Abstract
In this study, the effect of crosslinking density of an acrylic-melamine clearcoat on water vapor transmission rate was evaluated. Four types of acrylic resins with different OH contents were used as the main polymeric backbone of the acrylic-melamine clearcoat. Also, three different acrylics to melamine–formaldehyde ratios were used in the coating formulations to obtain various crosslinking densities. It was observed that other film properties such as surface hydrophilicity and molecular homogeneity had a crucial impact on the clearcoat water vapor transmission rate together with the crosslinking density. Surprisingly, results showed that samples with acrylic resin with less OH content and lower melamine–formaldehyde crosslinker had the lowest water vapor transmission rate among other samples. These results were in agreement with the water contact angle and the data deduced from dynamic mechanical thermal analysis (DMTA) results of clearcoats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A car body consists of different coating layers to protect it from the environment. These organic coatings are usually composed of up to five layers, namely a thin phosphate conversion coating, an electrodeposited one, a primer surfacer, a metallic basecoat, and finally a clear top coat. Among these, the clearcoat is the top layer that is exposed to degrading environments, including UV irradiation from the sunlight, destructive mechanical factors like carwash and stone chipping, and chemicals such as acid rain. Preventing water vapor permeation through the film has been effective to control the fate of the coating.1 Therefore, this test is crucial to perform and it can be found in the paint testing plan of almost every car manufacturer.
The clearcoat, like most of the organic coatings, is composed of a suitable binder that is responsible for film formation and adhesion to the surface.
Different resins are used as binders based on their properties and compatibility with other coating components including additives and solvents. Acrylic-melamine coatings are one of the most used clearcoat systems.1 Acrylic resins have excellent resistance to light initiated deterioration, which makes them a suitable component for clearcoat composition.2 The use of melamine–formaldehyde will cause the clearcoat to be cured and make a 3D crosslinked network. In a typical acrylic-melamine coating, a 30 to 70 ratio between melamine to acrylic resin is used.3 In this combination, two types of reactions may cause network formation; one is the condensation reaction between polyols of the acrylic resin and melamine alkoxy groups leading to the formation of etheric functional groups in the structure and the other is the self-condensation reaction between the functional groups in melamine resin.4 Low crosslink density causes the clearcoat film to be more flexible, while by increasing the crosslink density, the hardness would increase.4
Permeation through organic coatings, at least to some extent, is inevitable even through coating films without imperfections or macro cracks on them.5 Small molecules pass through the coating using free volume.5 Thomas Graham’s solution-diffusion theory concerns the small molecules permeating through nonporous membranes.6 According to this theory, permeation is related to permeant diffusion on one side of the membrane and then its solution through the membrane, which leads to desorption on the other side.6 Some small molecules like water vapor have a plasticizing effect on the coating film, and their permeation leads to more coating permeability.5,6
Besides structural properties, chemical groups in the polymer chains have an essential influence on the coating permeation behavior. Functional groups that increase the polymer polarity like hydroxyl groups cause the coating to be more hydrophilic, which can lead to more water permeation.6
Many researchers have studied the crosslinking effect on the coating permeation. Yari et al.7 investigated the weathering performance of the acrylic-melamine clearcoat with nanosilica particles. They observed that nanosilica particles caused incomplete curing of the coating, which decreased the coating crosslinking density and storage modulus. However, further investigation showed that later reactions occurred due to atmospheric exposure, which led to an increase in the crosslink density. Córdoba et al.8 synthesized high solid acrylic-melamine latex with crosslinking capability using miniemulsion polymerization. Thermal and chemical analysis showed that the desired crosslinking reaction occurred when coating film was exposed to a temperature range of 130–210°C. It was observed that while the content of both reactive groups influenced crosslinking density, e.g., hydroxyl and melamine functional groups, curing rate, and film transitions were mainly affected by the –OH content of the acrylic polymer. Simulation performed by Shen et al.9 presented that the introduction of crosslinks slows down the chain dynamics and thus leads to a slight increase in the glass transition temperature.9 They observed that the storage modulus, the loss modulus, and the loss tangent showed a positive exponential relation with the apparent crosslink density. Shahriari et al.10 evaluated the effect of crosslink density on water and oxygen permeability of sol–gel-based hybrid UV cured coating on biaxially oriented polypropylene (BOPP) substrate. Their results showed that increasing crosslinking density caused a lower water vapor transmission rate. Besides, a dense organic network was able to compensate partially for the lack of an inorganic network for enhancing barrier properties. Rezaei et al.11 observed the effect of a sol–gel-based hybrid UV cured coating on water vapor transmission rate and oxygen permeability. They observed that increasing the inorganic content caused a more dense network, which decreased the water vapor transmission rate and oxygen permeability by making a tortuous path for permeant species.
