Abstract
In the present work, the surface behavior of cotton fabrics coated with a series of bio-based polybenzoxazines is explored. Herein, we report the design and synthesis of bio-based benzoxazine monomers (C-x) using cardanol (C) and seven different amines (x = ba, ha, dda, oda, ddm, jef, and fa). The molecular structures of the benzoxazine monomers have been confirmed using FTIR and NMR analyses. The microstructure of the polybenzoxazine-coated cotton fabrics observed from FE-SEM reveals that formation of rough nanostructure is influenced by molecular structure of monomers. Further, surface analysis shows that poly(C-dda)-coated cotton fabric offers superior water contact angle (WCA = 155° ± 3) with low sliding angle (6°). Also, poly(C-dda)-coated cotton fabric delivers the lowest surface energy (14.1 mN/m) and high resistance against acidic and alkaline media. Subsequently, oil–water separation investigation shows that the poly(C-dda)-coated cotton fabric yields 99% of separation efficiency with flux value of 7200 L/m2h. Thus, the cardanol-based polybenzoxazine-coated cotton fabrics prepared in the present work can find application in the field of oil–water separation due to their superior water-repellent nature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton is a natural, biodegradable, soft, and low-cost cellulose fiber.1 The porous structure and water absorption nature of cotton make it an absorbent material for various medical and healthcare applications.2 Recently, cotton fabric was employed in immiscible oil–water separation after suitable surface modification.3,4,5,6 Superhydrophobic surfaces are developed through introducing nanostructure roughness and coating with low-surface-energy material.7,8 Polybenzoxazine (PBz), an advanced thermoset material, possesses low surface energy and offers superhydrophobicity over a wide pH range.9,10 Also, a low water sliding angle of 1° led to the self-cleaning property of PBz.11 Compared to fluoropolymers, PBz possesses several advantages in terms of easy synthesis, being versatile in monomer design, and low-cost.12,13 These admirable properties made PBz as a platform coating material for various textile fabrics.14,15
In 2015, Xin et al. demonstrated the oleophilicity of TiO2/PBz-coated cotton fabric and reported its efficiency of oil–water separation for the first time.16 Subsequently, in 2017, Advincula et al. studied and reported the anti-icing, anticorrosive, and superoleophilic behaviors of rubber-modified PBz/SiO2 nanocomposites.17 In 2018, Tan et al. reported the PBz/SiO2 nanocomposite-coated cotton fabric with a contact angle value of 156° and studied oil–water separation.18 Most of the reports describe only the superhydrophobic nature of PBz prepared from bisphenol-A (BPA) precursor. About 4.5 million tons of BPA is consumed every year for the preparation of conventional resins, namely epoxy, benzoxazine, polycarbonates, and polyacrylates. However, the genotoxicity and endocrine-disrupting activity of BPA cause serious health issues and consequently, BPA-based plastics are under scrutiny.19,20 Owing to this fact, BPA is increasingly replaced by similar non-toxic chemical precursors.21 In this regard, bio-based precursors receive great attention as a feedstock chemical to prepare industrially important polymers for various engineering applications.22,23
Cardanol-based benzoxazines have been studied significantly in recent years to replace traditional bisphenol-based benzoxazines.22,23,24 PBz derived from cardanol possessing long aliphatic chains has been proven already for its superior superhydrophobicity and lower surface energy.11,25 Very recently, cardanol–aniline-based PBz/SiO2-modified fabric with superhydrophobicity and superoleophilicity for oil/water separation was reported by Yao et al. in 2019.26 Though the coatings of nanoparticles like SiO2, TiO2, and ZnO along with PBz could impart microroughness on the surfaces in addition to lowering surface energy, their inclusion is still a tedious and a costly approach from the industrial perspective. The development of hierarchy rough nanostructure surfaces with low surface energy through coating skeletally modified benzoxazine has not yet been studied. Building molecular coating without any additional additives could result in the formation of nanostructures on the surfaces. Thus, to develop benzoxazine monomers from non-hazardous precursors and to distribute hierarchal rough nanostructure, cardanol-based benzoxazines are designed and prepared using different types of amines. In the present work, the benzoxazines prepared using cardanol and different amines (amine-containing fluorine atom, long aliphatic chains, aryl rings, polyethers) are coated over the cotton fabric and studied extensively for their surface property and oil–water separation behavior. The data resulted from various studies are discussed in detail and reported.
Experimental section
Materials
Cardanol was obtained from Satya cashew products, Nanganallur, Chennai, India. Butylamine (ba), heptylamine (ha), dodecylamine (dda), octadecylamine (oda), 4-fluoroaniline (fa), jeffamine (jef), and diaminodiphenylmethane (ddm) were obtained from Sigma-Aldrich, India. Paraformaldehyde, dioxane, ethyl acetate, tetrahydrofuran, and sodium hydroxide were obtained from Qualigens, India. Cotton fabric was procured from the textile industry in Erode, Tamilnadu, India.
Synthesis of cardanol-based benzoxazine monomers (C-x)
The benzoxazine monomers (C-x) from cardanol and different types of amines are prepared as per Scheme 1. For the preparation of monofunctional monomers, the long-chain aliphatic monoamines and fluorine-substituted aromatic monoamines are used along with cardanol. Similarly, cardanol-based bifunctional monomers are also obtained condensing with aliphatic long-chain diamine and aromatic diamine. In detail, to a solution of cardanol (5 g, 16.7 mmol) in 1,4-dioxane (8 mL), respective amines, namely butylamine [(ba) 1.2 g, 16.7 mmol] or heptylamine [(ha) 1.9 g, 16.7 mmol] or dodecylamine [(dda) 3.09 g, 16.7 m mol] or octadecylamine [(oda) 4.5 g, 16.7 mmol] or jeffamine [(jef) 1.9 g, 8.35 mmol] or diaminodiphenylmethane [(ddm) 1.66 g, 8.35 mmol] or fluoroaniline [(fa) 1.8 g, 16.7 mmol], were added along with the addition of an appropriate equivalent of paraformaldehyde (66.8 mmol) separately and allowed to warm at 60°C. Then, the temperature of the reaction was slowly raised to 110°C and stirred for 12 h. Meanwhile, the progress of the reaction was monitored by TLC. After the formation of the products, the reaction mixtures were cooled, quenched in water, and extracted using ethyl acetate. The presence of unreacted cardanol was removed by adding 1 M NaOH to the organic layer. Subsequently, the organic layer was dried over anhydrous sodium sulfate and evaporated under vacuum. The resultant monomers (C-ba, C-ha, C-dda, C-oda, C-jef, C-ddm, and C-fa) were named as per IUPAC nomenclature of benzoxazine chemistry. After ascertaining their structures through FTIR and NMR spectra, the prepared monomers were coated over cotton as described subsequently.
