Abstract
Herein, it is reported that synthesis of poly(styrene-co-butyl acrylate) latexes containing ZnO and TiO2 nanoparticles was carried out using miniemulsion polymerization. The synthesized latexes and films cast from them were characterized by dynamic light scattering (DLS), X-ray diffraction (XRD), scanning electron microscopy energy dispersive X-ray (SEM-EDAX), transmission electron microscopy (TEM), and thermogravimetric analysis (TGA). Stress–strain and DMA analysis was performed for the mechanical analysis of the films prepared from latexes. The particle sizes of the latexes were about 60 nm for pure latex and latex only containing ZnO and TiO2, while the particle size for latex containing nanoparticle mixture was measured as 120 nm. XRD, SEM-EDAX, and TEM analysis results showed that the nanoparticles were well dispersed in latex. When the mechanical analysis results were compared, latex films including 2% ZnO nanoparticles were found to be stronger. DMA analysis showed that the glass transition temperature (Tg) values of the polymers were between 0 and 10°C and the nanoparticles were not effective in changing the Tg values. Latex films were exposed to 254 nm UV light to determine their yellowing. According to the colorimetric measurement, ZnO nanoparticle-embedded films were found to be more resistant to yellowing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Emulsion polymerization, which includes techniques such as conventional emulsion polymerization, inverse emulsion polymerization, dispersion polymerization, microemulsion polymerization, miniemulsion, and suspension polymerization, is a preferred process in the production of many commonly used polymer applications such as binders, paints, plastics, adhesives, and so on.1,2,3
Although waterborne latexes, styrene acrylates, are widely preferred in various fields due to their transparency, chemical resistance, weather resistance, mechanical stability, adhesion, and film-forming properties, they do not have good ultraviolet (UV) protection properties for outdoor applications. To prevent UV deterioration, UV absorbers (benzotriazole, benzophenone, etc.), radical scavengers, radical quenchers, and UV protective agents (nano-cerium dioxide, nano-zinc oxide, and nano-titanium dioxide) are preferred as stabilizers.4,5,6,7,8,9 Many chemical approaches such as solution exfoliation, melt intercalation, and in situ polymerization (batch, solution, and heterogeneous polymerization techniques) are used for the preparation of nanocomposites. In recent years, waterborne polymeric nanocomposites prepared by emulsion, miniemulsion, or suspension polymerizations have attracted great attention in academia and industry due to being environmentally friendly and other advantages (cheap, clean, low viscosity, etc.).10,11,12
Studies on the synthesis of new nanocomposite polymers that greatly improved the properties of the pure polymer with the contributions of the nano-inorganic filler are of great interest for both academia and industry. Inorganic nano fillers such as nano-clay12,13,14,15,16,17,18,19,20,21, nano-ZnO9,11,22,23,24,25, nano-TiO24,26,27,28,29,30, nano-CeO25,31,32,33,34,35, and nano-iron oxide36,37,38 are mostly used either in pure form or in modified form. Due to the agglomeration and difficulties in the dispersion of nanoparticles in solvent medium and also melting incorporation, nanocomposite preparation has attracted a great deal of interest in the literature, especially since a Toyota research team reported the exfoliated Nylon 6/clay nanocomposite.39 For example, Erdem and co-workers prepared styrene/encapsulated titania nanoparticles with miniemulsion polymerization using cyclohexane26 and hexadecane27 co-stabilizers. Man et al.4 synthesized and characterized rutile TiO2/poly(methyl methacrylate(MMA)-butyl acrylate(BA)-acrylic acid(AA) nanocomposites via seeded emulsion polymerization and studied their UV-shielding property. González et al.30 synthesized poly(MMA/BA) copolymer-stabilized titania nanoparticles by miniemulsion polymerization and reported that increased TiO2 content decreased hybrid particle size and film roughness. Moreover, ZnO nanoparticles were incorporated into the MMA, BA, and perfluorooctyl acrylate (FOA) latexes by batch miniemulsion polymerization, and corrosion behavior was investigated by Chimenti et al.9 Danková et al.24 synthesized acrylate latex binders