Abstract
Eugenol (E) based mono-functional benzoxazine(E-x) monomers were prepared using different long-chain monoamines(x = ba, ha, dda, oda) and fluorine substituted aromatic monoamine (x = fa). The molecular structure of the monomers developed was characterized by FTIR and NMR spectral analysis. Further, the prepared monomers were coated over the cotton fabric and studied for their surface behavior. The poly(E-dda) coated cotton fabric exhibits the higher value of water contact angle (WCA = 151°) than that of other samples coated with polybenzoxazines(E-ba, E-ha,E-oda, and E-fa). Furthermore, poly(E-dda) coated cotton fabrics also displayed the lower value of surface energy of 15.6 mN/m with a lower sliding angle value(11°) than those of other coated cotton fabric samples. The formation of rough surfaces on the fabric was ascertained from microstructure analysis and thereby contributes to superhydrophobicity along with pH robustness. Subsequently, the oil-water separation efficiency and flux of the poly(E-dda) coated cotton fabric was found to be 98% and 5800 L/m2h respectively. It was also observed that the specimen of a glass substrate coated with poly(E-dda) exhibited the delayed ice formation. Data obtained from different studies, it is suggested that the eugenol-dodecylamine(E-dda) based benzoxazine can be effectively employed as an alternate to fluorine-based polymers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polybenzoxazines(PBz) emerging as an advance thermoset polymeric material with superior superhydrophobicity and low surface free energy [1, 2]. Polybenzoxazines also delivers low sliding contact angle (1°) and superior self-cleaning property[3]. Polybenzoxazines possesses several advantages such as ease of production, light weight and lower cost than that of fluoropolymers [4]. Introducing the roughness and lowering the surface energy are the two major routes to obtain superhydrophobic surfaces[5, 6]. Polybenzoxazine coated superhydrophobic surfaces offer a wide range of applications such as oil-water separation, anti-icing, and protecting surfaces corrosion [7, 8].
Gogoi et al. [9] during 2014 reported the PBz coated polyester with low surface energy behavior, and subsequently in the same year Tao Zhang [10] developed the super hydrophobic nature of PBz coated ramie fabrics. The oil-water separation tendency of polybenzoxazine was first explored by Zhong Xin et al. in 2015 [11], in which the oleophilictiy of PBz along with self-cleaning behavior was also demonstrated. Rigoberto C. Advincula et al. reported the anti-icing, anti-corrosive and superoleophilic behavior of rubber-modified PBz/SiO2nanocomposites in 2017 [12]. Recently, Tanet al reported the PBz/SiO2 nanocomposites coated cotton fabrics for oil-water separation with superhydrophobic surface (156°) [13]. All the reported studies suggest that the polybenzoxazine coated surfaces are customized with superhydrophobicity. However, most of these reports are related only with the significant behavior of bisphenol-A (BPA) based polybenzoxazines, which has much industrial importance. Though BPA has much industrial importance, its practice could cause endocrine disruption that might subsequently result in serious health issues [14, 15]. Hence, the development of non-toxic and environmentally friendly precursors are highly warranted to substitute the existing hazardous nature of materials. In this view, our research group have recently developed benzoxazines from alternate non-toxic phenol sources [16,17,18].
In the recent years, cardanol, eugenol, guiacol, rosin, sesamol, etc., received a significant attention from researchers to develop an environmentally friendly,sustainable and renewable bio-based benzoxazines [14, 15, 19,20,21,22]. Eugenol is a bio-phenolic compound extracted bio-sources particularly from clove oil, nutmeg, cinnamon, basil, and bay leaf [23]. In the molecular structure of eugenol, ortho and para positions are occupied by methoxy and allyl groups respectively [24, 25]. Eugenol has been recently used as a non-toxic feedstock chemical for the preparation of benzoxazines [16, 24, 26]. Benzoxazine ring-opening polymerization results in the cross-linking preferably at ortho and para position of the phenolic substrate [27]. However, in the case of eugenol less favored meta-position is only possible, which could results in less cross-linking density compared to those of various phenol derived benzoxazines [25, 27]. This limited cross-link density would imparts flexibility to the resulted matrices. As a result, the eugenol based benzoxazines are chosen in the present work as an effective coating material for the cotton fabrics to impart hydrophobic behaviour.
Recently,cardanol–aniline based polybenzoxazine/SiO2 modified fabric was studied for oil/water separation by Zhong Xin et al. in 2019 [28]. It was observed that the most of the previous reports, the micro roughness on the surfaces are created through coating polybenzoxazines hybrids containing SiO2,TiO2,ZnO nanoparticles [11, 12, 28]. However, the incorporation of expensive nanoparticles has limited feasibility towards industrial perspective commercial utility. Hence, the demand for robust, cost competitive and environmentally friendly polybenzoxazines coating without incorporation of nanoparticles is highly warranted.
Hence, in the present work benzoxazines based on eugenol and aliphatic monoamines containing different alkyl chain length having (4, 7, 12 and 18 carbons) were prepared and characterized using different sophisticated analytical techniques .The aliphatic chain moiety could favor hydrophobic behavior due to their non-polar nature [29]. The prepared benzoxazines were coated over cotton fabric and glass substrates. The surface morphology, water contact angle and oil-water separation behaviour of the cotton fabric coated with polybenzoxazines are studied and the results obtained are discussed in detail and reported. In addition, the anti-icing property of the glass surfaces coated with eugenol based polybenzoxazines are also explored and reported.
