Abstract
Cotton woven fabrics were first dyed with a graphene oxide (GO) aqueous dispersion using a simple industrialized exhaustion dyeing process. The resulting fabrics were then chemically reduced to prepare the electrically conductive cotton fabrics. They were characterized by the FE-SEM, XPS and Raman spectrum and evaluated with respect to whiteness, electrical surface resistance and electrochemical impedance spectroscopy. Some factors affecting the process such as the GO concentration, dyeing and reducing conditions, and nature and concentration of the reducing agent were also investigated. The results indicated that GO could be fixed on cotton fabric by a simple exhaustion dyeing process and converted into the reduced GO (RGO) with a reducing agent. Increasing the GO concentration or dyeing temperature and time decreased the whiteness and electrical surface resistance of the RGO-dyed fabric. However, higher pH showed a reverse effect. Na2S2O4 was found to be a stronger reducing agent for the conversation of GO into RGO on cotton fabric than ascorbic acid and thiourea. The reduction of GO-dyed fabric was easily completed by an increasing Na2S2O4 concentration at higher temperature. Moreover, an increasing number of dyeing cycles decreased the surface resistance and impedance modulus and increased the abrasion resistance of the RGO-dyed cotton fabric.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electronic textiles and wearable electronics are innovative textile materials, which generally include high-performance sportswear, wearable displays, portable power and embedded health monitoring devices, etc. They incorporate new classes of electrically conductive fibers with an array of novel functionalities, such as flexibility, stretch-ability and being lightweight, directly into the textile itself (Liu et al. 2012). It is well known that electrically conductive textiles can be prepared using metallic fibers mixed with textile fibers woven into the construction of the textile. Conductivity of fabrics has been also obtained by coating with the conjugated polymers, carbon black, carbon nanotubes, metal-based powders or indium tin oxide (Shaterikhalilabad and Yazdanshenas. 2013b). However, these technologies are not cost-effective for commercial production because they often contain expensive materials and complicated processes. On the other hand, a few attempts (Kaempgen et al. 2009; Pushparaj et al. 2007; Hu et al. 2011; Jost et al. 2011; Liu et al. 2012; Yu et al. 2011) have been made to integrate nanoscale materials into textiles to prepare flexible, thin and lightweight power conversion and storage devices.
Graphene as a two-dimensional, single-layer sheet of sp2 hybridized carbon atoms has attracted a great deal of attention because of its high electronic conductivity, good thermal stability, and excellent mechanical strength (Geim and Novoselov 2007; Dong et al. 2012). Furthermore, it has been used on cotton (Liu et al. 2012; Shaterikhalilabad and Yazdanshenas 2013a, b), polyester (Molina et al. 2013a) and acrylic (Fugetsu et al. 2010) textiles as an electronic conductive coating. Graphene is often generated using several methods such as chemical vapor deposition, micromechanical exfoliation of graphite, epitaxial growth on an electrically insulating surface and creation of colloidal suspensions by chemical reduction (Zhou et al. 2011; Molina et al. 2013a). The chemical reduction method is widely used owing to its simple procedure and low cost for the possible route for industrial production. More importantly, graphene converted with graphene oxide (GO) using a reducing agent is very suitable for textiles with low degradation temperature because chemical reduction can be realized at temperatures lower than 100°C (Shaterikhalilabad and Yazdanshenas 2013a). GO is heavily oxygenated graphene bearing hydroxyl and carboxyl groups and is highly dispersible in both aqueous and polar organic solvents (Fugetsu et al. 2010). It should be noted that GO has a similar structure to the vat dye used in the textile industry (Zhou et al. 2011). Therefore, GO sheets are fixed on the surface of different fibers using the conventional dyeing methods because of the attraction forces existing between them; subsequently, it is converted to graphene by the reducing agents used in