by semicontinuous emulsion polymerization using 1.5 wt% ZnO nanoparticles with respect to the polymer content. Chou and coworkers25 synthesized PMMA-b-PBA block copolymer latexes including zinc oxide/poly(sodium 4-styrenesulfonate). Recently, Eren and Can40 synthesized the zinc methacrylate-methylmethacrylate-butyl acrylate emulsions by semibatch emulsion polymerization and applied them to exterior paints and reported on the solubility of the ZnO nanoparticles in the medium of methacrylic acid monomer. Also, some authors preferred the combination of ZnO/TiO2 nanoparticles to achieve more efficient photocatalytic nanoparticles and they used ZnO/TiO2 nanoparticles combination for photocatalytic removal of a herbicide (Bentazon).41 Zhang et al.28 successfully synthesized nano ZnO/TiO2 coupled oxide photocatalyst by a two-step method and applied it for the degradation of methyl orange dye. Magnetic titanium dioxide/polystyrene/magnetite composite hybrid polymer particles were synthesized by Pickering miniemulsion polymerization in one single step and used for degrading methylene blue (MB) from solutions by Bonnefond et al.36
Unlike the above-mentioned literature, for the first time, in this study, it was reported that poly(Sty-co-BA-AA)/ZnO/TiO2/ZnO-TiO2 nanocomposite latexes were synthesized by miniemulsion polymerization in one-pot adding (batch-wise). Moreover, incorporation of nanoparticles into the polymer matrix without premodification was accomplished. Synthesized nanocomposite latexes and their films were characterized, and yellowing of the films was assessed.
Experimental
Materials
Styrene (Sty-, purchased from Poliya Company, Turkey), butyl acrylate (BA-supplied kindly from KEMPRO Company, Turkey) and acrylic acid (AA- purchased from ZAG Kimya, Turkey) monomers were used in the study after distillation in darkness under reduced pressure to remove the inhibitors and stored in a refrigerator before use. Ammonium persulfate (APS), sodium bicarbonate (SBC), and sodium dodecyl sulfate (SDS) were used as the initiator, to adjust the pH and emulsifier, respectively, and they were obtained from Merck. The hexadecane (HD) used in the study was purchased from Sigma-Aldrich and used as is. ZnO nanoparticle with 40-100 nm particle size and 10–25 m2/g surface area was supplied from Alfa Aesar. The rutile TiO2 nanoparticles with < 100 nm particle size and trace metal basis used in the study were Aldrich brand. Deionized water (Mes, MP Minipure, Turkey) was used as solvent throughout the study.
Experimental procedure
Synthesis studies of nanoparticle-added latexes containing various proportions of ZnO and TiO2 were carried out with a total ~ 30% solids. All studies were achieved using 50/50% styrene/butyl acrylate monomer ratios and 2 g of acrylic acid (neutralized completely with NaOH solution). To ensure that the ZnO and TiO2 nanoparticles remain stable in latex, the general method for the synthesis of latexes was applied as follows.
The emulsifier, nanoparticles (ZnO, TiO2, and ZnO/TiO2), monomers, hexadecane, and deionized water were weighed in appropriate amounts according to the total solid content (see Table 1) and were mixed overnight. The temperature was kept low to avoid polymerization. Then, the mixture was first subjected to ultrasonic treatment in the ultrasonic bath (Kudos, 53 kHz frequency) for 20 min, and then, it was mixed with the homogenizer (IKA T18 digital ULTRA TURRAX) at 8000 rpm to obtain pre-emulsion. The pre-emulsified colloidal mixture was transferred to the polymerization reactor, the temperature of which was adjusted to 75 °C, to fulfill the polymerization. All the experiments were carried out in batch-wise (one-pot synthesis). The system was stirred at 260 rpm with the help of a mechanical stirrer (Heidolph). After the system reached the desired temperature, the reaction was started by adding initiator (APS). Five hours later, the reaction was terminated by cooling the reactor. After the filtration of the latex with the glass frit of pore size 1 to separate the coagulum, samples were stored in closed containers to prevent evaporation in the dark, protected from sunlight.
To prepare latex films, 20 mL of synthesized latex was taken and poured into petri dishes and allowed to dry in the dark. Dried latex films were taken from petri dishes and kept for UV tests and mechanical analysis in a way that they do not receive light.
