Abstract
This study demonstrates the coating of poly(hydroxypropyl methacrylate) (PHPMA) thin films from the corresponding monomer HPMA using pulsed-initiated chemical vapor deposition (pulsed-iCVD) method. The advantages of pulsing the power delivered to the filament wires during iCVD through successive on–off duty cycles (DC) are outlined by considering the deposition rates, structure, and morphology of as-deposited PHPMA films. FTIR and XPS analysis of the as-deposited films verifies that pulsing the filament power produces thin films of PHPMA, which is structurally very similar to that synthesized using classical continuous power iCVD. However, there is up to 70% increase in deposition rate upon pulsing the filament power in comparison with the continuous power iCVD. AFM analysis of the as-deposited films shows that the film morphology can be controlled by varying the DC during pulsed-iCVD. At a DC of 85.7%, the most hydrophilic film is observed with a measured water contact angle of 31°.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thin films of functional polymers derived from acrylic or methacrylic monomers are desirable materials for many applications due to their useful and flexible properties. Desired functionalities can be imparted on a substrate surface with the help of thin polymeric films, which can be deposited using either solution-based or dry processing routes.1,2,3,4 The use of solvents in wet techniques causes both environmental problems and application difficulties on fragile surfaces. Most liquids cannot penetrate into deep holes and recessions, which reduces the coating uniformity for geometrically complex substrates. Initiated chemical vapor deposition (iCVD), which falls within the broader class of hot-wire CVD (HWCVD) techniques, is a dry alternative for coating functional polymeric thin films on virtually any surface. iCVD technique has been widely used and tested since its first description in 2003, and the studies highlighted the distinct advantages of the technique compared with other techniques.5,6,7,8
In iCVD, the polymerization takes place in the vapor environment and at low substrate temperatures, making it possible to obtain substrate-independent, conformal and uniform coatings.9,10 The activation energy required for the polymerization reactions is supplied by using the heated filament array, which is placed a few centimeters above the substrate surface, while the substrate is cooled to promote the physical adsorption of monomer molecules on its surface. The temperature of the filament array is adjusted just to dissociate the weak initiator molecules, while monomer molecules remain intact at such low temperatures. In this way, the retention of functional groups of starting monomers has been shown to be very high, which is advantageous to make use of the intended functionality in the as-deposited film.11 Plasma-enhanced CVD (PECVD) differs from iCVD in the way that the coating reactions are activated. In PECVD, energetic particles produced in the plasma discharge are used to initiate polymerization reactions. Usually, thin polymeric films obtained with PECVD are insoluble in water, have high mechanical strength, and are thermally stable due to their strong crosslinkages.12,13 However, in the case of conventional continuous wave plasma polymerization, it is difficult to obtain a pure and well-defined polymer because of severe fragmentation of the precursor within the electrical discharge.14,15 More recently, pulsing the plasma discharge has been shown to improve the deposition rates and structural retention of many monomers during PECVD, which can be ascribed to low levels of precursor fragmentation during the short on-period in association with conventional polymerization reaction pathways predominating during the pulsed plasma duty cycle off-period.16,17
In this study, we tried to extend the advantages of using pulsed-power in PECVD to iCVD by pulsing the electrical power supplied to the filament array during the deposition of poly(hydroxypropyl methacrylate) (PHPMA) from its corresponding monomer. PHPMA in the form of a thin film is a desirable material for many applications due to its key properties like being hydrophilic, bio-compatible, nontoxic, and stimuli-responsive.18,19,20 A comparison was made between the continuous and pulsed iCVD modes, by considering the deposition rates, structure, and morphologies of as-deposited PHPMA films.
Experimental
Materials
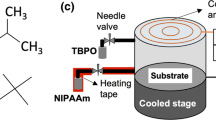
Hydroxypropyl methacrylate (HPMA) (Aldrich, 97%) and tertbutyl peroxide (TBPO) (Aldrich, 98%) were used as monomer and initiator, respectively, as-received without any purification. Films were deposited on flat silicon wafers (100, p-type), which were used as-received. The chemical structures of HPMA and TBPO are shown in Fig. 1a.