Experimental
Chemicals
Eugenol was obtained from Loba chemie laboratory chemicals and reagents, India. Butylamine (ba), heptylamine (ha), dodecylamine (dda), octadecylamine (oda), and 4-fluoroaniline (fa) were obtained from Sigma Alrich, India. Tetrahydrafuron, chloroform, paraformaldehyde, dioxane, and sodium hydroxide were obtained from Qualigens, India. Cotton fabric was procured from textile industry in Erode, Tamilnadu, India. Glass slides with dimension of 2 × 1 cm was collected from local stores, coimbatore, India.
Synthesis of Eugenol Based Benzoxazine Monomer (E-ba, E-ha, E-dda, E-oda, and E-fa)
The preparation of monofunctional benzoxazine monomers with long chain aliphatic amines and fluorine substituted aromatic amine are presented in Scheme 1. In detail, eugenol (5 g, 30.4 mmol) dissolved in 1,4-dioxane (8 mL), respective amines namely butylamine [(ba) 2.22 g, 30.4 mmol] or heptylamine [(ha) 3.50 g, 30.4 mmol] or dodecylamine [(dda) 5.63 g, 30.4 m mol], or octadecylamine [(oda) 8.20 g, 30.4 mmol], or fluoroaniline [(fa) 3.38 g, 30.4 mmol] were added along with the addition of paraformaldehyde in two equivalents separately reacted at 60 °C. Then the temperature of the reaction was slowly raised to 110 °C, allowed to reflux and stirred constantly for 12 h.Meantime, the progress of the reaction was monitored by TLC. After the completion of the reaction, the reaction products were cooled, quenched in water and extracted using ethylacetate. The unreacted eugenol was removed by adding 1 M NaOH to the organic layer. Subsequently, the organic layer was dried over anhydrous sodium sulphate and evaporated under vacuum. The benzoxazine monomers synthesized are named as E-ba, E-ha, E-dda, E-oda and E-fa by following the IUPAC nomenclature. After ascertaining for their structural confirmation from FTIR and NMR spectra, the prepared monomers were coated over cotton fabric as described subsequently.
Preparation of Benzoxazines Coated Cotton Fabric and Glass Surfaces
Cotton fabric was washed with distilled water and soaked in 2 M NaOH solution for 3 h to remove the waxy material and activate the surface. Later, the fabric was washed with distilled water and kept drying at 60 °C for 10 h. Then, the obtained cotton fabric was coated with different benzoxazines to modify the surface. Exactly 1 g of each benzoxazines monomer is allowed to dissolve separately in 10 mL of THF. To the separate solutions containing benzoxazines, the two piece of cotton fabrics were immersed for 20 min and subsequently cured at 180 °C for 5 min. The pristine cotton without polybenzoxazine also studied for comparison. For coating the glass substrate, the surface was cleaned in piranha solution followed by drying in a vacuum oven at 110 °C for 30 min. The solution containg 0.5 g of benzoxazines dissolved in THF were then the drop casted over the pre-cleaned glass (piranha etched) and cured for 1 h in hot air oven.
Characterisation
FTIR spectra measurements were carried out in Agilent Cary 630 FTIR Spectrometer. NMR spectra were recorded in Bruker (400 MHz) using deuterated chloroform (CDCl3) solvent and tetramethylsilane (TMS) as an internal standard. The curing behavior of the E-x benzoxazine monomers was determined using NETZSCH STA 449F3 Jupiter-German from room temperature to 300 °C.The morphology of the polybenzoxazine coated fabrics is analysed from an FEI QUANTA 200F high-resolution scanning electron microscope (HRSEM). The water contact angle measurements of the coated fabrics were conducted on a Data physics instrument (OCA 15, Germany) using a water drop (V = 10 µL). The oil-water separation is performed in filtration assembly without the aid of pressure using cotton fabrics of 2.5 cm diameter and 50% v/v of the oil-water mixtures (petrol/water, diesel/water, and engine oil/water).Separation efficiency (%) and flux were calculated using Eqs. 1 and 2. The cyclic test was conducted for 10 cycles using petrol/water mixture.
Anti-icing performances of the eugenol based polybenzoxazines were analyzed after coating over glass surfaces. The polybenzoxazines coated glass surfaces were subjected at − 5° C for 24 h. Later, 20 µL of water was dropped on the glass surfaces containing eugenol based polybenzoxazines. The process of ice formation over the surfaces was recorded using a digital camera for every 10 min and the results were analysed and compared in order to evaluate the anti-icing behavior.