Preparation of benzoxazine-coated cotton fabrics
Initially, waxy material over the cotton fabric surface was removed through soaking in 2 M NaOH solution for 3 h. Later, the fabrics were washed with distilled water and dried at 60°C for 10 h. After drying, the cotton fabrics were subjected to surface modification through coating the prepared benzoxazine monomers. Exactly 1 g of each benzoxazine monomer was dissolved separately in 10 mL of THF. Two sets of cotton fabrics were immersed in the solution for 20 min and subsequently cured at two different temperatures (180 and 250°C) separately for 5 min. The pristine cotton without polybenzoxazine coating was also studied and compared.
Characterization
FTIR spectra measurements were taken in Agilent Cary 630 FTIR Spectrometer. NMR spectra were recorded in Bruker (400 MHz) using deuterated chloroform (CDCl3) solvent and tetramethylsilane (TMS) as an internal standard. The curing profiles of the C-x benzoxazine monomers were determined using NETZSCH STA 449F3 Jupiter-German from room temperature to 300°C. The morphology of the polybenzoxazine-coated fabrics was identified from a ZEISS Sigma Field Emission scanning electron microscope (FE-SEM). The water contact angle measurements of the coated fabric were taken on a DataPhysics instrument (OCA 15, Germany) using a water drop (V = 10 μL) gently placed on the surface of the sample. The XPS was measured using an Omicron nanotechnology instrument at a pressure below 10−10 Torr. The tensile properties of the fabrics were measured using a universal testing machine (Tensile Tester Z10 of Zwick/Roell, Germany), with a constant rate of extension as per ASTM standard D5035-11 (Reapproved 2015). In accordance with the standard BS 3424-16:1995, the air permeability of the pristine cotton and the treated fabrics was tested using air permeability tester of P.S.I Sales Pvt Ltd, New Delhi, India.
Oil–water separation test
The oil–water separation experiment was performed without the aid of pressure using cotton fabrics (diameter = 0.04 m, area = 0.00125 m2) and 50% v/v of the oil–water mixture. Different oil–water mixtures such as petrol/water, diesel/water, and engine oil/water were used. The oil–water separation time was recorded in the hour after fixing the poly(C-x)-coated fabrics at the interface between the separation and filtration flask. The separation efficiency (%) was calculated using equation (1). The cyclic test was conducted for 10 cycles using petrol/water mixture. After each cycle, the membrane was washed with ethanol and dried at 30° for 2 h and subjected to oil–water flux experiments. Equation (2) is adopted to calculate the flux value in (liter/area in m2 x time in h).
Results and discussion
Spectral analysis
The prepared cardanol benzoxazines (C-ba, C-ha, C-dda, C-oda, C-jef, C-ddm, and C-fa) were analyzed initially using FTIR to validate the functional groups. Figure 1 shows the FTIR spectra of the observed benzoxazine monomers. Typically, bands appearing at 2923 cm−1 and 2848 cm−1 correspond to the asymmetric and symmetric stretching vibrations of methylene group (–CH2–), which in turn confirms the presence of alkyl chains present in cardanol and amine (ba, ha, dda, and oda) precursors27,28 The bands appearing at 1242 and 1105 cm−1 were attributed to the respective asymmetric and symmetric stretching vibrations of C–O–C bond present in the monomer benzoxazines.29 Further, the appearance of a peak at 1507 cm−1 corresponds to tri-substituted benzene rings. Also, the appearance of a peak at 960 cm−1, confirms the formation of benzoxazine monomers.30
Further 1H- and 13C-NMR analyses were performed in order to confirm the chemical structures of benzoxazine monomers. Figures 2a–2g show the respective 1H-NMR spectra of C-ba, C-ha, C-dda, C-oda, C-jef, C-ddm, and C-fa benzoxazine monomers. The formation of oxazine rings was confirmed by the presence of two singlets of methylene protons corresponding to (–O–CH2–N–) and (–N–CH2–Ar). Concerning the nature of precursors, the positions of the peak show minor shifts.31,32,33 The arylamine-condensed oxazine ring gives signals in the upfield region, whereas that of an aliphatic amine-based oxazine ring gives signals in the downfield region.
In the case of cardanol- and aliphatic monoamine-based benzoxazines [C-ba, C-ha, C-dda, and C-oda], the two singlets from the methylene protons of oxazine rings show signals at δ 3.9 and δ 4.9 ppm (Figs. 2a–2d) in 1H-NMR spectra. The terminal methyl protons of cardanol, ba, ha, dda, and oda moieties appear at δ 0.9 ppm.30 The major signal that appeared between δ 1.0 and 2.0 ppm corresponds to aliphatic chain protons of cardanol, ba, ha, dda, and oda moieties. The multiplet appearing at δ 2.7 ppm corresponds to –N–CH2– protons of ba, ha, oda, and dda.34 The 13C-NMR spectra of C-ba, C-ha, C-dda, and C-oda showed two signals at δ 52 (–N–CH2–Ar) and δ 82 ppm (–N–CH2–O–) (Figs. 3a–3d), which correspond to the methylene carbons of benzoxazine rings. The quaternary carbon of the benzoxazine ring adjacent to oxygen atom appeared in the deshielding region at δ 152 ppm.