Pulsed iCVD of PHPMA thin films
PHPMA thin films were deposited in a custom-built iCVD system, the schematic drawing of which is shown in Fig. 1b. 21 The system consists of a vacuum chamber, vacuum pump, chiller, precursor jars, feed lines, and control units for temperature, pressure, and mass flow. Real-time thickness monitoring was achieved using a laser interferometry system, which consists of a He-Ne laser (Uniphase) and a laser power meter (Thorlabs). The liquid monomer and the initiator were vaporized in separate temperature-controlled stainless-steel jars, and their vapors were delivered to the reactor with the aid of needle valves. For all runs, flowrates (FR) of monomer and initiator were kept constant at 0.5 and 1 sccm, respectively. Depositions were carried out at two different pressures (P), 170 and 300 mtorrs, which were measured and controlled using a capacitance manometer (MKS Baratron) and a butterfly valve (MKS 253). Substrate temperature (Ts) was set to the desired value using a water-circulated chiller. A filament array containing 12 parallel tungsten filaments (Alfa Aesar, 99.95%, 0.375 mm) was placed 20 mm above the substrate surface, and it was resistively heated to the desired temperature, which was measured by directly anchoring a K-type thermocouple (Omega) to one of the filaments. Different from classical iCVD, in which the filament is continuously heated, the power input to the filament wire was cycled between “on” and “off” periods (ton and toff, respectively), which we call pulsed-iCVD (depicted in Fig. 1c). For the pulsed experiments, the duty cycle (DC) and average power delivered to the system (PEQ) were calculated using the following expressions:
where PCN is power delivered to the system under continuous mode.
Effects of filament operation mode (either continuous or pulsed) and the duty cycle on the structure, morphology and deposition rate of as-deposited PHPMA were investigated at different reactor pressures and substrate temperatures. Details of the experimental runs are listed in Table 1.
Characterization of the as-deposited thin films
Chemical structure of the deposits was investigated by Fourier transform infrared spectroscopy (FTIR, Bruker Vertex 70) and X-ray photoelectron spectroscopy (XPS, Specs spectrophotometer with a monochromatized Al source). FTIR spectra presented in this study represent the average of 32 scans over the wavenumber range between 4,000 and 400 cm-1 at a resolution of 4 cm-1. Atomic force microscopy (AFM) (NT-MDT) was employed in semicontact mode to investigate the surface morphology of as-deposited PHPMA thin films. Water contact angle measurements were taken to determine the wettability of as-deposited PHPMA surfaces using a contact angle goniometer (Kruss Easy Drop).
Results and discussion
Deposition kinetics
In this section, it is aimed to reveal the effect of pulsing the filament power on the deposition rates, if any. Two sets of experiments were carried out, in which the duty cycle (DC) was set as an independent variable while keeping all other deposition parameters constant. The effect of DC, which was varied between 28.5% and 85.7%, on deposition rates at two different deposition pressures and at two different substrate temperatures is given in Figs. 2 and 3, respectively. For the deposition carried out at low pressure (Fig. 2), the deposition rate was found to increase with increasing DC. It is important to note here that the deposition rates observed in continuous mode, in which the filament power was constantly on during the deposition runs, were always lower than the deposition rates observed under pulsed power conditions. In iCVD, electrical power is used for resistive heating of the filaments. Under continuous power mode, the temperature of the filament is kept at the same value throughout the deposition runs. Pulsing the power supplied to the filament array results in a decrease in filament temperature each time the filament power was turned off. Figure 4 shows the temperature profile of the filament array at different duty cycles, and it is seen that at low duty cycle percentages, the minimum and average filament temperatures are low, as expected. The average filament temperature values given in Table 2 were calculated by integral time averaging of the filament temperatures measured during each run. When the power was turned on, the maximum temperature was reached in very short times. During the on period, at which the maximum filament temperature was observed, the rate of fragmentation reaction of initiator molecules into free radicals is highest. The proposed iCVD reaction mechanism, as studied extensively in the literature,22,23 consists of steps involving the dissociation of initiator near the heated filament array, adsorption of primary radicals formed after the thermal dissociation of initiator, and surface reaction between adsorbed initiator and monomer molecules. The decrease in filament temperature upon pulsing essentially decreases the rate at which the initiator dissociates, but at the same time it also decreases the actual surface temperature due to decreasing radiation heat load on the substrate surface. In our previous study, we showed that iCVD of PHPMA is surface-kinetics-controlled; that is, the surface temperature and the deposition rates were directly proportional.24 Therefore, the increase in deposition rates at higher duty cycles shown in Fig. 2 is expected because of increasing average filament and hence the surface temperatures. Under these conditions, the rate of surface reaction is most likely slower than that of the surface adsorption, so the increase in deposition rates is promoted.