Characterization
The solids content (SC) of the latexes was measured with Radwag MA 110R model infrared heater moisture analyzer. After separating the coagulum from the reaction product, the solid content of the latexes was determined taking into account the amount of auxiliary materials. Zeta potential values and the z average particle size (Di-nm) were measured with Zetasizer Nano ZS, Malvern Instruments. XRD diffraction analysis was performed with PANalytical—Empyrean using Cu Kα radiation (λ = 0.15406 nm, 40 mA, 45 kV, Step Size ° [2Th.] 0.0260). TGA analysis was performed at 10°C/min heating rate in 25–600°C temperature range with PerkinElmer Diamond instrument in nitrogen atmosphere. Electron diffraction analyses were performed by suspension preparation on carbon-coated copper grids and imaged with transmission electron microscope (TEM) using Jeol JSM 3010 300 kV, and TEM micrographs were obtained with JEOL JEM 1220. EDAX analyses of the latex films were examined by field emission–environmental scanning electron microscope–energy distribution spectrometry (FE-ESEM-EDS) (FEIQuanta 450 FEG) to obtain surface images and characterize the elemental composition. Stress–strain analysis was carried out with SHIMADZU AG-XD 50kN device with 5 mm/min shrinkage speed, and results were given as the average of three measurements. DMA analysis was carried out with SHIMADZU AG-XD 50kN device with 5 mm/min shrinkage speed and with PerkinElmer DMA 8000 device, with 3°C/min heating speed, 1 Hz vibration frequency, 0.05 mm/min shrinkage speed, and between − 60 and 100°C range, respectively. To examine the changes of latex films under UV light, the latex films were exposed to UV light of 254 nm for 96 h in the UV cabinet. Color measurements of samples were performed with Datacolor 400 brand color measurement instrument.
Results and discussion
When nanoparticle-filled latexes are obtained, it is expected that no coagulation occurs, the particles do not precipitate over time, and the resulting latex remains stable during the waiting period (such as no phase separation, solidification, film formation). At this point, miniemulsion polymerization was used as a preferable approach to obtain stable latex droplets. To this end, the effects of different parameters for the synthesis of ZnO and TiO2 nanoparticle-filled latexes were examined. Stability of the latexes and sedimentation problem of the nanoparticle were observed in the study conducted with different ratios of emulsifier and nanoparticle without acrylic acid (AA) and hexadecane (HD). In addition, since acrylic acid caused dissolution of ZnO nanoparticles and coagulation, neutralized acrylic acid was used in subsequent experiments.41 In polymerization experiments using acrylic acid and hexadecane, the best results were achieved using 8% AA, 4% HD, and 4% SDS (based on the amount of monomer). Furthermore, SBC and APS amounts were used as 0.5% and 0.75%, respectively, according to the monomer amount. Some properties of latexes with different ratios of ZnO and TiO2 nanoparticles prepared using these optimum conditions are given in Table 2.
The solids content, zeta potential values, and particle sizes of the synthesized latexes are shown in Table 2. Considering the recipe used in latex synthesis, the theoretically calculated amount of solid content was ≈ 30%. Table 2 shows that the results are very close to the theoretical results in all experiments and conversions are close to 100%. Particle sizes are given as a z-mean result. When the particle size analysis results are examined, the particle sizes of the latex containing ZnO and TiO2 nanoparticles alone are close to sample 1 (pristine polymer latex) and not dependent on the nanoparticle amount. In contrast, in the latexes containing both nanoparticles together (sample 6, 7 and 8), particle sizes were observed to increase up to 120 nm. Although it is known that the particle sizes increase with the addition of nanoparticles,10,30,33,42,43 there is no literature regarding the increase in particle size by adding ZnO and TiO2 nanoparticle together. Faucheu et al.43 reported an increase in particle size with increased amount of clay for clay/MMA-BA composite polymers prepared using water soluble initiator and miniemulsion polymerization. Similar cases were also reported by Leiza and co-workers. Interestingly, Romo-Uribe et al.44 reported that the latex (MMA/BA/AA) particle size remained about constant (140 nm) with nano-SiO2 concentrations. As shown in Table 2, zeta potential values indicate that stable latexes were obtained; especially, ZnO/TiO2 nanoparticle mixtures seem to be more effective on the stability of latexes.