According to the best of our knowledge, research focused on the water permeability of acrylic-melamine clearcoats is rare. In this study, we aim to investigate the water vapor permeation through acrylic-melamine clearcoat by focusing on the crosslinking density. To determine whether increasing the crosslinking density always means a better permeation resistance, an acrylic polyol with different OH contents and different acrylic polyol to melamine ratios were used.
Experimental
Materials
Four types of acrylic resins with different hydroxyl values (1.8, 2.7, 3.2, and 4.2%) were provided by Poly Resin Co, Iran. For abbreviation purposes, acrylic resins were shown, for example, as A1.8 to A4.2. SETAMINE US-138 and SETAMINE US-146 from Allnex were used as the melamine–formaldehyde co-curing resin (high reactive and low reactive fully butylated, respectively). Xylene and butyl acetate were used as solvents.
Preparation of acrylic-melamine clearcoat
The two factors that affect the crosslinking density change were monitored, namely the OH value variation and percentage of melamine–formaldehyde resin to acrylic.
The acrylic-melamine clearcoats were prepared with three different acrylic/melamine ratios. Firstly, a combination of 1 to 2.5 SETAMINE US-138 and 146 was added to the acrylic resin. Then solvents were added to maintain the viscosity of the mixture at 24 s by Zahn cup 4 at 23° ± 2°C. The mixture was then stirred to achieve a uniform solution. The exact composition of the materials is listed in Table 1. In this table, M indicates the melamine–formaldehyde content.
For example, sample M30A2.7 has 70 percent of acrylic resin with 2.7% OH content and 30 percent of melamine–formaldehyde resins mixture. Finally, each sample was applied on a cleaned glass plate by a doctor blade. After 15 min of flash off time, films were cured at 145°C for 20 min. The final dry film thickness was 45 ± 5 µm.
Characterization
The static contact angle measurements with water as the probe liquid were taken at room temperature using a CAM 200 optical contact angle meter (Data Physics Instruments). Ten measurements were taken, and the average values were reported. Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy (Thermo Fisher Scientific, USA) in the range of 4000–400 cm−1 with 2 cm−1 resolution was used to determine the functional groups of the clearcoats.
The solvent resistance of clearcoats using solvent rubs was carried out according to the ASTM D5402 standard, and the samples were observed for any changes in color or deformation. For measuring the hardness of the coatings, pendulum damping (ERICHSEN, Germany) was used in König mode according to ASTM D4366 standard. Also, microindentation hardness (Leica VHMT MOT) was applied to obtain Vickers Hardness with 245.2 mN force for 12 s. Pencil hardness test was used according to ASTM D3363 standard.
Dynamic mechanical thermal analysis (DMTA Tritec 2000, England) was performed to study the thermomechanical behavior of coatings. The temperature range between – 70 and 180°C with a 5°C/min heating rate and 1 Hz frequency was used in tension mode. Free films with 1 × 3 cm2 dimensions were used for this test.
The wet cup method was used to measure the water vapor transmission rate (WVTR) according to the ASTM D1653 standard. The test chamber in which cups were held was maintained at 30 ± 2°C and 50 ± 5% relative humidity. The gravimetric measurements were recorded for 42 days.
Results and discussion
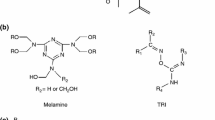
Automotive coatings must provide excellent resistance to chemical and mechanical damage in order to maintain a vehicle’s long-term appearance and satisfaction. Automotive consumers have become increasingly demanding of the performance and durability of their cars. The expectation of long-term ownership and the cost of today appear to be the primary influencing factors. Increasing attention is being given to the ability of coatings to retain a ‘new’ car appearance by resisting weathering, chemical, and physical damage, including scratch, chemical resistance, weathering, and water resistance. One fundamental property that has become a critical issue for today’s automotive original equipment manufacturers (OEM) is moisture resistance. Actually, water molecules can diffuse into the clearcoat and deliver themselves into the basecoat, at the interface of the basecoat, and clearcoat accumulation of water changes the appearance of coating and manifests as a defect. Over the last 50 years, the acrylic polyol-melamine formaldehyde resin system (Fig. 1) has been exclusively used for automotive topcoats. The moisture resistance of acrylic-melamine clearcoats has been a challenge during these years, and investigation of affecting parameters in the chemical structure of the clearcoat on the moisture resistance could provide valuable data, and thus this study has focused on it.