the textile industry (Fugetsu et al. 2010; Zhou et al. 2011; Shaterikhalilabad and Yazdanshenas 2013a). Although the coating of graphene on cotton fabric for electronic conductivity has been reported in previous studies (Krishnamoorthy et al. 2012; Liu et al. 2012; Shaterikhalilabad and Yazdanshenas 2013a; Samad 2014), the coating methods used were found to be hand operation or crude processes such as soaking (Shaterikhalilabad and Yazdanshenas 2013b; Sahito et al. 2015), brushing (Liu et al. 2012; Javed et al. 2014) or dipping (Krishnamoorthy et al. 2012; Samad 2014) without modern industrialized equipment, which may lead to an uneven and unfixed coating of RGO sheets on the cotton fiber surface, thus limiting their future commercial application. Additionally, some factors affecting RGO coating processes were investigated in the previous studies. However, the coating process parameters were not systematically optimized toward better electrical conductivity in the case of using industrialized equipment, which may reduce their practical importance. Thus, it is expected that production of large-scale RGO-coated cotton fabrics can be started by using industrialized processes and equipment for their increasing applications. In this present work, cotton woven fabric was selected to be dyed with GO aqueous dispersion using a simple industrialized exhaustion dyeing process because cotton fiber is a commonly used cellulose fiber in modern textile industries worldwide. It has a hierarchical network structure with a complicated surface morphology, hydrophilic groups and high porosity (Hu et al. 2010; Pasta et al. 2010; Li et al. 2015), which favor better adsorption of GO into cotton fiber from water than synthetic fibers. On the other hand, it is well known that the exhaust process is the most common and low cost method for dyeing textile materials. Moreover, textile substrates can be dyed in exhaust processes in almost any stage of their assembly into a textile product including fiber, yarn, fabric or garments (Perkins 2004). The GO-dyed cotton fabrics were then chemically reduced to prepare the electrically conductive fabrics. They were characterized by the FE-SEM, XPS and Raman spectrum and evaluated with respect to whiteness, electrical surface resistance and electrochemical impedance spectroscopy (EIS). Some factors affecting the process such as the GO concentration, dyeing and reducing conditions, and nature and concentration of reducing agent were also discussed. Consequently, the best dyeing and reducing conditions are demonstrated to optimize the electrical conductivity of the resulting cotton fabrics for the future industrialized applications.
Experimental section
Reagents and materials
Graphene oxide (GO) was purchased from The Sixth Element (Changzhou) Materials, Inc., China. Sodium dithionite (Na2S2O4), thiourea (CH4N2S) and ascorbic acid (C6H8O6) were of analytical grade and used as received. Commercially scoured and bleached cotton woven fabric (118.2 gm−2) was used in this study. This fabric was further treated with a solution containing 1.0 g l−1 Na2CO3 and 2.0 g l−1 soap at a boil for 30 min, then thoroughly washed with cold water and dried at ambient temperature before use. Deionized water was employed throughout the study.
Dyeing of cotton fabric with reduced GO (RGO)
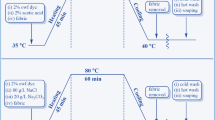
The GO aqueous dispersion was first prepared by mixing GO powders with the deionized water in an ultrasound bath for 30 min. A laboratory dyeing machine with 12 stainless steel dyeing tubes of 500 ml capacity (Datacolor International Applied Color System, Inc., USA) was used for dyeing. The known weight of the pretreated cotton fabric was dyed with different concentrations of GO aqueous dispersion at the appropriate temperature using a fabric/liquor ratio of 1:50 for a given time, and then the impregnated fabric was padded on a laboratory mangle (Mathis AG, Switzerland) with wet pick-up of 75–80 %. This procedure was repeated several times to increase the GO loading on cotton fabric. Afterward, 2.0 g of GO-dyed fabric was immersed in 100 ml aqueous solution containing varied concentrations of reducing agent at the design temperature for a certain time. Finally, the RGO-dyed fabric was rinsed thoroughly with warm deionized water and dried at 60 °C in an oven.