In the case of C-jef, the two singlets from the methylene protons of oxazine rings show signal at δ 3.5 and δ 4.0 ppm (Fig. 2e) in 1H-NMR spectrum. The terminal methyl protons of cardanol appeared at δ 0.9 ppm, whereas those of the methyl group of jeffamine appeared at δ 1.2 ppm. The major signals that appeared between δ 1.0 and 2.0 ppm correspond to aliphatic chain protons of both cardanols. The –N–CH2– protons showed a multiplet at δ 2.7 ppm. The 13C-NMR spectrum (Fig. 3e) of C-jef showed two signals at δ 52 (–N–CH2–Ar) and δ 82 ppm (–N–CH2–O–) corresponding to the methylene carbons of oxazine ring. The signal at δ 82 ppm (CH3–CH–N–) corresponds to the methyl-substituted secondary carbon. The quaternary carbon of the benzoxazine ring adjacent to oxygen atom appeared in the deshielding region at δ 152 ppm.
In the case of C-ddm and C-fa, the two singlets from the methylene protons of oxazine rings show signals at δ 4.5 and δ 5.5 ppm (Figs. 2f–3g) in 1H-NMR spectra. The terminal methyl protons of cardanol moiety appeared at δ 0.9 ppm. The signal at δ 3.9 ppm (Fig. 2f) corresponds to the signal of methylene protons of ddm (Ar–CH2–Ar). The major signals that appeared between δ 1.0 and 2.0 ppm correspond to aliphatic chain protons of cardanol moiety. The multiplet signals around δ 6.5–7.2 correspond to the aryl rings. The 13C-NMR spectra of C-dda and C-oda showed two signals at δ 50 (–N–CH2–Ar) and δ 80 ppm (–N–CH2–O–) (Figs. 3f–3g) corresponding to the methylene group of benzoxazine rings. The quaternary carbon of the benzoxazine ring adjacent to oxygen atom appeared in the deshielding region at δ 158 ppm. The methylene carbon bridging two aryl rings of ddm shows the signal at δ 40 ppm (Fig. 3f). The fluorine-substituted quaternary carbon of the aryl ring shows the signal in a high deshielding region at δ 162 ppm (Fig. 3g).
Curing behavior of benzoxazine monomers
The curing behaviors of the C-x benzoxazines were studied using DSC and observed thermograms are presented in Fig. 4a. The curing onset of the cardanol monomers prepared using aliphatic monoamines (ba, ha, dda, and oda) begins at 175°C and ends at 265°C. Among the aliphatic amines, C-ba benzoxazine prepared using butyl amine shows the lowest Tp at 228°C, whereas other benzoxazines, namely C-ha, C-dda, and C-oda, show Tp value at 237°C. On the other hand, the curing onset of the cardanol monomer C-fa prepared using aromatic monoamines starts at 195°C and ends at 270°C with Tp at 245°C. This increase in the value of Tp by about 15°C for C-fa when compared to those of aliphatic monoamines might be due to the electronic effect of aromatic rings.35 In addition, the curing behavior of diamine-based monomers, namely C-jef and C-ddm, was also studied. The C-jef monomer with long-chain aliphatic chain shows curing onset at 175°C and ends at 260°C with Tp at 230°C. On the other hand, the curing of C-ddm possessing aromatic diamines starts at 235°C and ends at 293°C with Tp at 272°C. Similar to cardanol monomers prepared using monoamines, the C-ddm monomers also show an increased Tp value of about 42°C when compared to that Tp value of C-jef. This might be due to the steric effect experienced by the C-ddm monomers similar to the case of linear aliphatic diamine-based benzoxazines36,37 in addition to the electronic effect of aromatic rings. Moreover, the aliphatic amines benzoxazine monomer liberates comparatively lower enthalpy than those of aromatic-based monomers.
Though the benzoxazine monomers show different curing temperatures with respect to their structure starting from 228 to 272°C, it is anticipated that after coating over the cotton fabric, the curing of benzoxazines could occur at relatively lower temperatures. Since cotton fabric surfaces are rich with polar functional groups, namely hydroxyl, carboxylic, etc., to further ascertain the assumption, the C-ddm monomer (Tp = 272°C) was coated over the cotton fabric and subjected to DSC analysis. The obtained DSC thermogram of C-ddm/cotton is presented in Fig. 4b in comparison with C-ddm. It is interesting to observe that the curing of C-ddm over the cotton fabric surface tends to occur at a lower temperature (Tp = 239°C). This shows that the curing temperature can be lowered in the presence of cotton surfaces. Thus, the benzoxazine-coated cotton fabrics were subjected to two different temperatures, namely 180°C and 250°C, and studied further.
Spectral analysis of the coated fabrics
Figures 5a and 5b represent the FTIR spectra of the cardanol-based polybenzoxazines coated on the cotton fabrics subjected at two different temperatures in comparison with pristine fabric. In the FTIR spectra, the peaks at 1712 and 1246 cm−1 of pristine fabric correspond to the carboxylic acid functional group and C–O–C linkage, respectively. Since the fabric was treated with sodium hydroxide, the primary hydroxyl groups might have been oxidized to the carboxylic group.38 After coating with benzoxazines, the fabrics were subjected at different temperatures, namely 180°C (Fig. 5a) and 250°C (Fig. 5b). In Figs. 5a and 5b, the benzoxazine-coated cotton fabrics show no distinct peaks for benzoxazines, which suggest the ring-opening reactions of oxazine groups.38 The peaks observed at 2917 cm−1 and 2844 cm−1 correspond to the asymmetric and symmetric stretching vibrations of a methylene group (–CH2–), respectively, of long alkyl side chains and oxazine rings.38 Thus, the FTIR spectra cured at 180°C (Fig. 5a) and 250°C (Fig. 5b) show similar vibration bands, which confirm the curing of benzoxazines. This result infers that subjecting cardanol benzoxazine monomer-coated cotton fabric at 180°C is sufficient to form polybenzoxazine coating over cotton surfaces.