The most striking and contradictory observation, on the other hand, is that the deposition rate under continuous power is about 41% lower than the rate of depositions carried out at 85.7% duty cycle. In the pulsed-PECVD method, the deposition rates usually decrease upon pulsing the discharge.25,26 The increase in the deposition rate under pulsed-power conditions can be attributed to the increased number of primary radicals targeting the substrate surface, due to their increased concentration during each on–off cycle. When the power is pulsed, the thermal equilibrium at the boundary layer around the heated filament is disturbed, promoting more initiator molecules entering the boundary layer. When the power is turned on, which is accompanied by a sharp increase in filament temperature, new radicals are formed at a high rate. This proposed phenomenon of course is dependent on the conditions in the CVD chamber. At higher pressures, for example, the initial increase in deposition rates with increasing DC was followed by a decrease in deposition rate after a certain DC value. The decrease in deposition rate at higher pressure may be attributed to an increase in residence time of reactants in the chamber, which may cause the rate of termination to increase, or it may promote a change in deposition mechanism, such as the onset of some gas-phase reactions at a lowered mean-free path.
The effect of DC on deposition rates at a low substrate temperature is shown in Fig. 3. A decrease in deposition rates at low substrate temperatures is expected since PHPMA deposition mechanism is a surface-kinetics limited type, as stated earlier. In that case, the highest deposition rates were observed at the lowest DC, where the average filament temperature was just 136°C. There is a close relation between decreasing the substrate temperature and increasing the chamber pressure; both increase the saturation ratio. It can be said that at an increased saturation ratio value, which is close to unity at low-temperature experiments carried out in this study, very short on-periods are enough to create necessary primary radicals out of the initiator molecules to start fast deposition reactions.
Structure and morphology of as-deposited films
Figure 5 shows a comparison of typical FTIR spectra of PHPMA deposited by iCVD under pulsed and continuous modes in comparison with the spectrum of the HPMA monomer. The iCVD films were grown under filament and substrate temperatures of 250°C and 30°C, respectively, at a pressure of 170 mTorr. It can be seen from the figure that the monomer and polymer spectra compare favorably, in that they both have the similar functional groups at the same wavenumbers, indicating a high degree of structural retention in the as-deposited thin films. There are mainly five main vibrational modes in all spectra: O–H stretching (3700–3050 cm−1), C–H stretching (3050–2800 cm−1), C=O stretching (1750–1690 cm−1), C–H bending (1500–1350 cm−1), and C–O stretching (1300–1200 cm−1). These peak assignments are based on literature data on the FTIR analysis of PHPMA.24,27 The monomer spectrum in Fig. 5c has peaks at 1640, 1396, 1265, 940, and 887 cm−1, which are characteristic absorbance bands for the C=C double bond . The lack of these peaks in the FTIR spectra of iCVD polymers indicates that the polymerization proceeded via activation of the acrylate C=C double bonds. In the spectra of iCVD PHPMA films, the broad peak centered at 3450 cm-1 and the strong peak centered at 1725 cm-1 clearly show the retention of hydroxyl and carbonyl functionalities, respectively. The strong resemblance between the spectra of films deposited under pulsed and continuous power conditions (Figs. 5a and 5b) shows that pulsing the power produces thin films of PHPMA, which is structurally very similar to that synthesized using classical continuous power iCVD. XPS analysis was carried out to further characterize the films deposited under pulsed mode. Figure 6 shows the XPS survey scan of PHPMA deposited under pulsed mode with a DC of 87.5. As expected from the chemical formula for the corresponding monomer HPMA, only carbon and oxygen peaks are observed in the spectrum. From the survey spectrum, the ratio of carbon/oxygen atomic concentration was 70.4/29.6, which is nearly identical to the theoretical ratio of 70/30 calculated from the chemical formula of PHPMA. Both FTIR and XPS analyses indicate that the functional groups are maintained to a high extent during pulsed CVD.