Characterization of RGO-dyed cotton fabric
Field-emission scanning electron microscopy (Hitachi S-4800, Japan) operating at 15 kV was used to observe the surface morphology of the RGO-dyed fabrics. Their binding energy analyses were performed on a PHI 5600 X-ray photoelectron spectrometer (Pekin-Elmer Inc., USA), and the binding energy of C1s was shifted to 284.8 eV as the reference. Raman spectra were recorded from 100 to 2700 cm; the fabric was further treated with a solution containing 1.0 g l−1 Na2CO3 and 2.0 g l−11 on a Renishaw 2000 Confocal Raman Microscope (Rhenishaw Instruments, England). Electrical surface resistance of the fabrics was measured through a standard two-probe method using a digital dual-display multimeter (Agilent U3402, USA). The measured distance of the fabric was 1.0 cm. An Alpha-A high-resolution dielectric, conductivity, impedance and gain phase modular measurement system with a ZGS active sample cell (Novocontrol BDS-50, Germany) was used to perform electrochemical impedance spectroscopy (EIS) analyses in the range of 10–107 Hz for the measurement of the electrical resistance. Whiteness of the dyed fabrics was tested using a WSB-2 fluorescent whiteness meter (Beijing Kangguang Optical Instrument Co., China) since the color can be employed as a tool for easy detection and simple evaluation of the amount or thicknesses of graphene and GO multilayer systems (Inhwa et al. 2011). The dry and wet rubbing fastness of the RGO-dyed fabric was examined by a rubbing fastness tester according to the ISO international standard (ISO105X12/D02).
Results and discussion
Dyeing of cotton fabric with GO
In order to meet the demands for rapid and effective GO dyeing of cotton fabric in future commercial applications, the pretreated cotton fabric was dyed at different conditions using an exhaustion process, and then the dyed cotton fabrics were reduced with Na2S2O4 for conversion into the graphitic-colored and electrically conductive fabrics. Their electrical surface resistance and whiteness were measured and shown in Fig. 1.
Figure 1a–c shows that the surface resistance and whiteness of the dyed cotton fabric gradually decreased with increasing GO concentration or dyeing temperature and time. However, an insignificant decrease in surface resistance was found when the GO concentration was over 0.50 %. This result suggested that cotton fabric was covered with GO sheets, thus leading to a high electrical conductivity. This is because GO sheets have various functional groups such as carboxyl, carbonyl and hydroxyl; epoxyl groups give them a hydrophilic nature (Si and Samulski 2008; Krishnamoorthy et al. 2012). This allows GO sheets to be readily soluble in water at molecular levels with high surface affinity for cellulose fibers owing to the attraction forces such as van der Waals forces and hydrogen bonds between them (Shaterikhalilabad and Yazdanshenas 2013b; Molina et al. 2013b). Consequently, when cotton fabric was immersed in the GO aqueous dispersion, GO sheets were strongly adsorbed on the fiber surface to form the thinnest layer possible, with a thickness equal to a single GO (Li et al. 2008; Cote et al. 2009; Fugetsu et al. 2010). Moreover, increasing the GO concentration could enhance the contact of GO molecules with cellulose fibers in the solution, thus resulting in a significant number of GO molecules adhered to the cellulose fibers. GO sheets have a greater tendency to disaggregate at high temperature or long dyeing time. Besides, temperature elevation is also favorable to the degree of swelling of cotton fiber in the aqueous medium because of its low crystallinity and the orientation of the molecular chains (Yi et al. 2012). On the other hand, Fig. 1d shows that higher pH increased the surface resistance and whiteness of the dyed cotton fabric. A reason for this is that cotton cellulose fiber usually exhibited a negative nature in the aqueous solution (Lim and Hudson 2004). GO is a heavily oxygenated graphene bearing hydroxyl and carboxyl groups (Fugetsu et al. 2010). Therefore, the GO molecule has low affinity for the cellulose fiber because of the enhanced charge repulsion between its ionized carboxyl groups and cellulose fiber at higher pH, especially in the alkaline pH range. On the contrary, weakening of the electrostatic interaction promoted GO molecules on the surface of the cellulose fiber at the lower pH level.