Scheme of crosslinking reaction between acrylic resin and melamine–formaldehyde12
Water contact angle
The water contact angle was measured to investigate the surface hydrophilicity or hydrophobicity and is listed in Table 2. According to the contact angle data, no significant difference between most of the samples was observed, and the water contact angle of samples was less than 90°, which shows the surface is hydrophilic.
Contact angles for acrylic resin with OH% of 1.8 (the least OH%) were somewhat bigger than that of other samples, which means that less polar groups are presented on the surface of these clearcoats. For other acrylic resins, the range was between 72° and 77°, which showed that samples are not too hydrophobic, and the difference in water permeability may not be due to the water repellency.
ATR-FTIR results
The ATR-FTIR spectra of the samples are presented in Fig. 2.
The peaks at around 750–760 cm−1 are assigned to the presence of the styrene monomer in the acrylic resins.13 The peaks at 815 and 1550 cm−1 are related to the stretching vibrations of the C–N bond in the melamine triazine ring.13 The characteristic peak of ethers was found at 1085 cm−1.14 Also, the peak before that at 1120 cm−1 was related to ether bond.15 The absorption peak at 1730 cm−1 related to the C=O carbonyl group was distinguishable in the spectra.16 Furthermore, the peaks presented at around 2850–2950 cm−1 are related to the C–H stretching.16 Comparing the A4.3 spectra with A1.8, it turns out that the peak related to the carbonyl group (1730 cm−1) has more intensity in A4.3 showing more acidic feature in the A4.3 acrylic resin.
Solvent resistance
After wiping out the solvent, samples were tested for aesthetic alteration and mechanical damage. None of the 12 samples showed any severe change in color shift or turbidity nor any deformations such as softening.
The hardness of the clearcoats
The pendulum dampening results were reported as time per second needed for the dissipation of the oscillation and are listed in Table 3.
According to Table 3, the A1.8 samples have the greatest hardness among other acrylic resin types. Also, it can be deduced from the data that adding more melamine–formaldehyde content to the samples caused a slight decrease in their hardness. A decrease in the pendulum dampening by increasing the melamine content could be due to the increase in dissipation ability of coating by increasing the inhomogeneity as will be shown in DMTA experiment [the width at half maximum of the peak (FWHM) increased by increasing the melamine content].
The force obtained in the microindentation test was 245.2 mN, and the applied time was 12 s. The test was repeated three times for each sample, and the average of the results is reported in Table 3 with the standard deviation. The microindentation results were obtained by dividing the force applied on the Vickers indenter by the area that force was applied. Unlike the hardness results obtained by the pendulum dampening test, here a clear deduction cannot be seen from the hardness data and differences were not significant, statistically. The film hardness results by pencil test were all in the range of 3H to 4H. This grade of pencil hardness is considered acceptable for automotive clearcoats.
Dynamic mechanical thermal properties (DMTA)
DMTA diagrams are represented in Fig. 3. They provide three main characteristics: the storage modulus (E’), the loss modulus (E’’), and the loss tangent (tan δ). Also, another crucial characteristic, which is determined from DMTA, is the glass transition temperature (Tg). The storage modulus data are essential because they are used to calculate the crosslink density of the clearcoats. Crosslink density can be calculated using equation (1) 17:
where the storage modulus E’ (dynes/cm2) is obtained in the rubber plateau, T is the absolute temperature in K corresponding to the storage modulus, and R is the universal gas constant (8.314 × 107 dynes/degrees K · mole in the cgs unit system).17\(\nu_{e}\) is reported in (\({\text{mol}}/{\text{cm}}^{3}\)) and its value varies from 5.5 × 10−4 \({\text{mol}}/{\text{cm}}^{3}\) for the coating with almost no crosslinking to 27 × 10−3 \({\text{mol/cm}}^{3}\) for highly crosslinked coatings.17 The crosslink densities calculated with storage modulus in rubber plateau are listed in Table 4.