Figure 7 shows the surface morphologies of PHPMA films deposited at different temperatures and duty cycles. It was observed that films deposited at low DC of 28.5% are featureless with very low RMS values of 0.50 nm (b) and 0.28 nm (d). On the other hand, films deposited at high DC of 85.7% possess coarse morphology. At such a high DC value, the films deposited at 15 and 30°C have RMS roughness values of 5.3 and 4.1 nm, respectively. At high DC, the deposition rates were found to be highly dependent on surface temperature as discussed above. The substantial change in deposition rates implies a change in deposition mechanism, which most likely has an effect on the change in film morphology. At high substrate temperature, the wormlike structures, which have been observed for some other films grown by iCVD in the literature,28 can be a result of the domination of film growth by initiation. With decreasing substrate temperature, the feature size increases indicating the decrease in nucleation sites at such low substrate temperatures. A similar dependence of surface morphology on substrate temperature was also observed for the PHPMA films deposited under continuous iCVD conditions.24
Figure 8 shows the change of water contact angle on iCVD-PHPMA surfaces with respect to DC. It was observed that WCA values of as-deposited polymers can be tuned by careful adjustment of DC. The change in film surface morphology is the most probable reason behind the changes in WCA values. With decreasing DC, films become smoother, which is accompanied by an increase in WCA. This observation is consistent with the Wenzel model, according to which the roughness and hydrophobicity are inversely related for hydrophilic surfaces, as in the case for PHPMA deposited in this study. The deposition strategy outlined in this study, i.e., pulsing the filament power during iCVD, can be applied for the CVD of functional polymers, which have been already deposited by various vapor phase coating strategies.29,30,31,32,33
Conclusions
This work demonstrated that pulsed-iCVD can be used to deposit polymeric thin films with desired functionalities by feeding a proper monomer and an initiator. Higher growth rates up to 70% could be achieved under pulsed-iCVD conditions compared to the rates observed in classical continuous power iCVD. The structure of the as-deposited films, on the other hand, is not affected much upon pulsing the power. The rate enhancement, due to the pulsing the power, is attributed to the change in the deposition mechanism during iCVD upon pulsing. Not only deposition rates but also film morphology was affected from DC, which in turn changed the film wettability. Most hydrophilic films were obtained at high duty cycles.