XRD results
XRD patterns of TiO2 and ZnO nanoparticles and latex films containing these nanoparticles are given in Fig. 1. As shown in Fig. 1, latex films containing TiO2 had diffraction peaks originating from TiO2, and the intensity of these peaks appropriately increased with TiO2 concentration. No diffraction peak was observed for the latexes including ZnO nanoparticle. This could be explained by the fact that ZnO nanoparticles randomly dispersed in latex and exfoliated nanocomposite formation.13,17 However, it was also reported by Youssef, et al, while diffraction peak was observed in high ZnO loadings, no diffraction peak was observed in low ZnO loadings.45 Additionally, diffraction peaks in the TiO2 containing film were sharper and shifted relative to the diffraction peaks of the TiO2 nanoparticles themselves which could be evaluated as an indication of aggregation of the TiO2.
TGA results
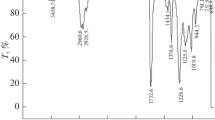
TGA analysis of filmed latex samples was performed to examine whether there was a change in the thermal stability of latex samples. TGA curves are given in Fig. 2. As shown in Fig. 2, latex and nanoparticle-added latex films were thought to exhibit two-stage degradation and the first degradation step was between 80 and 150°C and about 3% mass loss, which were due to water loss. When the detailed graph given in Fig. 2 for the second degradation step (225°C initial) was investigated, it was observed that the latex without nanoparticle was more easily degraded, and the ZnO nanoparticles increased thermal stability more than TiO2. This increased thermal stability could be attributed to crosslinking agent behavior of the ZnO nanoparticles.6,46 Likewise, it has been stated in the literature that ZnO nanoparticles are capable of self-crosslinking in latex containing neutralized carboxylic groups in the wet state and during drying at room temperature.47,48 Since sample 5 contains both ZnO and TiO2 nanoparticles, its thermal stability remained between the ZnO including latex film and TiO2 including latex film. When the residue amounts were compared, it was noted that latex films containing ZnO showed the highest thermal resistance.
SEM and TEM results
SEM images and EDAX analysis results of latex films containing ZnO and TiO2 nanoparticles are given in Fig. 3 with × 50k magnification. As seen from EDAX results in Fig. 3, it is clear that ZnO and TiO2 nanoparticles were incorporated into the latex. In addition, it was seen from the SEM images that the nanoparticles were distributed within the latex, with some being homogeneous in some places, and others demonstrating agglomeration in some places. The reason could be attributed to the aggregation of nanoparticles during film formation from the latex. However, as seen from the SEM images (Fig. 3c) of the latex containing nanoparticle mixture, it was seen that the nanoparticle distribution was more homogeneous; in other words, there was no agglomeration. This could be interpreted as a positive synergistic effect on nanoparticle distribution when nanoparticles were used together. When the TEM images, given in Fig. 4 are examined, it is clearly seen that the nanoparticle sizes vary in the range of 30–70 nm and the particles are dispersed in the latex even though there is agglomeration. It is also noteworthy that in Figs. 4a and 4b, the ZnO nanoparticles are encapsulated.42 Moreover, the dark lines between the polymer particles show the pinhole effect reported by Tauer et al.10,49 Additionally, images of the electron diffraction analysis in the TEM (selected area electron diffraction-SAED) are given for sample 3 (2% ZnO) and sample 5 (2% TiO2) (see Figs. 4e and 4f). Considering the copolymer composition (component that can make diffraction), diffraction rings with different images evidently demonstrate that latex samples contain ZnO and TiO2 nanoparticles.