According to Table 4, crosslink density increased with melamine–formaldehyde increasing from M23 to M43 series. This increase can effectively influence the water vapor transmission rate if the clearcoats have structural homogeneity combined with high crosslink density. Loss tangent graphs are presented in Fig. 3.
The height and width of the tan δ graph are representative of the structural homogeneity. Polymeric compounds without crosslinks and crystallinity that have narrow molecular weight distribution show narrow tan δ peaks. The presence of crosslinks or inhomogeneity in the polymer molecular structure broadens the tan δ peaks.17 The data obtained from loss tangent graphs are listed in Table 5.
According to Table 5, increasing the hydroxyl group content in acrylic resins from A1.8 to A4.3 and increasing the melamine–formaldehyde in the clearcoats composition caused an increase in both the glass transition temperature and the width at half maximum of the peak. These characteristics demonstrate structural inhomogeneity, which can affect water vapor transmission. It is necessary to mention that, about automotive clearcoats, the Tg in the range of 50°C is low and it can be attributed to incomplete curing due to the lack of use of an acid catalyst. Using some method to improve curing, such as using a catalyst or increasing of time and temperature of curing, can be helpful to increase glass transition temperature of automotive clearcoats.
Water vapor transmission rate
According to the wet cup method to acquire water vapor transmission rate (WVTR), a certain amount of water was poured in each cup, and free films of the clearcoats were placed on top of them. After placing the sealing gasket, the whole set was weighed. The cups were checked for weight change with different intervals for a total of 42 days. The results are presented in Fig. 4.
According to the ASTM D1653 standard, if the weight change of cups has linear behavior, the water vapor transmission would be the slope of those lines in \(\left( {\frac{g}{{m^{2} \times 24h}}} \right)\).18 The WVT values are listed in Table 6.
Table 6 shows that M23A1.8 and M30A1.8 have the least water vapor transmissions. According to Table 5, the M23A1.8 sample also has the least width at half peak height among other samples. Besides, the M23A1.8 and M30A1.8 samples had the highest water contact angle (Table 2), which indicates their lower hydrophilicity.
Conclusions
In this research, acrylic-melamine clearcoats were investigated. Water contact angle results showed that the samples with the least melamine–formaldehyde hardener content (M23 series) and hydroxyl content of 1.8 acrylic resin (lowest one) had the least hydrophilicity. They also had better coating hardness. According to the DMTA analysis, although the M43 series had the most crosslinking density, especially the M43A3.2 and M43A4.3 samples, they also had the least molecular homogeneity according to their loss tangent data. The M23A1.8 and M23A2.7 showed the most structural homogeneity in this test. The WVTR of the samples was directly related to their homogeneity. The M23A1.8 and M23A2.7 samples had the least WVTR compared to other samples, which demonstrate crosslinking density is an essential factor in the coating permeation behavior, but surface characteristics like hydrophilicity and structural homogeneity have significant impacts on the clearcoats permeation. In other words, the nature of the crosslinked networks determines the clearcoat behavior toward permeating water vapor molecules. It is possible that adding less polar crosslinking groups may benefit resistance against permeants.