Surface properties
The values of water contact angle (WCA) of the cardanol polybenzoxazine-coated cotton fabrics are presented in Fig. 6. The WCA of poly(C-ba)-, poly(C-ha)-, poly(C-dda)-, poly(C-oda)-, poly(C-jef)-, poly(C-ddm)-, and poly(C-fa)-coated cotton fabrics subjected at 180°C is 140°, 145°, 155°, 153°, 151°, 132°, and 153°, respectively. On the other hand, the poly(C-ba)-, poly(C-ha)-, poly(C-dda)-, poly(C-oda)-, poly(C-jef)-, poly(C-ddm)-, and poly(C-fa)-coated cotton fabrics subjected at 250°C show WCAs as 129°, 140°, 149°, 144°, 132°, 138°, and 149°, respectively. The polybenzoxazine-coated fabrics subjected at 250°C show lower contact angle values and higher standard deviation than those of fabrics cured at 180°C. This result infers that the fabrics cured at low temperatures have a uniform layer of polybenzoxazines that delivers hydrophobicity. The lower and inconsistent contact angle values for fabrics subjected at 250°C are due to the formation of polar functional groups (–OH, –COOH, etc.) on the cotton fabric surfaces when subjected to high temperature.39 These results suggest that cotton fabrics coated with cardanol-based benzoxazines cured at low temperature (180°C) itself are sufficient to achieve better hydrophobic surfaces. Hence, the fabrics subjected at 180°C are subsequently used for further studies. Moreover, the poly(C-dda)-coated cotton fabric exhibits similar superhydrophobicity as equivalent to that of cotton fabrics coated with PDMS.3
It is well known that the aliphatic chain could favor superhydrophobic behavior due to the nonpolar nature.38 Hence, cardanol possessing long aliphatic chain in meta position and aliphatic long chain monoamines having different carbon chain lengths (4, 7, 12, and 18) are chosen to prepare benzoxazine. Generally, cardanol is a mixture with a saturated long chain and single unsaturated long chain at C8, two unsaturated carbons at C8 and C11, and three unsaturated carbons at C8, C11, and C14.40,41 However, in the present case, we deliberately considered that the cardanol has a complete saturated carbon chain. Among the polybenzoxazine (C-ba, C-ha, C-dda, C-oda)-coated fabrics, the WCA value of the fabric coated with poly(C-dda) was observed to be higher (155°C) (Fig. 6). This phenomenon shows that the presence of cardanol (15 carbons)- and dodecylamine (12 carbons)-based benzoxazines with (15Cs-12Cs) carbon chain length provides higher values of contact angle than those of other aliphatic amine derived polybenzoxazines. Replacing dda with oda possessing a comparatively long carbon chain with 18Cs results in lower contact angle value (Fig. 7). This might be due to the formation of steric hindrance, which could alter the molecular orientation and thereby reduce the WCA. It is important to notice that the aliphatic amines and cardanol-derived monofunctional benzoxazines are found to be unsymmetric with respect to their long carbon chains.
Further, to study the WCA behavior of polybenzoxazines with symmetric carbon chain, two bifunctional benzoxazines (C-jef and C-ddm) were prepared using jeffamine (jef) and diaminodiphenylmethane (ddm) along with cardanol. Interestingly, the WCA value of poly(C-jef) is observed to be higher (153° @ 180°C) than poly(C-ddm) (132° @ 180°C)-coated cotton fabrics (Fig. 6). In the case of C-jef-based benzoxazine, the presence of polyether linkage with more methylene carbons between the two amine terminals is strong enough to prevent the steric effect rendered by the long aliphatic chain (C15) of cardanol (Fig. 7). However, the poly(C-ddm) with two phenyl rings connected to a methylene carbon could experience more steric effect and thereby the aliphatic chains of cardanol tend to orient away from one another and lead to reduction in the WCA (Fig. 7). Finally, the WCA value of fluorine atom-containing cardanol-based polybenzoxazine-coated cotton fabric was observed to be 153°. These results suggest that the value of contact angle of poly(C-dda) is greater than those of unsymmetric long-chain monoamine-cardanol-based benzoxazines, symmetric difunctional cardanol-based benzoxazines, and fluorine-containing cardanol-based benzoxazines.
The WCA values confirm that the alkyl chains of the benzoxazines play a significant role in increasing the superhydrophobicity of the coated cotton fabric. In general, the low-surface-free-energy materials like Teflon usually offer higher hydrophobicity. Hence, it is highly desirable to evaluate the surface free energy (SFE) of the cardanol-based polybenzoxazine-coated cotton fabrics. The surface free energy of the fabrics is calculated using the equation of state and presented in Fig. 8. The surface free energies observed using water for the fabrics coated with poly(C-ba), poly(C-ha), poly(C-dda), poly(C-oda), poly(C-jef), poly(C-ddm), and poly(C-fa) are 12.7, 17.0, 14.1, 15.3, 17.0, 17.8, and 17.7 mN/m, respectively. However, the surface free energy of pristine fabric measured is 26.2 mN/m using water. The observed results are in accordance with the WCA results. Thus, the poly(C-dda)-coated fabric offers higher WCA and lower SFE, which is desirable for coating materials.