Reduction of GO-dyed cotton fabric
To select the most effective reducing agent and optimize the process variables for reduction of the GO-dyed cotton fabric, three reducing agents including ascorbic acid, thiourea and Na2S2O4 were used at different conditions to examine their influence on the surface resistance and whiteness of the RGO-dyed cotton fabric, and the results are presented in Fig. 2.
Figure 2a clearly shows that the GO-dyed cotton fabric reduced with Na2S2O4 had a much higher electrical surface resistance than those with the other two reducing agents under the same conditions, although their whiteness fluctuated slightly. This was similar to the result reported by Shaterikhalilabad and Yazdanshenas (2013b). The mechanism for the chemical reduction of graphene oxide is often regarded as two-step SN2 nucleophilic reactions followed by one step of thermal elimination. Na2S2O4 is an efficient reducing agent owing to its lower electrode potential (E SO −23 /S2O −24 = −1.12 V) than ascorbic acid and thiourea in alkaline solution (Zhou et al. 2011; Shaterikhalilabad and Yazdanshenas 2013a). And it is ready to dissociate two protons functioning as a nucleophile. Epoxide and hydroxyl groups of GO molecules are attacked by a nucleophile with a back-side SN2 nucleophile reaction, resulting in the formation of an intermediate and water, and then the intermediate may undergo thermal elimination to produce the reduced GO (Gao et al. 2010; Zhou et al. 2011; Shaterikhalilabad and Yazdanshenas 2013a). Accordingly, Na2S2O4 was used as the best reducing agent to examine the effect of process variables on the electronic conductivity of the RGO-dyed cotton fabric. Figure 2b, c shows that the surface resistance and whiteness of the RGO-dyed cotton fabric gradually decreased with an increasing Na2S2O4 concentration and reduction temperature, and the decreasing tendency became level at 3.0 % Na2S2O4 concentration or at higher than 50 °C. This result indicated that a higher Na2S2O4 concentration and temperature enhanced the darker color and the formation of RGO on cotton fabric. A reason is that the electrical conductivity is perhaps the best indicator of the extent to which the GO has been reduced to graphene (Khan et al. 2012). When the GO-dyed cotton fabric was reduced for 40 min, the lowest surface resistance and whiteness were achieved. Further increasing the reduction time resulted in a small change in its surface resistance and whiteness.
Characterization of GO-dyed cotton fabric before and after reduction
FE-SEM observation
Figure 3a shows the SEM image of the original cotton fiber, which appears as a flat ribbon-like structure with occasional convolutions along its length. Some RGO sheets could be observed on cotton fibers after GO dyeing and reduction, as seen in Fig. 3b, c. A higher magnification (2000×) FE-SEM image of the RGO-dyed cotton (Fig. 3d) show that the wrinkled RGO sheets agglomerated and formed a hierarchical structure, thus increasing the surface roughness, which was similar to the SEM observation by Krishnamoorthy et al. (2012) and Ge et al. (2014).
XPS analysis
According to the survey scan from Fig. 4a, the GO-dyed cotton fabric surface was composed mainly of carbon and oxygen elements (the hydrogen element is not detectable in XPS analysis). The contents of carbon and oxygen were 71.27 and 28.27 %, respectively. After reduction, the content of carbon for the GO-dyed cotton fabric increased to 82.47 %, whereas its oxygen content exhibited a reverse trend. More importantly, the high-resolution carbon C1s spectrum was resolved into four symmetric Gaussian components, C1 (C–C), C2 (C–O), C3 (C=O) and C4 (O–C–O) (Tang et al. 2012; Waltman et al. 1993; Stankovich et al. 2007) (Fig. 4b, c) for the dyed cotton fabric. After reduction, its main peak related to the C–C remains was slightly changed, but the intensity of the three main peaks related to the oxygen group was much smaller. These results are consistent with the above elemental analysis data and indicated a significant de-oxygenation by the reduction process for GO-dyed cotton fabric.