Results and Discussion
Spectral Analysis
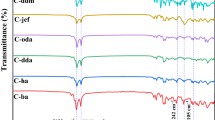
To validate the structures of the prepared benzoxazines (E-ba, E-ha, E-dda, E-oda and E-fa), spectral analysis such as FTIR and NMR were carried out. Figure 1 shows the FT-IR spectra of the benzoxazine monomers. Typically, bands appeared at 2915 and 2840 cm− 1 were assigned as asymmetric and symmetric stretching vibrations respectively, representing the methylene groups (–CH2–) of alkyl side chains of the ba, ha,dda and oda moieties [30, 31]. With an increase in the alkyl chain length, the vibration band also became stronger and confirms their presence. These stretching bands are not observed in the case of E-fa benzoxazine, due to the presence of aryl ring of 4-fluoroaniline.The broad bands appeared between 1242 and 1190 cm− 1 were attributed to the asymmetric stretching vibrations of C–O–C groups present in the benzoxazines monomer [32]. Further, the band appeared at 1142 cm− 1 corresponds to the symmetric vibration of C–O–C groups present in the benzoxazines monomer[32]. The appearance of the sharp band at 1501 cm− 1 corresponds to tri-substituted benzene rings of eugenol moiety. Further, the appearance of a peak at 912 cm− 1, confirms the formation of benzoxazine monomers [2, 33].
The 1H-NMR analysis was carried out to confirm the molecular structure of benzoxazine monomers. Figure 2a–e shows the 1H-NMR spectra of E-ba, E-ha, E-dda, E-oda and E-fa benzoxazine monomers. The formation of oxazine ring was confirmed by the presence of two singlets of methylene protons corresponding to (–O–CH2–N–) and –N–CH2–Ar). With respect to the nature of precursors, the peak positions corresponding to (–O–CH2–N–) and –N–CH2–Ar) methylene signals could undergo minor shifts [34,35,36]. The aryl amine condensed oxazine ring gives signal at downfield region, whereas that of aliphatic amine based oxazine ring gives signal at upfield region.
In the case of eugenol and aliphatic monoamine based benzoxazines [E-ba, E-ha, E-dda and E-oda], the two singlets from the methylene protons of oxazine ring show signals at δ 3.8 and δ 4.8 ppm (Fig. 2a–d) in 1H-NMR spectra. The signal representing terminal methyl protons of ba, ha, dda and oda moieties appears at δ 0.9 ppm [33]. The major signals that appeared in between δ 1.0 and 2.0 ppm correspond to the aliphatic chain protons of ba, ha, dda and oda moieties. The singlet appeared at δ 2.8 ppm corresponds to N-CH2- protons of ba, ha, oda and dda [18]. In the case of E-fa, the two singlets from the methylene protons of the oxazine ring appeared at 4.5 and 5.5 ppm (Fig. 2e) in 1H-NMR spectra. The multiplet signals around δ 6.5–7.2 corresponds to the aryl rings. The signal appeared at δ 3.2 (Fig. 2a–e) corresponds to the methylene proton (=CH–CH2–Ar)of eugenol that present in between the terminal alkene carbon and aromatic ring. The terminal protons from the ethene chain of eugenol moiety appeared at δ 5.0 ppm (Fig. 2a–e).
Analysis of Curing Behaviour of Benzoxazines
The curing process of eugenol monomers were analyzed using differential scanning calorimeter (DSC). Figure 3 presents the DSC thermograms of eugenol based benzoxazines from room temperature to 300 °C. In Fig. 3, the appearance of broad endothermic peaks indicates the occurrence of either softening or melting process. It is interesting to note that the melting/softening process is highly influenced by the amine chain length. As the chain length of the amine increases, the temperature corresponding to the endothermic nature was decreased [37, 38]. Thus, the E-dda and E-oda with long aliphatic chain shows broad endothermic peaks comparatively below 150 °C, whereas that of E-ba and E-ha monomers with shorter chain length delivers endothermic peaks nearly around 240 °C.
Similarly, the peak maxima of broad exothermic peaks (Tp) associated with the ring-opening polymerization of benzoxazine monomers are also influenced by the change in the aliphatic chain length of of the monomers (Fig. 3). The monomer prepared using butylamine requires higher temperature for polymerization (Tp = 261 °C) than that of others. Subsequently, the other benzoxazine monomers prepared by aliphatic amines with seven, twelve and eighteen carbon alkyl chain namely E-ha, E-dda and E-oda possess the Tp at 245 °C, 215 and 227 °C respectively. This phenomenon could be attributed to various factors such as molecular structure, flexibility, and the concentration of reactive functional groups. As the chain length increases, the molecular size also increases,which in turn decreases the reactivity. However, the flexibility and mobility of monomers get increased with an increase in aliphatic chain length, which allows the occurrence of softening and facilitates the ease of ring-opening reaction at lower temperatures. The value of Tp was found to be decreased with an increase in the aliphatic chain of amines. However, the eugenol monomer prepared using octadecyl amine (18 carbon) shows slightly increased Tp(227 °C) than that of monomer prepared from dodcely amine(12 carbon). This might be attributed to the cause of steric effect, as noticed in the case of linear aliphatic amine-based benzoxazines [39,40,41,42,43]. On the other hand, the E-fa monomer shows Tp at 227 °C. Moreover, the aliphatic amine condensed benzoxazine monomers release comparatively lower enthalpy than that of aromatic based monomer.