In order to validate the suitability of the developed fabric toward oil–water separation application, the SFE behavior was measured using diesel. The values of SFE measured using diesel for the fabrics coated with poly(C-ba), poly(C-ha), poly(C-dda), poly(C-oda), poly(C-jef), poly(C-ddm), and poly(C-fa) are 24.1, 25.3, 27.7, 27.7, 26.9, 27.5, and 27.7 mN/m, respectively. Besides, the surface free energy of pristine fabric with diesel was found to be 10.7 mN/m. These results suggest that the SFE of poly(C-dda)-coated fabric was significantly higher with diesel than those of other coated fabrics. Generally, oils have small surface free tension ranging from 20 to 30 mN/m2. In particular, the surface tension of diesel oil is 24.14 mN/m2, which is almost equivalent to that of cotton fabric surfaces free energy coated with cardanol-based polybenzoxazines prepared in the present work. Hence, it is suggested that the polybenzoxazine-coated fabrics developed in the present work can be used as a cost-competitive sustainable filtration material for oil–water separation application similar to that of material reported earlier.42
Morphology of the polybenzoxazine-coated cotton fabrics
The morphology of pristine cotton and cardanol-based benzoxazine-coated cotton fabrics was investigated using field emission scanning electron microscope (FE-SEM) and is presented in Fig. 9. The pristine cotton fabric shows a relatively smooth and grooved surface along with the fiber. However, after coating with cardanol-based polybenzoxazines, the surfaces become rough and have asperity textured morphology. The unevenness in the surface was closely observed in the case of fabric coated with poly(C-dda) when compared to that of other aliphatic amine cardanol benzoxazines. Interestingly, the cotton fabric coated with bifunctional polybenzoxazines with symmetric structure shows nanopillared rough surface morphology more randomly in the case of poly(C-jef). On the other hand, the poly(C-ddm)-coated fabrics deliver epicuticular morphology. Based on the experimental results and theories, it is strongly believed that the formation hierarchically rough structured surface plays an important role in enhancing the water-repelling behavior.43
Inspired from the lotus leaf effect, superhydrophobic cotton surface has been developed using cardanol-based benzoxazines as biomimetic materials. The contact angle values observed in the present work are promising because of the formation of rough surfaced fabrics, besides lowering of SFE. The long aliphatic chain moieties of benzoxazines led to the protuberances and air beneath textured surfaces, which in turn stack the water droplet to be sited on the top without contacting fabric surface.43 Thus, the reduced interfacial interaction creates the Cassie–Baxter state of interaction between the fabric and water, which contributes to enhanced water contact angles. Finally, the strong adhesive forces of polybenzoxazine with cotton and subsequent non-adhesiveness in the presence of water rendered by the long alkyl chains delivered superhydrophobic surfaces similar to those modified by silanes with different alkyl (methyl, propyl, octyl, dodecyl, octadecyl) chains.44 The cardanol-based long-chain polybenzoxazines coating developed in the present work is cost-effective when compared with those of long-chain silane derivatives.44
XPS analysis
The cotton surface coated with poly(C-dda) contributes to an enhanced superhydrophobicity and lower surface free energy. Hence, in order to study the interaction of poly(C-dda) with cotton fabric, XPS analysis has been carried out. The resulted XPS spectra for pristine cotton and poly(C-dda)-coated cotton are presented in Fig. 10a.
During the general scan, the appearance of peaks at 283 and 531 eV for pristine fabric shows the existence of carbon and oxygen, respectively.34 However, in the case of poly(C-dda)-coated cotton, the appearance of an extra signal at 399 eV corresponding to the nitrogen supports the presence of polybenzoxazine coating.34 Accordingly, the elemental profiles are found to be varied significantly. The pristine cotton possesses 26.9% of oxygen and 73.1% of carbon, whereas poly(C-dda)-coated cotton exhibits 17.6% of oxygen, 80.7% of carbon, and 1.63% of nitrogen. Besides, the deconvoluted XPS curves presented in Fig. 10b represent the change in the binding energy of carbon atom of pristine fabric after being coated with poly(C-dda). The pristine atom delivers peaks at 286.0, 284.1, and 282.4 eV, which are all attributed to the presence of carbon at three different bonding environments, namely C=O, C–O, and C–C. However, on the other hand, the poly(C-dda)-coated fabric shows a major peak at 282.3 eV and a minor peak at 283.3 eV, which correspond to the binding energy curves of sp3 and sp2 carbons, respectively. Further, the absence of binding energy signals of polar functional groups such as C=O and C–O infers that the polar groups present in the fabric might be crosslinking with the coated polybenzoxazine during the thermal treatment. Also, the O1s peak binding energy signals presented in Fig. 10c show the significant difference between pristine fabric and poly(C-dda)-coated fabrics. In addition, the appearance of N1s binding energy signal presented in Fig. 10d indicates that the polybenzoxazine has been coated on the cotton fabric. Thus, the coating of poly(C-dda) on cotton fabric favors more hydrophobic and low surface free energy nature.
Oil–water separation
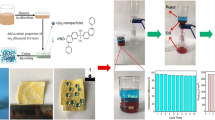
Further, the oil–water separation efficiency was analyzed for all the cardanol-based polybenzoxazine-coated cotton fabrics (Fig. 11a). Initially, the oil–water in equal volume was prepared using commercially available engine oil (20W40 grade with a density of 0.85 kg/L,). Due to the high density, water existed as a bottom layer (Fig. S1a). To bring up the water layer above, high-density dichloromethane (d = 1.33 g/cm3; 50 mL) was added, which subsequently moved down as a lower layer along with oil. Also, an equal volume of water was once again added to maintain the volume ratio (Fig. S1b). Once the oil present in DCM layer met the cotton fabrics coated with cardanol-based polybenzoxazines, the penetration of oil was observed quickly and it was collected in the filtration flask under gravity (Fig. S1c). Even after the complete removal of the oil layer, the water layer stayed over the fabrics, which indicated the low surface free energy and superhydrophobic nature of coated cotton fabric (Fig. S1d). Among the cardanol-based polybenzoxazine-coated cotton fabric, the poly(C-dda) was found to be the most effective (98%) in separating oil–water mixture (Fig. 11a). This may be explained due to its superior WCA (155°) and the lower SFE (14.1 mN/m) against water. As discussed earlier, the SFE became 27.7 mN/m after coating with poly(C-dda) (Fig. 8). However, before coating, the SFE was observed to be 10.7 mN/m. These significant changes in SFE suggest that the surface becomes superhydrophobic and thereby retards water penetration. Further, the oil–water separation was carried out using two different mixtures, namely water/petrol (density = 0.77 kg/L) and water/diesel (density = 0.85 kg/L). Comparatively, petrol–water mixture separation was found to be more efficient than those of diesel–water and oil–water separations. Once again, the poly(C-dda)/cotton fabric showed higher separation efficiency (99%) from both types of the mixture (Fig. 11a, Video. S1) than other polybenzoxazine-coated fabrics. This infers that the poly(C-dda)/cotton could suit a wide range of industrial oils. Further, the flux behavior was tested for the poly(C-dda)-coated cotton fabric to ascertain the commercial viability of benzoxazine-coated cotton fabrics. Hence, the oil–water experiment was repeated for 10 cycles using a petrol–water mixture to ascertain the cycling performance. After each cycle, the fabric was washed with ethanol and dried for subsequent usage.