Raman analysis
Figure 5 shows that the D and G bands of the GO-dyed cotton fabric were centered at 1345 and 1593 cm−1, respectively. After reduction, its Raman spectrum also contained both D and G bands (shifted to 1584 and 1352 cm−1, respectively). However, the ratio of the intensities (I D/I G) was significantly increased from 0.94 for GO to 1.64 for RGO. This change indicated that some new and small sp2 domains were formed, and the average size of the crystalline RGO increased after reduction (Stankovich et al. 2007; Li et al. 2014).
Effect of dyeing process cycles
A series of GO-dyed cotton fabrics was prepared using different numbers of dyeing processes, and then reduced with Na2S2O4. The electrical resistance of the resulting RGO-dyed cotton fabrics was tested before and after rubbing, and the results are presented in Fig. 6. Furthermore, their EIS measurements were carried out in the 10–107 Hz frequency range, and the results are shown in Fig. 7.
Figure 6 shows that there is a significant decrease of the surface resistance of the RGO-dyed cotton fabrics from the first to the third dyeing process. The surface resistance slightly decreased with further increasing, and the lowest surface resistance value of 1.43 kΩ cm−1 was achieved at the seventh dyeing process. On the other hand, Fig. 7 shows that the original cotton fabric has |Z| values higher than 108 Ω cm−2 for low frequencies. The |Z| values can be observed with varying frequency. When cotton fabric was dyed with GO and successively reduced with Na2S2O4, a |Z| decrease of about six orders of magnitude was obtained (185 Ω cm2 for 10.0 Hz). It is worth noting that the |Z| value decreases with an increasing number of dyeing processes. When the seventh layer of RGO was deposited on the fabric, the |Z| value lowered to 5.5 Ω cm2 for 10.0 Hz. According previous works (Molina et al. 2013b; Shaterikhalilabad and Yazdanshenas 2013a; Fugetsu et al. 2010), the electrical conductivity increases with the increasing graphene content of the substrate, which can be attributed to the improved orientation and structure of the graphene layer. In the first dyeing process, there may be a poor contact between the RGO sheets on the fabric, thus limiting their electrical conduction. Increasing the number of dyeing cycles could add more RGO sheets on the fabric surface to obtain a better RGO sheet-to-sheet connection for constructing an infinite network of connected paths, favoring the electrical conductivity of the fabrics. Besides, Fig. 6 also shows that after dry rubbing, a similar surface resistance value was obtained for all the fabrics dyed with different RGO layers. However, wet rubbing increased the surface resistance, and the difference in surface resistance between the original and dyed fabrics declined as the number of dyeing processes increased. Little change in the surface resistance was found at the seventh dyeing process. These finds revealed that the fabrics with more RGO dyeings have excellent rubbing fastness, indicating better wear resistance of the RGO-dyed cotton fabrics.
Conclusions
Cotton fabric could be dyed with GO aqueous dispersion using a simple conventional exhaustion dyeing and chemical reducing process to prepare the electrically conductive cotton fabric. SEM, XPS and Raman spectrum analysis confirmed that wrinkled RGO sheets were formed on the surface of cotton fibers after GO dyeing and reduction. Increasing GO concentration or dyeing temperature and time could enhance the GO content on the dyed cotton fabrics and decreased their electrical surface resistance. However, increasing pH increased the surface resistance and whiteness of the dyed cotton fabric because of the enhanced charge repulsion between the GO molecule and cotton fiber. Furthermore, three reducing agents including ascorbic acid, thiourea and Na2S2O4 showed a significant reducing effect on the transformation of GO into RGO on cotton fabric. Na2S2O4 had a stronger reduction performance than the other two reducing agents at the same conditions. A higher Na2S2O4 concentration and elevation of temperature decreased the surface resistance and whiteness of the dyed cotton fabric, thus leading to an increase in its RGO content and electrical conductivity. Little change in its surface resistance was observed when the Na2S2O4 concentration was over 3.0 %, especially at higher than 50 °C. The lowest surface resistance and whiteness were obtained when the GO-dyed cotton fabric was reduced with Na2S2O4 for 40 min. An increasing number of dyeing cycles resulted in a decrease of the surface resistance and impedance modulus of the RGO-dyed fabric. The lowest surface resistance and impedance modulus were 1.43 kΩ cm−1 and 5.5 Ω cm2 for 10.0 Hz, respectively, at the seventh dyeing cycles. Dry and wet rubbing caused a slight increase in their surface resistance, particularly for the fabric with more RGO dyeings, demonstrating that the obtained RGO layers on the surface of the cotton fabric were very flexible and had better abrasion resistance. To sum up, the accepted electrically conductive cotton fabric was found when it was prepared with a 0.50 % GO aqueous dispersion and 3.0 % Na2S2O4 as a reducing agent at the desired temperature and time using a simple exhaustion dyeing process.