Mechanical properties of the latex films
Stress–strain analysis and DMA (dynamic mechanical analysis) were performed as three parallel studies to determine the mechanical properties of the films obtained from the synthesized latex. When Table 3 and Fig. 5 are examined together, it is understood that ZnO-embedded latex films had the highest mechanical strength due to crosslinking effect mentioned in thermal properties section, and considering samples 6, 7, and 8, ZnO was more effective than TiO2 in view of increasing the mechanical properties of latex films. Table 3 shows that the maximum force was already observed in sample 3 containing 2% ZnO nanoparticle. Although it was understood that the results of the pristine latex film (sample 1) showed good resistance compared to the nanocomposite films, the film thickness should not be neglected. In particular, the mechanical strength of latexes containing 1% ZnO and 1% TiO2 nanoparticle samples seems to be quite good considering the film thicknesses. The modulus vs temperature curves of the obtained latex films are given in Fig. 6a. As shown in Fig. 6a, there was a slight decrease in the modulus values of the latex films incorporated into the nanoparticle, and this decrease was not as much in both the glassy state and the rubbery region for the films containing 2% ZnO. This decrease appeared to be greater in films alone containing TiO2 and the mixture (1% ZnO and 1% TiO2). While the mechanical properties of latex films containing nanoparticles were expected to increase, this loss in modulus was thought to be related to decrease in molecular weight with the addition of nanoparticles.13,50 The curves of Tan δ vs temperature of latex films are given in Fig. 6. As shown in Fig. 6, the temperature at which polymer films began to soften, i.e., Tg values, was almost the same. The ~ Tg value calculated from the Fox equation for the Sty-BA-AA (sodium acrylate in this study) copolymer composition in this study is around + 15°C. As shown in Fig. 5.7, in the synthesized latex films, a transition from glassy to rubbery was observed below this temperature (0–10°C). Nanoparticles appeared to slightly widen the curve. This can be interpreted as a decrease in glass transition temperature, especially for samples 5 and 7. This can be explained by the fact that the incorporated nanoparticles supplied plasticizing properties to the latex films, even to a small extent.50
Yellowing test of the latex films
Color measurement results of latex films kept under UV light for 96 h are presented in Table 4. Given delta b values in Table 4 were measured based on measuring the difference in color between exposed and masked portions of the same film. The value of −b means that the samples move away without yellowing, and + b values mean that the samples turn yellow.30,51 When Table 4 is examined according to this definition, yellowing occurred in sample 1, which is pristine latex film. Except for sample 4, it is seen that the latex film samples with nanoparticle are effective against yellowing and move away from yellowing. It is concluded that the most effective condition against yellowing was in sample 6; less than 2% TiO2 and less than 1% ZnO nanoparticles were not effective against yellowing and ZnO was more effective than TiO2 against yellowing.
Conclusions
The composite latexes containing the ZnO and TiO2 nanoparticles were investigated to meet the conditions (emulsifier amount, HD amount, AA amount), such as the formation of coagulum, the nanoparticles not collapsing over time, and the stability of the obtained latex during the waiting period. As a result of the work done:
-
AA should be used in a neutralized way
-
Stable latexes cannot be obtained when using HD less than 4%
-