Figure 11b illustrates the flux values of poly(C-dda)-coated cotton using a petrol–water mixture for different cycles. The flux value of the poly(C-dda) observed for the first cycle is 7200 L/m2h, whereas after 10 cycles the flux becomes 6300 L/m2h showing about 98% effectiveness. However, the flux value for poly(C-dda)-coated cotton fabric placed on the sintered disk shows only 430 L/m2h and 95% efficiency (Fig. S2). Thus, it is observed that the highly desired separation was performed in absence of sintered disk (Fig. S3). As stated earlier, the superhydrophobic and superoleophilic properties of poly(C-dda)/cotton fabric enabled petrol to permeate quickly and collect into filtration flask while keeping water on the coated fabric. The observed results are consistent with that of superhydrophobic membrane prepared using polycaprolactone and beeswax, which delivers 98% separation.45 Beeswax contains long-chain methylene [–(CH2)–] carbons, which is responsible for the superhydrophobic nature. Similarly, in the present work, long chain associated with both alkyl amine and cardanol renders superior superhydrophobic behavior with low surface energy, which forms hierarchal morphology on the outer surfaces of the fabric. In addition, the durability of poly(C-dda)-coated fabric was analyzed in a wide pH range. Since the real-time application of oil/water separation includes either acidic or alkaline water, it is desirable to test the contact angle behavior in a wide pH range from 2 to 12 (Fig. 12). From Fig. 12, the poly(C-dda)-coated cotton fabric retains stable superhydrophobicity from pH 2 to 12. Even at extreme acidic and alkali conditions such as pH = 2 and 12, the observed WCAs are 149 ± 2° and 148 ± 2°, respectively. Further, the water sliding angle (WSA) of poly(C-dda) was also observed to be 6°, which was less than bisphenol-A-based polybenzoxazine-coated cotton fabric (9°) reported earlier.34 Inset of Fig. 12 shows superior hydrophobic behavior against a wide variety of liquids including saltwater, acid, base, milk, tea, coffee, and natural extracts.
Contact angle behavior of poly(C-dda)-coated fabric at different pH values and inset showing hydrophobic nature against variety of substrates [(1) NaCl solution, (2) 1 M HCl, (3) 1 M NaOH, (4) lemon juice, (5) honey, (6) turmeric extract, (7) chilly extract, (8) milk, (9) tea, and (10) coffee extracts]
The self-cleaning performance of pristine cotton and poly(C-dda)/cotton fabrics was studied using natural turmeric powder. The pristine fabric is quickly contaminated while drizzling water drops over the fabric surface containing turmeric powder (Fig. 13). In contrast, the poly(C-dda)-coated fabric surfaces sprinkled with turmeric powder, allow the powder to roll down while drizzling the water drops and left a clean surface (Fig. 13, Video S2). Thus, the coating of poly(C-dda)-coated fabric shows the self-cleaning surface in addition to the durable superhydrophobic nature. Further, the intrinsic properties of the cotton fabric such as tensile strength and air permeability were analyzed. The tensile strength of pristine cotton is 13.3 MPa, whereas the poly(C-dda)/cotton shows 24.5 MPa. The enhancement in the tensile strength after coating with poly(C-dda) is due to improvement in the stiffness. Further, the air permeability of the pristine fabric was 96 mm/s, whereas that of the poly(C-dda)-coated fabric was 64 mm/s which in turn confirms the coating. On the whole, poly(C-dda)-coated cotton fabric retains strong resistance against a wide range of pH and WSA, which endows the utility for oil/water separation.
Conclusion
The fabrication of superhydrophobic/superoleophilic cotton fabric was achieved using cardanol-based benzoxazine monomers. To develop nanostructure roughness over the cotton surface, the cardanol-based benzoxazines were prepared along with different types of amines (aliphatic monoamines, diamine, aromatic monoamine, and diamine). The prepared cardanol-based benzoxazines displayed a variety of morphologies when coated over cotton fabrics. Among those coated on cotton fabrics, the cardanol-dodecylamine (C-dda)-based benzoxazines possess the highest value of water contact angle (155°) followed by cardanol-jeffamine and cardanol-4-fluoroaniline moieties delivering contact angle values as 153°. The morphology observed in the SEM images also indicates that the closely distributed roughness was achieved with poly(C-dda)-coated cotton fabrics compared to those of other benzoxazine-coated fabrics. Finally, among the benzoxazine-coated cotton fabrics, the poly(C-dda) delivers better oil–water separation efficiency (99%) with greater cyclic repeatability. Thus, the bio-based cardanol-derived polybenzoxazine prepared in the present study can be used as an effective hydrophobic/oleophilic material for oil–water separation applications.
Supporting information
Experiment setup describing oil–water separation is provided in Supporting Information.
