, J, Esteves, MF, Fernández, J, Bonastre, J, Cases, F, “Polyaniline Coated Conducting Fabrics. Chemical and Electrochemical Characterization.” Eur. Polym. J. 47 2003–2015 (2011)
Shen, W, Dong, Y, Cui, G, Li, B, “Optimized Preparation of Electrically Conductive Cotton Fabric by an Industrialized Exhaustion Dyeing with Reduced Graphene Oxide.” Cellulose, 23 (5) 3291–3300 (2016)
Montazer, M, Nia, ZK, “Conductive Nylon Fabric Through In Situ Synthesis of Nano-silver: Preparation and Characterization.” Mater. Sci. Eng. C Mater. Biol. Appl., 56 341–347 (2015)
Nasirizadeh, N, Dehghani, M, Yazdanshenas, ME, “Preparation of Hydrophobic and Conductive Cotton Fabrics Using Multi-wall Carbon Nanotubes by the Sol–Gel Method.” J. Sol–Gel Sci. Technol., 73 (1) 14–21 (2014)
Dai, SX, Li, YY, Du, ZL, Carter, KR, “Electrochemical Deposition of ZnO Hierarchical Nanostructures from Hydrogel Coated Electrodes.” J. Electrochem. Soc., 160 (4) D156–D162 (2013)
Kelly, JJ, West, AC, “Copper Deposition in the Presence of Polyethylene Glycol: II. Electrochemical Impedance Spectroscopy.” J. Electrochem. Soc., 145 (10) 3477 (1998)
Izaki, M, Omi, T, “Transparent Zinc Oxide Films Prepared by Electrochemical Reaction.” Appl. Phys. Lett., 68 (17) 2439–2440 (1996)
Yoshida, T, Komatsu, D, Shimokawa, N, Minoura, H, “Mechanism of Cathodic Electrodeposition of Zinc Oxide Thin Films from Aqueous Zinc Nitrate Baths.” Thin Solid Films, 451–452 166–169 (2004)
Wei, S, Lian, J, Chen, X, Jiang, Q, “Effects of Seed Layer on the Structure and Property of Zinc Oxide Thin Films Electrochemically Deposited on ITO-Coated Glass.” Appl. Surf. Sci., 254 (20) 6605–6610 (2008)
Sun, S, Jiao, S, Zhang, K, Wang, D, Gao, S, Li, H, Wang, J, Yu, Q, Guo, F, Zhao, L, “Nucleation Effect and Growth Mechanism of ZnO Nanostructures by Electrodeposition from Aqueous Zinc Nitrate Baths.” J. Cryst. Growth, 359 15–19 (2012)
Zhou, R, Wang, X, Zhou, R, Weerasinghe, J, Zhang, T, Xin, Y, Wang, H, Cullen, P, Wang, H, Ostrikov, K, “Non-thermal Plasma Enhances Performances of Biochar in Wastewater Treatment and Energy Storage Applications.” Front. Chem. Sci. Eng., (2021). https://doi.org/10.1007/s11705-021-2070-x
Gültekin, ND, “Investigation of Thermal and Electrical Conductivity Properties of Carbon Black Coated Cotton Fabrics.” Marmara Univ. J. Sci., 27 (3) 91–94 (2015)
Xue, CH, Yin, W, Zhang, P, Ji, PT, Jia, ST, “UV-Durable Superhydrophobic Textiles with UV-Shielding Properties by Introduction of ZnO/SiO2 Core/Shell Nanorods on PET Fibers and Hydrophobization.” Colloids Surf. A Physiochem. Eng. Asp., 427 7–12 (2013)
Miyachi, M, Yamanoi, Y, Shibata, Y, Matsumoto, H, Nakazato, K, Konno, M, Inoue, Y, Nishihara, H, “Surfactant-Enhanced Performance of a Bio-conjugated Photodetecto Composed of Photosystem I Coupled to a Gold Nanoparticles.” Chem. Commun., 46 2557–2559 (2010)
Shelke, NT, Karche, BR, “Ultraviolet Photosensor Based on Few Layered Reduced Graphene Oxide Nanosheets.” Appl. Surf. Sci., 418 374–379 (2017)
Acknowledgments
The authors are grateful to Ms. A. Senthilnathan, Mr. Sandun Dissanayake, and Ms. D. Chandrakumara for their kind support during this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MMMGPGM and NR conceived the idea and designed the project. UGME carried out the experiments with different contributions and wrote the first draft of the manuscript. KEDYTD edited and reviewed the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ekanayake, U.G.M., Dayananda, K.E.D.Y.T., Rathuwadu, N. et al. Fabrication of multifunctional smart polyester fabric via electrochemical deposition of ZnO nano-/microhierarchical structures. J Coat Technol Res 19, 1243–1253 (2022). https://doi.org/10.1007/s11998-021-00606-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-021-00606-6