Curing Analysis of the Coated Fabrics
Figure 4 represents the FTIR spectra of the eugenol based benzoxazines coated cotton fabrics. For comparison, the FTIR spectrum of pristine cotton fabric is also provided in the supporting information (Fig. S1). For pristine cotton fabric, the peaks appeared at 1712 and 1246 cm− 1 correspond to the carboxylic acid functional group and C-O-C linkage respectively. Since the fabric was treated with sodium hydroxide, the primary hydroxyl groups might have been oxidized to the carboxylic group [29]. After coating with benzoxazines, the fabrics were cured at 180 °C and subjected to FTIR analysis. In Fig. 4, the absence of peak at 912 cm− 1 represents that the respective benzoxazine monomers have undergone ring-opening polymerization over cotton fabrics [29]. The peaks observed at 2919 and 2846 cm− 1 correspond to the asymmetric and symmetric stretching vibrations of a methylene group (–CH2–) respectively, arised due to the presence of long alkyl side chains [29].
Water Contact Angle (WCA) and Surface Free Energy
To study the water repellent characteristic of the fabrics, the values of water contact angle (WCA) are measured and are presented in Fig. 5. The WCA values of poly(E-ba), poly(E-ha), poly(E-dda), poly(E-oda) and poly(E-fa) coated cotton fabrics are 118°, 132°, 151°, 136° and 140° respectively. Compartively, the values of WCA of the fabric coated with poly(E-dda) was found to be higher (151 °C) (Fig. 5) than those of other samples. This infers that the optimum aliphatic chain length required to achieve superhydrophobicity using bio-based eugenol benzoxazine with dodecylamine (12 Carbon atoms). Earlier, the values of higher contact angle was achieved with stearic acid possessing higher packing density. The superhydrophobic nature was also achieved using stearic acid through the well-ordered self-assembly of carbon chains in an upright position [44]. However, cotton fabric coated with eugenol-benzoxazine prepared with octadecylamine(18 C) shows lower contact angle value than that of dodecyl aliphatic amine (12Cs). This might be due to steric hindrance, which could altered the molecular orientation and thereby results in a lower value of WCA. In order to validate the role of precursors (eugenol and dodecylamine), the eugenol was replaced by phenol and corresponding phenol-dodecylamine benzoxazine (P-dda) was studied (Fig. S2, 1H-NMR). Further, the phenol-dodecylamine benzoxazine (P-dda) coated cotton fabric shows WCA value of 142° (Fig. S3). Finally, the cotton fabric coated with fluoro-aniline based eugenol benzoxazine also shows the lower value of WCA (140°) than that of the poly(E-dda) coated cotton fabric (151°). These results infer that the synergistic contribution of eugenol and dodecylamine precursors towards superhydrophobicity than that of fluoro-aniline based benzoxazine [(poly(E-fa)].
Data obtained from WCA studies infer that the alkyl chains attached to the polybenzoxazines play a significant role in influencing the surface properties of the coated cotton fabrics. In general, hydrophobic substrates usually possess the lower values of surface free energy. It is already ascertained that the polybenzoxazines are low surface energy materials with inherent hydrophobicity [45]. Hence it is highly desirable to evaluate the surface free energy of the cotton fabric coated with eugenol based benzoxazines. The values of surface free energy of the fabrics are calculated using the equation of state and are presented in Fig. 6. The value of the surface free energy of pristine cotton fabric was found to be 26.2 mN/m using water. The values of surface free energy observed using water for the cotton fabrics coated with poly(E-ba), poly(E-ha), poly(E-dda), poly(E-oda) and poly(E-fa) are 20.3, 17.0, 15.6, 17.8, and 17.6mN/m respectively. From the results obtained for different benzoxazines coated samples, the surface free energy of poly(E-dda) coated fabric was significantly lower (15.6 mN/m) than that of other benzoxazine coated cotton fabrics. This value is even lower than the value reported for Teflon [4, 46].
It is well known that the oils have lower surface tension ranging from 20 to 30 mN/m2. Hence, it is highly desirable to predict the surface free energy of material using oil to ascertains their utility in oil-water separation. The surface free energy of pristine cotton fabric was observed to be 10.7mN/m using diesel, where as the surface free energies of cotton fabrics coated with poly(E-ba), poly(E-ha), poly(E-dda), poly(E-oda) and poly(E-fa) are 24.6, 26.0, 27.7, 26.5, and 27.6 mN/m respectively. These results suggest that the value of surface free energies of cotton fabrics coated with polybenzoxazines have almost equivalent to that of diesel oil, which has the value of 25.8mN/m [44]. Hence, it is suggested that the benzoxazine coated cotton fabrics developed in the present work can be used as cost-competitive sustainable filtration material for oil-water separation application similar to the other coating materials reported earlier [47,48,49].
Morphology of the Fabrics
The morphology of eugenol based benzoxazines coated cotton fabric was investigated using field emission scanning electron microscope (FE-SEM) and the results obtained are presented in Fig. 7 in comparison with pristine cotton fabric. The morphology of eugenol based benzoxazines coated cotton fabrics, show rough and asperity textured surfaces. The surface roughness was closely observed to increase with the increase in the length of carbon chain of the benzoxazines. Thus, poly(E-oda) coated cotton fabric shows more roughness due to the cluster of the long-chain moiety of octadecyl chain. It is well known that the formation hierarchically rough dual scale structured surface plays an important role to enhance the water-repelling behavior [50]. Thus, the formation of the rough surface aided by the coating of eugenol based benzoxazines contributed to an enhanced values of water contact angle. The polybenzoxazines coated cotton fabric surfaces possess protuberances and air beneath textured surfaces over the fabric, which in turn stack the water droplet to be sited on the top without contacting the fabric surfaces [50]. Thus, the reduced interfacial interaction influences the Cassie-Baxter state of attraction between the fabric surface and water drop. This behavior contributes to superhydrophobic behaviour due to the strong intra-molecular hydrogen bonding resulted between polybenzoxazine and cotton fabric. Further, the water repellent behavior imparted by the long alkyl chains is similar to the trichlorosilane with different alkyl (methyl, propyl, octyl, dodecyl, octadecyl) chain [51]. It is important to note that eugenol based long-chain benzoxazines prepared in the present work are cost-effective when compared with those of long-chain silane and fluorine derivatives [51].