References
Cote LJ, Kim F, Huang J (2009) Langmuir–Blodgett assembly of graphite oxide single layers. J Am Chem Soc 131:1043–1049
Dong Z, Jiang C, Cheng H, Zhao Y, Shi G, Jiang L, Qu L (2012) Facile fabrication of light, flexible and multifunctional graphene fibers. Adv Mater 24:1856–1861
Fugetsu B, Sano E, Yu H, Mori K, Tanaka T (2010) Graphene oxide as dyestuffs for the creation of electrically conductive fabrics. Carbon 48:3340–3345
Gao J, Liu F, Liu Y, Ma N, Wang Z, Zhang X (2010) Environment-friendly method to produce graphene that employs vitamin C and amino acid. Chem Mater 22:2213–2218
Ge B, Zhang Z, Zhu X, Men X, Zhou X, Xue Q (2014) A graphene coated cotton for oil/water separation. Compos Sci Technol 102:100–105
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191
Hu L, Pasta M, Mantia FL, Cui L, Jeong S, Deshazer HD, Choi JW, Han S, Cui Y (2010) Stretchable, porous, and conductive energy textiles. Nano Lett 10:708–714
Hu L, Chen W, Xie X, Liu N, Yang Y, Wu H, Yao Y, Pasta M, Alshareef HM, Cui Y (2011) Symmetrical MnO2–carbon nanotube–textile nanostructures for wearable pseudocapacitors with high mass loading. ACS Nano 5:8904–8913
Inhwa J, Rhyee JS, Son JY, Ruoff RS, Rhee KY (2011) Colors of graphene and graphene-oxide multilayers on various substrates. Nanotechnology 23:25708–25715
Javed K, Galib CMA, Yang F (2014) A new approach to fabricate graphene electro-conductive networks on natural fibers by ultraviolet curing method. Synth Met 193:41–47
Jost K, Perez CR, McDonough JK, Presser V, Heon M, Dion G, Gogotsi Y (2011) Carbon coated textiles for flexible energy storage. Energy Environ Sci 4:5060–5067
Kaempgen M, Chan C, Ma J, Cui Y, Gruner G (2009) Printable thin film supercapacitors using single-walled carbon nanotubes. Nano Lett 9:1872–1876
Khan U, Young K, O‘Neill A, Coleman JN (2012) High strength composite fibres from polyester filled with nanotubes and graphene. J Mater Chem 22:12907–12914
Krishnamoorthy K, Navaneethaiyer U, Mohan R, Lee J, Kim SJ (2012) Graphene oxide nanostructures modified multifunctional cotton fabrics. Appl Nanosci 2:119–126
Li X, Zhang G, Bai X, Sun X, Wang X, Wang E, Dai H (2008) Highly conducting graphene sheets and Langmuir–Blodgett films. Nat Nanotechnol 3:538–542
Li R, Chen C, Li J, Xu L, Xiao J, Yan D (2014) A facile approach to superhydrophobic and superoleophilic graphene/polymer aerogels. J Mater Chem A 9:3057–3064
Li B, Dong Y, Li L (2015) Preparation and catalytic performance of Fe(III)-citric acid-modified cotton fiber complex as a novel cellulose fiber-supported heterogeneous photo-Fenton catalyst. Cellulose 22:1295–1309
Lim SH, Hudson SH (2004) Application of a fibre-reactive chitosan derivative to cotton fabric as a zero-salt dyeing auxiliary. Rev Prog Color Relat Top 120:108–113
Liu W, Yan X, Lang J, Chao P, Xue Q (2012) Flexible and conductive nanocomposite electrode based on graphene sheets and cotton cloth for supercapacitor. J Mater Chem 22:17245–17253
Molina J, Fernández J, del Río AI, Bonastre J, Cases F (2013a) Chemical and electrochemical study of fabrics coated with reduced graphene oxide. Appl Surf Sci 279:46–54