References
Sasaki, K, Tenjimbayashi, M, Manabe, K, Shiratori, S, “Asymmetric Superhydrophobic/Superhydrophilic Cotton Fabrics Designed by Spraying Polymer and Nanoparticles.” ACS Appl. Mater. Interfaces, 8 651–659 (2016)
Lahiri, SK, Zhang, P, Zhang, C, Liu, L, “Robust Fluorine-Free and Self-Healing Superhydrophobic Coatings by H3BO3 Incorporation with SiO2-Alkyl-Silane@PDMS on Cotton Fabric.” ACS Appl. Mater. Interfaces, 11 10262–10275 (2019)
Ge, M, Cao, C, Liang, F, Liu, R, Zhang, Y, Zhang, W, Zhu, T, Yi, B, Tang, Y, Lai, Y, “A “PDMS-in-Water” Emulsion Enables Mechanochemically Robust Superhydrophobic Surfaces with Self-healing Nature.” Nanoscale Horiz., 5 65–73 (2020)
Dong, X, Gao, S, Huang, J, Li, S, Zhu, T, Cheng, Y, Zhao, Y, Chen, Z, Lai, Y, “A Self-roughened and Biodegradable Superhydrophobic Coating with UV Shielding, Solar-induced Self-healing and Versatile Oil-Water Separation Ability.” J. Mater. Chem. A., 7 2122–2128 (2019)
Gao, S, Dong, X, Huang, J, Li, S, Li, Y, Chen, Z, Lai, Y, “Rational Construction of Highly Transparent Superhydrophobic Coatings Based on a Non-particle, Fluorine-Free and Water-Rich System for Versatile Oil-Water Separation.” Chem. Eng. J., 333 621–629 (2018)
Bhushan, B, “Bioinspired Oil-Water Separation Approaches for Oil Spill Clean-up and Water Purification.” Philos. Trans. R. Soc. A Math. Phys. Eng. Sci., 377 20190120 (2019)
Xue, Z, Wang, S, Lin, L, Chen, L, Liu, M, Feng, L, Jiang, L, “A Novel Superhydrophilic and Underwater Superoleophobic Hydrogel-Coated Mesh for Oil/Water Separation.” Adv. Mater., 23 4270–4273 (2011)
Xue, Z, Cao, Y, Liu, N, Feng, L, Jiang, L, “Special Wettable Materials for Oil/Water Separation.” J. Mater. Chem. A., 2 2445–2460 (2014)
Wang, CF, Wang, YT, Tung, PH, Kuo, SW, Lin, CH, Sheen, YC, Chang, FC, “Stable Superhydrophobic Polybenzoxazine Surfaces over a Wide pH Range.” Langmuir, 22 8289–8292 (2006)
Rajamanikam, R, Pichaimani, P, Kumar, M, Muthukaruppan, A, “Optical and Thermomechanical Behavior of Benzoxazine Functionalized ZnO Reinforced Polybenzoxazine Nanocomposites.” Polym. Compos., 3 1881–1889 (2017)
Zhang, W, Lu, X, Xin, Z, Zhou, C, “Development of a Superhydrophobic Polybenzoxazine Surface with Self-cleaning and Reversible Water Adhesion Properties.” RSC Adv., 6 106054–106063 (2016)
Wang, CF, Su, YC, Kuo, SW, Huang, CF, Sheen, YC, Chang, FC, “Low-Surface-Free-Energy Materials Based on Polybenzoxazines.” Angew. Chem. Int. Ed., 45 2248–2251 (2006)
Revathi, R, Prabunathan, P, Kumar, M, Alagar, M, “Studies on Graphene Oxide–Reinforced Polybenzoxazine Nanocomposites.” High Perform. Polym., 28 425–435 (2016)
Gogoi, N, Rastogi, D, Jassal, M, Agrawal, AK, “Low-Surface-Energy Materials Based on Polybenzoxazines for Surface Modification of Textiles.” J. Text Inst., 105 1212–1220 (2014)
Zhang, T, Yan, H, Fang, Z, Yuping, E, Wu, T, Chen, F, “Superhydrophobic and Conductive Properties of Carbon Nanotubes/Polybenzoxazine Nanocomposites Coated Ramie Fabric Prepared by Solution-Immersion Process.” Appl. Surf. Sci., 309 218–224 (2014)
Zhang, W, Lu, X, Xin, Z, Zhou, C, “A Self-cleaning Polybenzoxazine/TiO2 Surface with Superhydrophobicity and Superoleophilicity for Oil/Water Separation.” Nanoscale, 7 (46) 19476–19483 (2015)
Caldona, EB, De Leon, ACC, Thomas, PG, Naylor, DF, Pajarito, BB, Advincula, RC, “Superhydrophobic Rubber-Modified Polybenzoxazine/SiO2 Nanocomposite Coating with Anticorrosion, Anti-Ice, and Superoleophilicity Properties.” Ind. Eng. Chem. Res., 56 1485–1497 (2017)
Li, Y, Yu, Q, Yin, X, Xu, J, Cai, Y, Han, L, Huang, H, Zhou, Y, Tan, Y, Wang, L, Wang, H, “Fabrication of Superhydrophobic and Superoleophilic Polybenzoxazine-Based Cotton Fabric for Oil–Water Separation.” Cellulose, 25 6691–6704 (2018)
Lehmler, HJ, Liu, B, Gadogbe, M, Bao, W, “Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013–2014.” ACS Omega, 3 6523–6532 (2018)
Okada, H, Tokunaga, T, Liu, X, Takayanagi, S, Matsushima, A, Shimohigashi, Y, “Direct Evidence Revealing Structural Elements Essential for the High Binding Ability of Bisphenol A to Human Estrogen-Related Receptor-γ.” Environ. Health Perspect., 116 32–38 (2008)
Manoj, M, Kumaravel, A, Mangalam, R, Prabunathan, P, Hariharan, A, Alagar, M, “Exploration of High Corrosion Resistance Property of Less Hazardous Pyrazolidine-Based Benzoxazines in Comparison with Bisphenol-F Derivatives.” J. Coat. Technol. Res., (2020). https://doi.org/10.1007/s11998-019-00312-4
Hariharan, A, Prabunathan, P, Kumaravel, A, Manoj, M, Alagar, M, “Bio-Based Polybenzoxazine Composites for Oil-Water Separation, Sound Absorption and Corrosion Resistance Applications.” Polym. Test., 86 106443 (2020)
Hariharan, A, Prabunathan, P, Subramanian, SS, Kumaravel, M, Alagar, M, “Blends of Chalcone Benzoxazine and Bio-benzoxazines Coated Cotton Fabrics for Oil-Water Separation and Bio-silica Reinforced Nanocomposites for Low-k Applications.” J. Polym. Environ., 28 598–613 (2020)
Prabunathan, P, Vasanthakumar, A, Manoj, M, Hariharan, A, Alagar, M, “Polypyrrole Inter-layered Low Temperature Curing Benzoxazine Matrices with Enhanced Thermal and Dielectric Properties.” J. Polym. Res., 27 1–14 (2020)