Oil-Water Separation
Further, the oil-water separation efficiency was measured for the poly(E-dda)/cotton fabric (Fig. 8a), because of its higher contact angle value and lower surface free energy. Three different types of oil-water mixtures were prepared using an equal volume of water and petroleum derivatives such as engine oil (20W40 grade with a density of 0.85 kg/L), diesel and petrol. Due to the high density of water, the oil layer exists upper. To bring up the water layer above, high-density dichloromethane (DCM) (d = 1.33 g/cm3)(50 ml) was added, which dissolves the petrol and brings down. Further, the extra volume of water was added to maintain the ratio of water and DCM (Fig. S4a). Once, the petrol present in DCM meet the polybenzoxazine coated cotton fabrics, the oil starts to penetrate immediately and collected in the filtration flask through gravity (Fig. S4b). After the complete removal of the oil layer, the water layer is expelled and stayed over the fabrics which indicate the superhydrophobic and low surface free energy nature of poly(E-dda) coated cotton fabric (Fig. S4c).
Among the different types of the oil-water mixture studied, the higher separation efficiency of about 98% was observed for petrol-water than that of diesel-water and engine oil-water mixtures. This infers that the poly(E-dda)/cotton fabric can suit the separation of a wide range of industrial oils. Further, the flux behavior was calculated using Eq. 2, for the poly(E-dda) coated cotton fabric to ascertain the commercial viability of polybenzoxazines coated cotton fabrics with petrol-water mixture. Initally, the flux value for poly(E-dda) coated cotton fabric placed on the sintered disc of separting flask shows only 680 L/m2h (Fig. S5). However, flux value of poly(E-dda) coated cotton without support of sintered disc was observed to be 5800 L/m2h (Fig. 8b). Upon subsequent cycles, the flux value reduces and becomes 5100 L/m2h after 10 cycles. This results infer that the highly desired separation was performed in absence of sintered disc (Fig. S6). The superoleophilic property of poly(E-dda)/cotton fabric facilitate rapid penetration of petrol. The observed results are consistent with thesuperhydrophobic membrane prepared from polycaprolactone and beeswax, which also shows 98% separation [52]. Beeswax contains long-chain methylene [–(CH2)–] carbons, which is responsible for the superhydrophobic nature.
In addition to water, the contact angle behavior of poly(E-dda) coated cotton fabric in different pH medium (pH 1–14) was performed. The observed results of WCA behavior are presented in Fig. 9a. The consistency in the WCA values of poly(E-dda) coated cotton fabric at different pH suggests that the coated fabric possesses the resistance to both acid and alkali conditions. Further, the value of water sliding angle (WSA) for poly(E-dda) was also determined and found to be 11°. Figure 9b shows superior hydrophobic behavior against a wide variety of liquids. In order to find the surface topology of the fabrics, AFM analysis was performed for both pristine and poly(E-dda) coated fabrics. The 3D image resulted from AFM analysis for both pristine and poly(E-dda) coated cotton fabrics are presented in Fig. 10. The pristine fabric shows average roughness (Ra) as 10.84 nm (Fig. 10a), whereas that of the fabric coated with poly(E-dda) was found to be about 34.40 nm (Fig. 10b). The results from AFM studies suggest that the surface roughness of the cotton fabric increased after coating with poly(E-dda). Thus, the robust poly(E-dda) coated cotton fabric can be used in oil/water separation of different environments.
Anti-Icing Behaviour
To study the anti-icing performance, glass surfaces were coated and cured with prepared benzoxazines and subseqeuntly exposed in refrigeration under − 5 °C. For reference, the glass surface without coating was also exposed. All the glass substrates were kept in the freezer for 24 h before anti-icing tests. At such a cooling state, a water droplet of 20 µL was added over all the surfaces to evaluate the anti-icing behavior. The surfaces of the water droplet on a glass surface show transparent ellipsoid shape (Fig. 11). Further, the anti-icing performances on the glass surfaces were monitored through the evolution of water droplets at a regular interval of 10 min duration. After subjected for 20 min, the transparency of the water droplet were starts to lose due to the crystallization. However, poly(E-dda) and poly(E-fa) coated surfaces don’t facilitate the crystallization of water on the surfaces upto 20 min. After 40 min, the water droplets on surfaces gradually shrunk because of the sublimation of ice bulk and the vaporization of water droplets. However, the water droplet on the surface of poly(E-dda) coated substrate still shows transparency. After 60 min the uncoated glass surfaces and polybenzoxazines coated glass surfaces tend to show the transformation of water droplet into a frozen state excluding the poly(E-dda) coated surface. The poly(E-dda) superhydrophobic surfaces on glass substrate thus exhibit a better anti-icing property than that of other benzoxazines coated surfaces. In the sub-zero environment, heterogeneous nucleation mainly occurs near the liquid-solid interfaces and free energy barrier (ΔG). According to nucleation theory, the surface with a higher contact angle leads to a larger ΔG and a smaller nucleation rate [53]. Also, the formation of Cassie’s state between the rough surfaces and water drop i.e., the existence of trapped air pockets at the solid-liquid interfaces reduces the heat transfer during the cooling process [54]. As a result, the poly(E-dda) coated glass possesses the highest water contact angle (Fig. S7) due to the formation of enhanced intermolecular hydrogen bonding led to protuberance. Thus, the air beneath the protuberance avoids the contact between the water and surface, which in turn delays the formation of the ice.