It is concluded that 4% SDS amount is sufficient for obtaining 30% solid latex, and latexes synthesized under these conditions are stable from the zeta potential data
Results obtained from SEM-EDAX and TEM analysis show that nanoparticles were dispersed in latex. It is concluded that nanoparticles partially increase the thermal stability of latex films. From the stress–strain analysis results of the latex films, especially ZnO nanoparticles caused an increase in strength due to ZnO crosslinking effect. It is understood from the DMA analysis results that the films began to change from glassy to rubber in the range of 0–10°C, and nanoparticles did not change this value much. As a result of the yellowing test, ZnO- and TiO2-doped latex films showed better resistance to yellowing caused by UV light and ZnO was more effective for yellowing than TiO2.
References
Erbil, Y, Vinyl Acetate Emulsion Polymerization and Copolymerization with Acrylic Monomers. CRC Press, Boca Raton (2000)
Lovell, PA, El-Aasser, MS, Emulsion Polymerization and Emulsion Polymers. Wiley, Hoboken (1997)
Dieter. U, Takamura, K, Polymer Dispersions and Their Industrial Applications. Wiley VCH Verlag GmbH & Co, KGaA (2002)
Man, YK, Mu, LY, Wang, Y, Lin, SH, Rempel, GL, Pan, QM, “Synthesis and Characterization of Rutile Titanium Dioxide/Polyacrylate Nanocomposites for Applications in Ultraviolet Light-Shielding Materials.” Polym. Comp., 36 (1) 8–16 (2015)
Aquirre, M, Paulis, M Leiza, JR, "Waterborne Acrylic/CeO2 Nanocomposites for UV Blocking Clear Coats," In: Khan, S, Akhtor, K (Eds) Cerium Oxide—Applications and Attributes, Intechopen (2018)
Tang, EJ, Liu, H, Sun, LM, Zheng, EL, Cheng, GX, “Fabrication of Zinc Oxide/poly(styrene) Grafted Nanocomposite Latex and its Dispersion.” Europ. Polym. J., 43 (10) 4210–4218 (2007)
Hu, J, Chen, M, Wu, LM, “Organic-inorganic Nanocomposites Synthesized via Miniemulsion Polymerization.” Polym. Chem., 2 (4) 760–772 (2011)
Hu, J, Zhou, Y, “The Properties of Nano(ZnO-CeO2)@polysiloxane Core–shell Microspheres and Their Application for Fabricating Optical Diffusers.” Appl. Surf. Sci., 365 166–170 (2016)
Chimenti, S, Vega, JM, Aguirre, M, García-Lecina, E, Díez, JA, Grande, H-J, Paulis, M, Leiza, JR, “Effective Incorporation of ZnO Nanoparticles by Miniemulsion Polymerization in Waterborne Binders for Steel Corrosion Protection.” J. Coat. Technol. Res., 14 (4) 829–839 (2017)
Delibas, A, Yildiz, U, Tauer, K, “Composite Latex Production with High Solid Content.” J. Appl. Polym. Sci., 136 18 (2019)
Tang, EJ, Dong, SY, “Preparation of Styrene Polymer/ZnO Nanocomposite Latex via Miniemulsion Polymerization and its Antibacterial Property.” Colloid Polym. Sci., 287 (9) 1025–1032 (2009)
Diaconu, G, Paulis, M, Leiza, JR, “High Solids Content Waterborne Acrylic/Montmorillonite Nanocomposites by Miniemulsion Polymerization.” Macromol. React. Eng., 2 (1) 80–89 (2008)
Zengeni, E, Hartmann, PC, Pasch, H, “Highly Filled Polystyrene/Laponite Hybrid Nanoparticles Prepared Using the Ad-Miniemulsion Polymerisation Technique.” Macromol. Chem. Phys., 214 (1) 62–75 (2013)
Bonnefond, A, Paulis, M, Bon, SAF, Leiza, JR, “Surfactant-free Miniemulsion Polymerization of n-BA/S Stabilized by NaMMT: Films with Improved Water Resistance.” Langmuir, 29 (7) 2397–2405 (2013)
Herrera, NN, Letoffe, JM, Putaux, JL, David, L, Bourgeat-Lami, E, “Aqueous Dispersions of Silane-functionalized Laponite Clay Platelets. A First Step toward the Elaboration of Water-based Polymer/Clay Nanocomposites.” Langmuir, 20 (5) 1564–1571 (2004)
Kang, BA, Schrade, A, Xu, Y, Chan, YT, Ziener, U, “Synthesis and Characterization of Dually Labeled Pickering-type Stabilized Polymer Nanoparticles in a Downscaled Miniemulsion System.” Langmuir, 28 (25) 9347–9354 (2012)
Yılmaz, O, Cheaburu, CN, Gülümser, G, Vasile, C, “On the Stability and Properties of the Polyacrylate/Na-MMT Nanocomposite Obtained by Seeded Emulsion Polymerization.” Europ. Polym. J., 48 (10) 1683–1695 (2012)