Molina J, Fernández J, del Río AI, Bonastre J, Cases F (2013b) Electrochemical characterization of reduced graphene oxide-coated polyester fabrics. Electrochim Acta 93:44–52
Pasta M, Mantia FL, Hu L, Deshazer HD, Cui Y (2010) Aqueous supercapacitors on conductive cotton. Nano Res 3:452–458
Perkins WS (2004) Textile coloration and finishing. China Textile Press, Beijing, p 175
Pushparaj VL, Shaijumon MM, Kumar A, Murugesan S, Ci LJ, Vajtai R, Linhardt RJ, Nalamasu O, Ajayan PM (2007) Flexible energy storage devices based on nanocomposite paper. Proc Natl Acad Sci 104:13574–13577
Sahito IA, Sun KC, Arbab AA (2015) Integrating high electrical conductivity and photocatalytic activity in cotton fabric by cationizing for enriched coating of negatively charged graphene oxide. Carbohydr Polym 130:299–306
Samad YA (2014) Non-destroyable graphene cladding on a range of textile and other fibers and fiber mats. RSC Adv 4:16935–16938
Shaterikhalilabad M, Yazdanshenas ME (2013a) Fabricating electroconductive cotton textiles using grapheme. Carbohydr Polym 96:190–195
Shaterikhalilabad M, Yazdanshenas ME (2013b) Preparation of superhydrophobic electroconductive graphene-coated cotton cellulose. Cellulose 20:963–972
Si Y, Samulski ET (2008) Synthesis of water soluble graphene. Nano Lett 8:1679–1682
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565
Tang H, Ehlert GJ, Lin Y, Henry A (2012) Highly efficient synthesis of graphene nanocomposites. Nano Lett 12:84–90
Waltman RJ, Pacansky J, Bates CW (1993) X-ray photoelectron spectroscopic studies on organic photoconductors: evaluation of atomic charges on chlorodiane blue and p-(diethylamino)benzaldehyde diphenylhydrazone. Chem Mater 5:1799–1804
Yi S, Dong Y, Li B, Ding Z, Huang X, Xue L (2012) Adsorption and fixation behaviour of CI Reactive Red 195 on cotton woven fabric in a nonionic surfactant Triton X-100 reverse micelle. Color Technol 128:306–314
Yu G, Hu L, Vosgueritchian M, Wang H, Xie X, McDonough JR, Cui X, Cui Y, Bao Z (2011) Solution-processed graphene/MnO2 nanostructured textiles for high performance electrochemical capacitors. Nano Lett 11:2905–2911
Zhou T, Chen F, Liu K, Deng H, Zhang Q, Feng J, Fu Q (2011) A simple and efficient method to prepare graphene by reduction of graphite oxide with sodium hydrosulfite. Nanotechnology 22:2362–2365
Acknowledgments
The authors thank the Tianjin Municipal Science and Technology Committee for a Research Program of Application Foundation and Advanced Technology (11JCZDJ24600). This research was also supported in part by the Innovation and Pioneering Talents Plan of Jiangsu Province (2015–2026) and Shaoxing Public-benefit Project (2014B70006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, W., Dong, Y., Cui, G. et al. Optimized preparation of electrically conductive cotton fabric by an industrialized exhaustion dyeing with reduced graphene oxide. Cellulose 23, 3291–3300 (2016). https://doi.org/10.1007/s10570-016-1006-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-1006-y