Conclusions
The superhydrophobic/superoleophilic cotton fabric was developed using eugenol based benzoxazine monomers. To develop nanostructure roughness over the cotton surface, the eugenol based benzoxazines were prepared along with different types of monoamines. The eugenol based benzoxazines developed in the present work displayed a varied morphology when coated over cotton fabrics. Among the benzoxazine coated cotton fabrics, the eugenol-dodecylamine(E-dda) based benzoxazine coated fabric exhibits the highest water contact angle (151°) and lowest surface free energy. The morphology observed in the SEM images infer the formation of roughness brought by polybenzoxazine coatings, which in turn contributes to superhydrophobicity. Finally, among the benzoxazines coated cotton fabrics, the poly(E-dda) delivers better oil-water separation efficiency (98%) with greater cyclic repeatability and anti-icing behavior. Thus, the poly(E-dda) prepared in the present study can be used as an effective hydrophobic/oleophilic material for oil-water separation and anti-icing applications.
References
Wang CF, Wang YT, Tung PH et al (2006) Stable superhydrophobic polybenzoxazine surfaces over a wide pH range. Langmuir 22:8289–8292. https://doi.org/10.1021/la061480w
Rajamanikam R, Pichaimani P, Kumar M, Muthukaruppan A (2017) Optical and thermomechanical behavior of benzoxazine functionalized ZnO reinforced polybenzoxazine nanocomposites. Polym Compos 38:1881–1889. https://doi.org/10.1002/pc.23758
Zhang W, Lu X, Xin Z, Zhou C (2016) Development of a superhydrophobic polybenzoxazine surface with self-cleaning and reversible water adhesion properties. RSC Adv 6:106054–106063. https://doi.org/10.1039/C6RA22524A
Wang CF, Su YC, Kuo SW et al (2006) Low-surface-free-energy materials based on polybenzoxazines. Angew Chem Int Ed 45:2248–2251. https://doi.org/10.1002/anie.200503957
Xue Z, Wang S, Lin L et al (2011) A novel superhydrophilic and underwater superoleophobic hydrogel-coated mesh for oil/water separation. Adv Mater 23:4270–4273. https://doi.org/10.1002/adma.201102616
Xue Z, Cao Y, Liu N et al (2014) Special wettable materials for oil/water separation. J Mater Chem A 2:2445–2460
Broje V, Keller AA (2006) Improved mechanical oil spill recovery using an optimized geometry for the skimmer surface. Environ Sci Technol 40:7914–7918. https://doi.org/10.1021/es061842m
Dubansky B, Whitehead A, Miller JT et al (2013) Multitissue molecular, genomic, and developmental effects of the deepwater horizon oil spill on resident Gulf killifish (Fundulus grandis). Environ Sci Technol 47:5074–5082. https://doi.org/10.1021/es400458p
Gogoi N, Rastogi D, Jassal M, Agrawal AK (2014) Low-surface-energy materials based on polybenzoxazines for surface modification of textiles. J Text Inst 105:1212–1220. https://doi.org/10.1080/00405000.2014.882042
Zhang T, Yan H, Fang Z et al (2014) Superhydrophobic and conductive properties of carbon nanotubes/polybenzoxazine nanocomposites coated ramie fabric prepared by solution-immersion process. Appl Surf Sci 309:218–224. https://doi.org/10.1016/j.apsusc.2014.05.013
Zhang W, Lu X, Xin Z, Zhou C (2015) A self-cleaning polybenzoxazine/TiO2 surface with superhydrophobicity and superoleophilicity for oil/water separation. Nanoscale 7:19476–19483. https://doi.org/10.1039/c5nr06425b
Caldona EB, De Leon ACC, Thomas PG et al (2017) Superhydrophobic rubber-modified polybenzoxazine/SiO2 nanocomposite coating with anticorrosion, anti-ice, and superoleophilicity properties. Ind Eng Chem Res 56:1485–1497. https://doi.org/10.1021/acs.iecr.6b04382
Li Y, Yu Q, Yin X et al (2018) Fabrication of superhydrophobic and superoleophilic polybenzoxazine-based cotton fabric for oil–water separation. Cellulose 25:6691–6704. https://doi.org/10.1007/s10570-018-2024-8
Lehmler HJ, Liu B, Gadogbe M, Bao W (2018) Exposure to Bisphenol A, Bisphenol F, Bisphenol S in U.S. adults and children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega 3:6523–6532. https://doi.org/10.1021/acsomega.8b00824
Okada H, Tokunaga T, Liu X et al (2008) Direct evidence revealing structural elements essential for the high binding ability of bisphenol a to human estrogen-related receptor-γ. Environ Health Perspect 116:32–38. https://doi.org/10.1289/ehp.10587