Ruggerone, R, Plummer, CJG, Herrera, NN, Bourgeat-Lami, E, Månson, J-AE, “Highly Filled Polystyrene–laponite Nanocomposites Prepared by Emulsion Polymerization.” Europ. Polym. J., 45 (3) 621–629 (2009)
Plummer, CJG, Ruggerone, R, Bourgeat-Lami, E, Manson, JAE, “Small Strain Mechanical Properties of Latex-based Acrylic Nanocomposite Films.” Polym., 52 (9) 2009–2015 (2011)
Diaconu, G, Micusik, M, Bonnefond, A, Paulis, M, Leiza, JR, “Macroinitiator and Macromonomer Modified Montmorillonite for the Synthesis of Acrylic/MMT Nanocomposite Latexes.” Macromol., 42 (9) 3316–3325 (2009)
Sheibat-Othman, N, Cenacchi-Pereira, AM, Dos Santos, AM, Bourgeat-Lami, E, “A Kinetic Investigation of Surfactant-free Emulsion polymerization of Styrene Using Laponite Clay Platelets as Stabilizers.” J. Polym. Sci. Part A-Polym. Chem., 49 (22) 4771–4784 (2011)
Christopher, G, Anbu Kulandainathan, M, Harichandran, G, “Highly Dispersive Waterborne Polyurethane/ZnO Nanocomposites for Corrosion Protection.” J. Coat. Technol. Res., 12 (4) 657–667 (2015)
Gu, X, Chen, G, Zhao, M, Watson, SS, Nguyen, T, Chin, JW, Martin, JW, “Critical Role of Particle/Polymer Interface in Photostability of Nano-filled Polymeric Coatings.” J. Coat. Technol. Res., 9 (3) 251–267 (2012)
Danková, M, Kalendová, A, Machotová, J, “Waterborne Coatings based on Acrylic Latex Containing Nanostructured ZnO as an Active Additive.” J. Coat. Technol. Res., 17 (2) 517–529 (2020)
Chou, IC, Lee, CF, Chiu, WY, “Preparation of Novel Suspensions of ZnO/Living Block Copolymer Latex Nanoparticles via Pickering Emulsion Polymerization and Their Long Term Stability.” J. Polym. Sci. Part A: Polym. Chem., 49 (16) 3524–3535 (2011)
Erdem, B, Sudol, ED, Dimonie, VL, El-Aasser, MS, “Encapsulation of Inorganic Particles via Miniemulsion polymerization. I. Dispersion of Titanium Dioxide Particles in Organic Media Using OLOA 370 as Stabilizer.” J. Polym. Sci. Part A: Polym. Chem., 38 (24) 4419–4430 (2000)
Erdem, B, Sudol, ED, Dimonie, VL, El-Aasser, MS, “Encapsulation of Inorganic Particles via Miniemulsion Polymerization. II. Preparation and Characterization of Styrene Miniemulsion Doplets Containing TiO2 Particles.” J. Polym. Sci. Part A: Polym. Chem., 38 (24) 4431–4440 (2000)
Zhang, M, Gao, G, Li, C-Q, Liu, F-Q, “Titania-Coated Polystyrene Hybrid Microballs Prepared with Miniemulsion Polymerization.” Langmuir, 20 (4) 1420–1424 (2004)
Wu, YF, Zhang, Y, Xu, JX, Chen, M, Wu, LM, “One-Step Preparation of PS/TiO2 Nanocomposite Particles via Miniemulsion Polymerization.” J. Colloid Interf. Sci., 343 (1) 18–24 (2010)
Gonzalez, E, Bonnefond, A, Barrado, M, Barrasa, AMC, Asua, JM, Leiza, JR, “Photoactive Self-cleaning Polymer Coatings by TiO2 Nanoparticle Pickering Miniemulsion Polymerization.” Chem. Eng. J., 281 209–217 (2015)
Ma, Q, Izu, N, Masuda, Y, “Ceria Polymer Hybrid Nanoparticles and Assembled Films for Coating Applications.” ACS Appl. Nano Mater., 1 (5) 2112–2119 (2018)
İncel, A, Güner, T, Parlak, O, Demir, MM, “Null Extinction of Ceria@Silica Hybrid Particles: Transparent Polystyrene Composites.” ACS Appl. Mater. Interfaces, 7 (49) 27539–27546 (2015)
Aguirre, M, Paulis, M, Leiza, JR, “Particle Nucleation and Growth in Seeded Semibatch Miniemulsion Polymerization of Hybrid CeO2/Acrylic Latexes.” Polym., 55 (3) 752–761 (2014)
Zgheib, N, Putaux, J-L, Thill, A, D’Agosto, F, Lansalot, M, Bourgeat-Lami, E, “Stabilization of Miniemulsion Droplets by Cerium Oxide Nanoparticles: a Step Toward the Elaboration of Armored Composite Latexes.” Langmuir, 28 (14) 6163–6174 (2012)
Garnier, J, Warnant, J, Lacroix-Desmazes, P, Dufils, P-E, Vinas, J, van Herk, A, “Sulfonated Macro-RAFT Agents for the Surfactant-free Synthesis of Cerium Oxide-based Hybrid Latexes.” J. Colloid Interf. Sci., 407 273–281 (2013)