References
Lv, X, Guo, P, Liu, H, Cui, L, Cui, X, “Preparation of Paraffin-Based Phase-Change Microcapsules and Application in Geopolymer Coating.” J. Coat. Technol. Res., 15 (4) 867–874 (2018)
Robinson, K, Khan, M, de Paz Banez, M, Wang, X, Armes, S, “Controlled Polymerization of 2-Hydroxyethyl Methacrylate by ATRP at Ambient Temperature.” Macromolecules, 34 (10) 3155–3158 (2001)
Ozaydin-Ince, G, Gleason, KK, “Thermal Stability of Acrylic/Methacrylic Sacrificial Copolymers Fabricated by Initiated Chemical Vapor Deposition.” J. Electrochem. Soc., 157 (1) D41 (2009)
Yang, R, Asatekin, A, Gleason, KK, “Design of Conformal, Substrate-Independent Surface Modification for Controlled Protein Adsorption by Chemical Vapor Deposition (CVD).” Soft Matter, 8 (1) 31–43 (2012)
Bose, R, Nejati, S, Lau, KK, “Initiated Chemical Vapor Deposition (iCVD) of Hydrogel Polymers.” ECS Trans., 25 (8) 1229 (2009)
Sreenivasan, R, Gleason, KK, “Overview of Strategies for the CVD of Organic Films and Functional Polymer Layers.” Chem. Vapor Deposit., 15 (4–6) 77–90 (2009)
Alf, ME, Asatekin, A, Barr, MC, Baxamusa, SH, Chelawat, H, Ozaydin-Ince, G, Petruczok, CD, Sreenivasan, R, Tenhaeff, WE, Trujillo, NJ, “Chemical Vapor Deposition of Conformal, Functional, and Responsive Polymer Films.” Adv. Mater., 22 (18) 1993–2027 (2010)
Yagüe, JL, Coclite, AM, Petruczok, C, Gleason, KK, “Chemical Vapor Deposition for Solvent-Free Polymerization at Surfaces.” Macromol. Chem. Phys., 214 (3) 302–312 (2013)
Asatekin, A, Barr, MC, Baxamusa, SH, Lau, KK, Tenhaeff, W, Xu, J, Gleason, KK, “Designing Polymer Surfaces via Vapor Deposition.” Mater. Today, 13 (5) 26–33 (2010)
Wang, M, Wang, X, Moni, P, Liu, A, Kim, DH, Jo, WJ, Sojoudi, H, Gleason, KK, “CVD Polymers for Devices and Device Fabrication.” Adv. Mater., 29 (11) 1604606 (2017)
Şimşek, B, Karaman, M, “Initiated Chemical Vapor Deposition of Poly(hexafluorobutyl acrylate) Thin Films for Superhydrophobic Surface Modification of Nanostructured Textile Surfaces.” J. Coat. Technol. Res., 17 (2) 381–391 (2020)
Gürsoy, M, “Fabrication of Poly(N-isopropylacrylamide) with Higher Deposition Rate and Easier Phase Transition by Initiated Plasma Enhanced Chemical Vapor Deposition.” Plasma Chem. Plasma Process., 40 1063–1079 (2020)
Vasudev, MC, Anderson, KD, Bunning, TJ, Tsukruk, VV, Naik, RR, “Exploration of Plasma-Enhanced Chemical Vapor Deposition as a Method for Thin-Film Fabrication With Biological Applications.” ACS Appl. Mater. Interfaces, 5 (10) 3983–3994 (2013)
Bodas, DS, Desai, SM, Gangal, S, “Deposition of Plasma-Polymerized Hydroxyethyl Methacrylate (HEMA) on Silicon in Presence of Argon Plasma.” Appl. Surface Sci., 245 (1–4) 186–190 (2005)
Gürsoy, M, Uçar, T, Tosun, Z, Karaman, M, “Initiation of 2-Hydroxyethyl Methacrylate Polymerization by Tert-Butyl Peroxide in a Planar PECVD System.” Plasma Processes Polym., 13 (4) 438–446 (2016)
Ryan, M, Hynes, A, Badyal, J, “Pulsed Plasma Polymerization of Maleic Anhydride.” Chem. Mater., 8 (1) 37–42 (1996)
Wu, Q, Gleason, KK, “Plasma-Enhanced CVD of Organosilicate Glass (OSG) Films Deposited from Octamethyltrisiloxane, Bis(trimethylsiloxy) Methylsilane, and 1,1,3,3-Tetramethyldisiloxane.” Plasmas Polym., 8 (1) 31–41 (2003)
Sakai, Y, Matuguchi, M, Sadaoka, Y, Hirayama, K, “A Humidity Sensor Composed of Interpenetrating Polymer Networks of Hydrophilic and Hydrophobic Methacrylate Polymers.” J. Electrochem. Soc., 140 (2) 432 (1993)