Hariharan A, Prabunathan P, Kumaravel A et al (2020) Bio-based polybenzoxazine composites for oil-water separation, sound absorption and corrosion resistance applications. Polym Test 86:106443. https://doi.org/10.1016/j.polymertesting.2020.106443
Hariharan A, Prabunathan P, Subramanian SS et al (2020) Blends of chalcone benzoxazine and bio-benzoxazines coated cotton fabrics for oil–water separation and bio-silica reinforced nanocomposites for low-k applications. J Polym Environ 28:598–613. https://doi.org/10.1007/s10924-019-01629-2
Manickam M, Pichaimani P, Arumugam H, Muthukaruppan A (2019) Synthesis of nontoxic pyrazolidine-based benzoxazine-coated cotton fabric for oil-water separation. Ind Eng Chem Res 58:21419–21430. https://doi.org/10.1021/acs.iecr.9b03440
Liu X, Li Z, Zhan G et al (2019) Bio-based benzoxazines based on sesamol: synthesis and properties. J Appl Polym Sci 136:48255. https://doi.org/10.1002/app.48255
Zhang Y, Liu X, Zhan G et al (2019) Study on the synergistic anticorrosion property of a fully bio-based polybenzoxazine copolymer resin. Eur Polym J 119:477–486. https://doi.org/10.1016/j.eurpolymj.2019.07.020
Liu X, Zhang R, Li T et al (2017) Novel fully biobased benzoxazines from rosin: synthesis and properties. ACS Sustain Chem Eng 5:10682–10692. https://doi.org/10.1021/acssuschemeng.7b02650
Prabunathan P, Vasanthakumar A, Manoj M et al (2020) Polypyrrole inter-layered low temperature curing benzoxazine matrices with enhanced thermal and dielectric properties. J Polym Res 27:1–14. https://doi.org/10.1007/s10965-020-2022-z
Khalil AA, Rahman UU, Khan MR et al (2017) Essential oil eugenol: sources, extraction techniques and nutraceutical perspectives. RSC Adv 7:32669–32681. https://doi.org/10.1039/c7ra04803c
Thirukumaran P, Shakila A, Muthusamy S (2014) Synthesis and characterization of novel bio-based benzoxazines from eugenol. RSC Adv 4:7959–7966. https://doi.org/10.1039/c3ra46582a
Dumas L, Bonnaud L, Olivier M et al (2015) Eugenol-based benzoxazine: from straight synthesis to taming of the network properties. J Mater Chem A 3:6012–6018. https://doi.org/10.1039/c4ta06636g
Thirukumaran P, Parveen AS, Sarojadevi M (2014) Synthesis and copolymerization of fully biobased benzoxazines from renewable resources. ACS Sustain Chem Eng 2:2790–2801. https://doi.org/10.1021/sc500548c
Krishnan S, Arumugam H, Chavali M, Muthukaruppan A (2019) High dielectric, low curing with high thermally stable renewable eugenol-based polybenzoxazine matrices and nanocomposites. J Appl Polym Sci 136:1–11. https://doi.org/10.1002/app.47050
Yao H, Lu X, Xin Z et al (2019) A durable bio-based polybenzoxazine/SiO2 modified fabric with superhydrophobicity and superoleophilicity for oil/water separation. Sep Purif Technol 229:115792. https://doi.org/10.1016/j.seppur.2019.115792
Thennarasu P, Prabunathan P, Senthilkumar M (2018) Development of biomass-derived functionalized activated carbon-coated and polyaniline-grafted cotton fabric with enhanced ultraviolet resistance. J Ind Text 47:1609–1625. https://doi.org/10.1177/1528083717702008
Rao BS, Palanisamy A (2011) Monofunctional benzoxazine from cardanol for bio-composite applications. React Funct Polym 71:148–154. https://doi.org/10.1016/j.reactfunctpolym.2010.11.025
Lu R, Gan W, Wu BH et al (2005) C-H stretching vibrations of methyl, methylene and methine groups at the vapor/Alcohol (n = 1–8) interfaces. J Phys Chem B 109:14118–14129. https://doi.org/10.1021/jp051565q
Rao BS, Palanisamy A (2012) A new thermo set system based on cardanol benzoxazine and hydroxy benzoxazoline with lower cure temperature. Prog Org Coatings 74:427–434. https://doi.org/10.1016/j.porgcoat.2012.01.006
Parveen AS, Thirukumaran P, Sarojadevi M (2014) Low dielectric materials from fluorinated polybenzoxazines. Polym Adv Technol 25:1538–1545. https://doi.org/10.1002/pat.3398
Bonnaud L, Chollet B, Dumas L et al (2019) High-performance bio-based benzoxazines from enzymatic synthesis of diphenols. Macromol Chem Phys 220:1800312. https://doi.org/10.1002/macp.201800312
Gomez IJ, Arnaiz B, Cacioppo M et al (2018) Nitrogen-doped carbon nanodots for bioimaging and delivery of paclitaxel. J Mater Chem B 6:7634–7639. https://doi.org/10.1039/x0xx00000x
Lin RC, Kuo SW (2018) Well-defined benzoxazine/triphenylamine-based hyperbranched polymers with controlled degree of branching. RSC Adv 8:13592–13611. https://doi.org/10.1039/c8ra00506k