Bonnefond, A, Ibarra, M, Gonzalez, E, Barrado, M, Chuvilin, A, Maria Asua, J, Ramon Leiza, J, “Photocatalytic and Magnetic Titanium Dioxide/Polystyrene/Magnetite Composite Hybrid Polymer Particles.” J. Polym. Sci. Part A: Polym. Chem., 54 (20) 3350–3356 (2016)
Mori, Y, Kawaguchi, H, “Impact of Initiators in Preparing Magnetic Polymer Particles by Miniemulsion Polymerization.” Colloids Surf. B: Biointerfaces, 56 (1) 246–254 (2007)
Staudt, T, Machado, TO, Vogel, N, Weiss, CK, Araujo, PHH, Sayer, C, Landfester, K, “Magnetic Polymer/Nickel Hybrid Nanoparticles via Miniemulsion Polymerization.” Macromol. Chem. Phys., 214 (19) 2213–2222 (2013)
Kojima, Y, Usuki, A, Kawasumi, M, Okada, A, Fukushima, Y, Kurauchi, T, Kamigaito, O, “Mechanical Properties of Nylon 6-clay Hybrid.” J. Mater. Res., 8 (5) 1185–1189 (1993)
Eren, M, Can, HK, “Preparation of Zinc Methacrylate-methylmethacrylate-butyl Acrylate Emulsions and Their Application in Exterior Paints.” Prog. Org. Coat., 135 424–437 (2019)
Gholami, M, Shirzad-Siboni, M, Farzadkia, M, Yang, J-K, “Synthesis, Characterization, and Application of ZnO/TiO2 Nanocomposite for Photocatalysis of a Herbicide (Bentazon).” Desalin. Water Treat., 57 (29) 13632–13644 (2016)
Aguirre, M, Barrado, M, Iturrondobeitia, M, Okariz, A, Guraya, T, Paulis, M, Leiza, JR, “Film Forming Hybrid Acrylic/ZnO Latexes with Excellent UV Absorption Capacity.” Chem. Eng. J., 270 300–308 (2015)
Faucheu, J, Gauthier, C, Chazeau, L, Cavaille, JY, Mellon, V, Bourgeat-Lami, E, “Miniemulsion Polymerization for Synthesis of Structured Clay/Polymer Nanocomposites: Short Review and Recent Advances.” Polym., 51 (1) 6–17 (2010)
Romo-Uribe, A, Arcos-Casarrubias, JA, Hernandez-Vargas, ML, Reyes-Mayer, A, Aguilar-Franco, M, Bagdhachi, J, “Acrylate Hybrid Nanocomposite Coatings Based on SiO2 Nanoparticles by In-situ Batch Emulsion Polymerization.” Prog. Org. Coat., 97 288–300 (2016)
Youssef, A, El-Nagar, I, El-Torky, A and El-Hakim, AEFA, "Preparation and Characterization of PMMA Nanocomposites Based on ZnO-NPs for Antibacterial Packaging Applications." Proc. World Congress on New Technologies, (2019)
Sonawane, SH, Teo, BM, Brotchie, A, Grieser, F, Ashokkumar, M, “Sonochemical Synthesis of ZnO Encapsulated Functional Nanolatex and Its Anticorrosive Performance.” Ind. & Eng. Chem. Res., 49 (5) 2200–2205 (2010)
Lee, DI, “The Effects of Latex Coalescence and Interfacial Crosslinking on the Mechanical Properties of Latex Films.” Polym., 46 (4) 1287–1293 (2005)
Pinprayoon, O, Saiani, A, Groves, R, Saunders, BR, “Particulate Ionomer Films Prepared from Dispersions of Crosslinked Polymer Colloids: a Structure–Property Study.” J. Colloid Interf. Sci., 336 (1) 73–81 (2009)
Schellenberg, C, Tauer, K, Antonietti, M, “Film Formation of Polymeric Emulsions: Structure Set-up and the Pinhole Effect Characterized by Microscopic Techniques.” J. Dispersion Sci. Technol., 20 (1–2) 177–186 (1999)
Samakande, A, Sanderson, RD, Hartmann, PC, “Encapsulated Clay Particles in Polystyrene by RAFT Mediated Miniemulsion Polymerization.” J. Polym. Sci. Part A: Polym. Chem., 46 (21) 7114–7126 (2008)
Ruot, B, Plassais, A, Olive, F, Guillot, L, Bonafous, L, “TiO2-Containing Cement Pastes and Mortars: Measurements of the Photocatalytic Efficiency Using a Rhodamine B-based Colourimetric Test.” Solar Energy, 83 (10) 1794–1801 (2009)
Acknowledgments
Thanks to Scientific Research Projects Unit of Yozgat Bozok University (Grant no. 6602c-FEF/16-12) for the financial support. Thanks to Dr. Ramazan Coşkun for his valuable comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Delibaş, A. Synthesis of (Sty-co-BA-AA) latexes including ZnO and TiO2 nanoparticle by miniemulsion polymerization. J Coat Technol Res 18, 717–727 (2021). https://doi.org/10.1007/s11998-020-00436-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-020-00436-y