Bahar, T, Tuncel, A, “Immobilization of α-Chymotrypsin Onto Newly Produced Poly(hydroxypropyl methacrylate–co-methacrylic acid) Hydrogel Beads.” React. Funct. Polym., 44 (1) 71–78 (2000)
Zhao, C, Li, L, Zheng, J, “Achieving Highly Effective Nonfouling Performance for Surface-Grafted Poly (HPMA) via Atom-Transfer Radical Polymerization.” Langmuir, 26 (22) 17375–17382 (2010)
Karaman, M, Çabuk, N, “Initiated Chemical Vapor Deposition of pH Responsive Poly(2-diisopropylamino)ethyl Methacrylate Thin Films.” Thin Solid Films, 520 (21) 6484–6488 (2012)
Ozaydin-Ince, G, Gleason, KK, “Transition Between Kinetic and Mass Transfer Regimes in the Initiated Chemical Vapor Deposition From Ethylene Glycol Diacrylate.” J. Vacuum Sci. Technol. A Vacuum Surfaces Films, 27 (5) 1135–1143 (2009)
Xu, J, Gleason, KK, “Conformal Polymeric Thin Films by Low-Temperature Rapid Initiated Chemical Vapor Deposition (iCVD) Using Tert-Butyl Peroxybenzoate as an Initiator.” ACS Appl. Mater. Interfaces, 3 (7) 2410–2416 (2011)
Sevgili, E, Karaman, M, “Initiated Chemical Vapor Deposition of Poly(hydroxypropyl methacrylate) Thin Films.” Thin Solid Films, 687 137446 (2019)
Gürsoy, M, Karaman, M, “Hydrophobic Coating of Expanded Perlite Particles by Plasma Polymerization.” Chem. Eng. J., 284 343–350 (2016)
Gürsoy, M, Karaman, M, “Improvement of Wetting Properties of Expanded Perlite Particles by an Organic Conformal Coating.” Prog. Org. Coat., 120 190–197 (2018)
Ali, A, Pareek, P, Sewell, L, Schmid, A, Fujii, S, Armes, S, Shirley, I, “Synthesis of Poly(2-hydroxypropyl methacrylate) Latex Particles via Aqueous Dispersion Polymerization.” Soft Matter, 3 (8) 1003–1013 (2007)
Perrotta, A, Christian, P, Jones, AO, Muralter, F, Coclite, AM, “Growth Regimes of Poly(perfluorodecyl acrylate) Thin Films by Initiated Chemical Vapor Deposition.” Macromolecules, 51 (15) 5694–5703 (2018)
Carletto, A, Badyal, JPS, “Ultra-High Selectivity Pulsed Plasmachemical Deposition Reaction Pathways.” Phys. Chem. Chem. Phys., 21 (30) 16468–16476 (2019)
Muralter, F, Greco, F, Coclite, AM, “Applicability of Vapor-Deposited Thermoresponsive Hydrogel Thin Films in Ultrafast Humidity Sensors/Actuators.” ACS Appl. Polymer Mater., 2 (3) 1160–1168 (2019)
Yılmaz, K, Şakalak, H, Gürsoy, M, Karaman, M, “Initiated Chemical Vapor Deposition of Poly(ethylhexyl acrylate) Films in a Large-Scale Batch Reactor.” Ind. Eng. Chem. Res., 58 (32) 14795–14801 (2019)
Çıtak, E, Testici, H, Gürsoy, M, Sevgili, E, Dağı, HT, Öztürk, B, Karaman, M, “Vapor Deposition of Quaternary Ammonium Methacrylate Polymers with High Antimicrobial Activity: Synthetic Route, Toxicity Assessment, and Durability Analysis.” J. Vacuum Sci. Technol. A, 38 (4) 043203 (2020)
Yartaşı, Y, Karaman, M, “Plasma Enhanced Chemical Vapor Deposition of Poly(cyclohexyl methacrylate) as a Sacrificial Thin Film.” Plasma Chem. Plasma Process., 40 (1) 357–369 (2020)
Acknowledgments
This study was supported by Konya Technical University Scientific Research Foundation with a grant number of 18201085.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mercan, E.S., Karaman, M. Coating of hydrophilic poly(hydroxypropyl methacrylate) thin films via pulsed-initiated chemical vapor deposition method. J Coat Technol Res 18, 1261–1268 (2021). https://doi.org/10.1007/s11998-021-00486-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-021-00486-w