Uyar T, Hacaloglu J, Ishida H (2013) Synthesis, characterization, and thermal properties of alkyl-functional naphthoxazines. J Appl Polym Sci 127:3114–3123. https://doi.org/10.1002/app.37692
Ishida H (2011) Overview and historical background of polybenzoxazine research. Elsevier B.V, New York
Liu J, Lu X, Xin Z, Zhou C (2016) Surface properties and hydrogen bonds of mono-functional polybenzoxazines with different N-substituents. Chin J Polym Sci (English Ed) 34:919–932. https://doi.org/10.1007/s10118-016-1810-8
Allen DJ, Ishida H (2009) Effect of phenol substitution on the network structure and properties of linear aliphatic diamine-based benzoxazines. Polymer 50:613–626. https://doi.org/10.1016/j.polymer.2008.11.007
Rao BS, Surendra P (2016) Synthesis and characterization of difunctional benzoxazines from aromatic diester diamine containing varying length of aliphatic spacer group: polymerization, thermal and viscoelastic characteristics. Eur Polym J 77:139–154. https://doi.org/10.1016/j.eurpolymj.2016.02.003
Allen DJ, Ishida H (2007) Polymerization of linear aliphatic diamine-based benzoxazine resins under inert and oxidative environments. Polymer 48:6763–6772. https://doi.org/10.1016/j.polymer.2007.09.003
Agag T, Akelah A, Rehab A, Mostafa S (2012) Flexible polybenzoxazine thermosets containing pendent aliphatic chains. Polym Int 61:124–128. https://doi.org/10.1002/pi.3156
Arumugam H, Krishnan S, Chavali M, Muthukaruppan A (2018) Cardanol based benzoxazine blends and bio-silica reinforced composites: thermal and dielectric properties. New J Chem 42:4067–4080. https://doi.org/10.1039/c7nj04506a
Prabunathan P, Alagar M (2017) Polybenzoxazine-based organic-inorganic nanohybrid materials for high performance engineering applications. In: Ishida H (ed) Advanced and emerging polybenzoxazine science and technology. Elsevier, New York, pp 801–834
Prabunathan P, Thennarasu P, Song JK, Alagar M (2017) Achieving low dielectric, surface free energy and UV shielding green nanocomposites: via reinforcing bio-silica aerogel with polybenzoxazine. New J Chem 41:5313–5321. https://doi.org/10.1039/c7nj00138j
Ge M, Cao C, Liang F et al (2020) A “pDMS-in-water” emulsion enables mechanochemically robust superhydrophobic surfaces with self-healing nature. Nanoscale Horizons 5:65–73. https://doi.org/10.1039/c9nh00519f
Lahiri SK, Zhang P, Zhang C, Liu L (2019) Robust fluorine-free and self-healing superhydrophobic coatings by H3BO3 incorporation with SiO2–Alkyl-Silane@PDMS on cotton fabric. ACS Appl Mater Interfaces 11:10262–10275. https://doi.org/10.1021/acsami.8b20651
Sasaki K, Tenjimbayashi M, Manabe K, Shiratori S (2016) Asymmetric superhydrophobic/superhydrophilic cotton fabrics designed by spraying polymer and nanoparticles. ACS Appl Mater Interfaces 8:651–659. https://doi.org/10.1021/acsami.5b09782
Jeevahan J, Chandrasekaran M, Britto Joseph G et al (2018) Superhydrophobic surfaces: a review on fundamentals, applications, and challenges. J Coatings Technol Res 15:231–250
Jin M, Li S, Wang J et al (2012) Controllable fabrication of organosilane nano-architectured surfaces with tunable wettability. Appl Surf Sci 258:7552–7555. https://doi.org/10.1016/j.apsusc.2012.04.084
Reshmi CR, Sundaran SP, Juraij A, Athiyanathil S (2017) Fabrication of superhydrophobic polycaprolactone/beeswax electrospun membranes for high-efficiency oil/water separation. RSC Adv 7:2092–2102. https://doi.org/10.1039/c6ra26123j
Jiang D, Fan P, Gong D et al (2016) High-temperature imprinting and superhydrophobicity of micro/nano surface structures on metals using molds fabricated by ultrafast laser ablation. J Mater Process Technol 236:56–63. https://doi.org/10.1016/j.jmatprotec.2016.05.009
He M, Wang J, Li H, Song Y (2011) Super-hydrophobic surfaces to condensed micro-droplets at temperatures below the freezing point retard ice/frost formation. Soft Matter 7:3993–4000. https://doi.org/10.1039/c0sm01504k
Acknowledgements
The authors thank the PSG Management for their financial and moral support. The authors also acknowledge the SIF, VIT-Vellore for providing NMR facility.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dinesh Kumar, G., Prabunathan, P., Manoj, M. et al. Fluorine Free Bio-Based Polybenzoxazine Coated Substrates for Oil-Water Separation and Anti-Icing Applications. J Polym Environ 28, 2444–2456 (2020). https://doi.org/10.1007/s10924-020-01782-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01782-z