Prabunathan, P, Thennarasu, P, Song, JK, Alagar, M, “Achieving Low Dielectric, Surface Free Energy and UV Shielding Green Nanocomposites via Reinforcing Bio-silica Aerogel with Polybenzoxazine.” New J. Chem., 41 5313–5321 (2017)
Yao, H, Lu, X, Xin, Z, Zhang, H, Li, X, “A Durable Bio-based Polybenzoxazine/SiO2 Modified Fabric with Superhydrophobicity and Superoleophilicity for Oil/Water Separation.” Sep. Purif. Technol., 229 115792 (2019)
Rao, BS, Palanisamy, A, “Monofunctional Benzoxazine from Cardanol for Bio-composite Applications.” React. Funct. Polym., 71 148–154 (2011)
Lu, R, Gan, W, Wu, BH, Zhang, Z, Guo, Y, Wang, HF, “C-H Stretching Vibrations of Methyl, Methylene and Methine Groups at the Vapor/Alcohol (n = 1–8) Interfaces.” J. Phys. Chem. B, 109 14118–14129 (2005)
Rao, BS, Palanisamy, A, “A New Thermo Set System Based on Cardanol Benzoxazine and Hydroxy Benzoxazoline with Lower Cure Temperature.” Prog. Org. Coat., 74 427–434 (2012)
Parveen, AS, Thirukumaran, P, Sarojadevi, M, “Low Dielectric Materials from Fluorinated Polybenzoxazines.” Polym. Adv. Technol., 25 1538–1545 (2014)
Bonnaud, L, Chollet, B, Dumas, L, Peru, AA, Flourat, AL, Allais, F, Dubois, P, “High-Performance Bio-Based Benzoxazines from Enzymatic Synthesis of Diphenols.” Macromol. Chem. Phys., 220 1800312 (2019)
Zhang, T, Wang, J, Feng, T, Wang, H, Ramdani, N, Derradji, M, Xu, X, Liu, W, Tang, T, “A Novel High Performance Oxazine Derivative: Design of Tetrafunctional Monomer, Step-wise Ring-opening Polymerization, Improved Thermal Property and Broadened Processing Window.” RSC Adv., 5 33623–33631 (2015)
Lin, RC, Kuo, SW, “Well-defined Benzoxazine/Triphenylamine-Based Hyperbranched Polymers with Controlled Degree of Branching.” RSC Adv., 8 13592–13611 (2018)
Manickam, M, Pichaimani, P, Arumugam, H, Muthukaruppan, A, “Synthesis of Nontoxic Pyrazolidine-Based Benzoxazine-Coated Cotton Fabric for Oil-Water Separation.” Ind. Eng, Chem. Res., 58 21419–21430 (2020)
Shukla, S, Lochab, B, “Role of Higher Aromatic Content in Modulating Properties of Cardanol Based Benzoxazines.” Polymer (Guildf), 99 684–694 (2016)
Rao, BS, Surendra, P, “Synthesis and Characterization of Difunctional Benzoxazines from Aromatic Diester Diamine Containing Varying Length of Aliphatic Spacer Group: Polymerization, Thermal and Viscoelastic Characteristics.” Eur. Polym. J., 77 139–154 (2016)
Allen, DJ, Ishida, H, “Polymerization of Linear Aliphatic Diamine-Based Benzoxazine Resins Under Inert and Oxidative Environments.” Polymer (Guildf), 48 6763–6772 (2007)
Thennarasu, P, Prabunathan, P, Senthilkumar, M, “Development of Biomass-Derived Functionalized Activated Carbon-Coated and Polyaniline-Grafted Cotton Fabric with Enhanced Ultraviolet Resistance.” J. Ind. Text., 47 1609–1625 (2018)
Basak, S, Samanta, KK, Chattopadhyay, SK, “Fire Retardant Property of Cotton Fabric Treated with Herbal Extract.” J. Text. Inst., 106 1338–1347 (2015)
Balachandran, VS, Jadhav, SR, Vemula, PK, John, G, “Recent Advances in Cardanol Chemistry in a Nutshell: From a Nut to Nanomaterials.” Chem. Soc. Rev., 42 427–438 (2013)
Arumugam, H, Krishnan, S, Chavali, M, Muthukaruppan, A, “Cardanol Based Benzoxazine Blends and Bio-silica Reinforced Composites: Thermal and Dielectric Properties.” New J. Chem., 42 4067–4080 (2018)
Cheng, M, He, H, Zhu, H, Guo, W, Chen, W, Xue, F, Zhou, S, Chen, X, Wang, S, “Preparation and Properties of pH-Responsive Reversible-Wettability Biomass Cellulose-Based Material for Controllable Oil/Water Separation.” Carbohydr. Polym., 203 246–255 (2019)
Jeevahan, J, Chandrasekaran, M, Britto Joseph, G, Durairaj, RB, Mageshwaran, G, “Superhydrophobic Surfaces: A Review on Fundamentals, Applications, and Challenges.” J. Coat. Technol. Res., 15 231–250 (2018)
Jin, M, Li, S, Wang, J, Liao, M, Zhao, Y, “Controllable Fabrication of Organosilane Nano-architectured Surfaces with Tunable Wettability.” Appl. Surf. Sci., 258 7552–7555 (2012)
Reshmi, CR, Sundaran, SP, Juraij, A, Athiyanathil, S, “Fabrication of Superhydrophobic Polycaprolactone/Beeswax Electrospun Membranes for High-Efficiency Oil/Water Separation.” RSC Adv., 7 2092–2102 (2017)
Acknowledgments
The authors thank the DST-SERB (TAR/2019/000234), India, and the PSG Management for their financial and moral support. Also, the authors acknowledge the SIF, VIT-Vellore, for providing NMR facility and CLIF, University of Kerala, for providing XPS facility. In addition, the authors acknowledge Mr. M. Venkatesan, SIF, VIT, for his continuous support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (MP4 122323 kb)
Supplementary material 3 (MP4 79158 kb)
Rights and permissions
About this article
Cite this article
Prabunathan, P., Elumalai, P., Dinesh Kumar, G. et al. Antiwetting and low-surface-energy behavior of cardanol-based polybenzoxazine-coated cotton fabrics for oil–water separation. J Coat Technol Res 17, 1455–1469 (2020). https://doi.org/10.1007/s11998-020-00365-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-020-00365-w