References
Streitberger, HJ, Dössel, KF, Automotive Paints and Coatings: Second Edition. (Streitberger H-J, Dssel K-F, eds.). Wiley, Weinheim (2008). https://doi.org/10.1002/9783527622375
Ryntz RA, Yaneff P V. Coatings of Polymers And Plastics. 3rd ed. (Tracton A, ed.). Marcel Dekker, New York (2003). https://doi.org/10.1201/9780203912379
Lambourne, R, Strivens, TA, Paint and Surface Coatings: Theory and Practice. Woodhead Pub. Ltd, Sawston (1999). https://www.sciencedirect.com/book/9781855733480/paint-and-surface-coatings. Accessed August 22, 2018
Haseebuddin, S, Raju, KVSN, Yaseen, M, “Crosslink Density and Cure Window of Oligourethane Diol/Melamine High-Solids Coatings.” J. Coat. Technol., 70 (4) 35–42 (1998). https://doi.org/10.1007/BF02697814
Wicks, ZW, Jones, FN, Pappas, SP, Organic Coatings: Science and Technology. 3rd ed, Wiley, New York (2007). https://www.wiley.com/en-us/Organic+Coatings%3A+Science+and+Technology%2C+3rd+Edition-p-9780471698067. Accessed August 5, 2018
Sangaj, NS, Malshe, VC, “Permeability of Polymers in Protective Organic Coatings.” Prog. Org. Coat, 50 (1) 28–39 (2004). https://doi.org/10.1016/j.porgcoat.2003.09.015
Yari, H, Moradian, S, Tahmasebi, N, “The Weathering Performance of Acrylic Melamine Automotive Clearcoats Containing Hydrophobic Nanosilica.” J. Coat. Technol Res., 11 (3) 351–360 (2014). https://doi.org/10.1007/s11998-013-9541-z
Córdoba, CA, Ronco, LI, Barrios, CE, Gugliotta, LM, Minari, RJ, “High Solid Acrylic-Melamine Latexes with Tunable Crosslinking Capability.” Macromol. React. Eng., 13 (2) 1800063 (2019). https://doi.org/10.1002/mren.201800063
Shen, J, Lin, X, Liu, J, Li, X, “Effects of Cross-Link Density and Distribution on Static and Dynamic Properties of Chemically Cross-Linked Polymers.” Macromolecules., 52 (1) 121–134 (2019). https://doi.org/10.1021/acs.macromol.8b01389
Shahriari, L, Mohseni, M, Yahyaei, H, “The Effect of Cross-Linking Density on Water Vapor and Oxygen Permeability of Hybrid UV Cured Nano Coatings.” Prog. Org. Coat., 134 66–77 (2019). https://doi.org/10.1016/j.porgcoat.2019.04.068
Rezaei, M, Mohseni, M, Yahyaei, H, “A Study on Water and Oxygen Permeability of BOPP Coated with Hybrid UV Cured Nanocoatings.” Prog. Org. Coat., 99 72–79 (2016). https://doi.org/10.1016/j.porgcoat.2016.05.006
Córdoba, CA, Collins, SE, Passeggi, MCG, Vaillard, SE, Gugliotta, LM, Minari, RJ, “Crosslinkable Acrylic-Melamine Latex Produced by Miniemulsion Polymerization.” Prog. Org. Coat., 118 82–90 (2018). https://doi.org/10.1016/j.porgcoat.2018.01.013
Chang, W, Yu, C, et al., “A Critical Evaluation of Spectral Library Searching for the Application of Automotive Paint Database.” Forensic Sci., 2 (1) 47–57 (2003)
Lee, S, Nguyen, T, Byrd, E, Martin, J, “Quantitative Study of Water Transport During the Hydrolysis of Polymer Coatings Exposed to Water Vapor.” J. Mater. Res., 18 (09) 2268–2275 (2003). https://doi.org/10.1557/JMR.2003.0316
Sharmin, E, Imo, L, Ashraf, SM, Ahmad, S, “Acrylic-Melamine Modified DGEBA-Epoxy Coatings and Their Anticorrosive Behavior.” Prog. Org. Coat., 50 (1) 47–54 (2004). https://doi.org/10.1016/j.porgcoat.2003.10.003
Cakić, SM, Ristić, IS, Jašo, VM, “Investigation of the Curing Kinetics of Alkyd–Melamine–Epoxy Resin System.” Prog. Org. Coat., 73 415–424 (2012). https://doi.org/10.1016/j.porgcoat.2011.03.016
Koleske, JV (ed.) Paint and Coating Testing Manual, 15th ed., Gardner-Sward Handbook, ASTM International, West Conshohocken (2012). https://doi.org/10.1520/MNL17-2ND-EB
ASTM D1653-13, Standard Test Methods for Water Vapor Transmission of Organic Coating Films. https://www.astm.org/Standards/D1653.htm. Accessed August 7, 2018.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nasirzadeh, M., Yahyaei, H., Lashgari, S.M. et al. Attributing the crosslinking density to water vapor transmission rate of an acrylic-melamine automotive clearcoat. J Coat Technol Res 18, 239–246 (2021). https://doi.org/10.1007/s11998-020-00400-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-020-00400-w