Abstract
Plant-based proteins have shown great potential as an alternative substitute for animal proteins to meet the increasing global demand. Nevertheless, several limitations mitigate plant-based protein application and utilization. As a panacea to meeting the market demand, it is imperative to modify plant-based proteins to produce improved quality and techno-functionalities compared to conventional animal protein ingredients. Enzymatic, chemical, and physical modifications have been used for plant-based proteins, which have shown exciting results in improving their techno-functional properties, digestibility, and inherent allergenicity. Among these modification methods, the low-cost, limited time, high sensitivity, and high reproducibility give enzymatic modification leverage over chemical and physical methods. This review gave a concise summary of the advantages and disadvantages of enzymatic modifications. The efficacy of enzymatic modification in producing protein ingredients from plant sources with improved techno-functional properties, digestibility, and alleviated allergenicity was discussed. Furthermore, the application of enzymatic modification in the production of bioactive compounds with health-beneficial properties adds in no small measure to the novelty of this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global demand for plant-based proteins has grown tremendously over the years owing to several factors, including sustainability, minimal environmental impact, and other beneficial health claims (Akharume et al., 2021). Although plant-based proteins are deficient in one or more essential amino acids, they have found wide applications in human diet supplementation, pet foods, and pharmaceuticals, in addition to producing functional and value-added products (Gençdağ et al., 2021; Hewage et al., 2022; Ismail et al., 2020; Nadeeshani et al., 2022). However, the application of plant proteins, particularly in food industries, has been stunted owing to their low techno-functional properties, inherent allergenicity, and poor digestibility, especially when compared to animal proteins. Protein modification has been implemented to circumvent these limitations by conformation modification or polypeptide chain fragmentation. Conformation modification could either be through thermal or non-thermal processes (Nasrabadi et al., 2021), which excessive polypeptide unfolding, aggregation, and irregular crosslinking has been reported in the former.
Chemical modifications, including acylation, methylation, phosphorylation, sulfation, farnesylation, ubiquitination, glycosylation, etc., are the most common non-thermal protein modifications for plant-based proteins, which have yielded promising results for improving protein functionality for food and non-food applications (Akharume et al., 2021; Boutureira & Bernardes, 2015; Bandara et al., 2018). Nonetheless, chemical modification has been associated with environmental pollution, difficulty in recycling, and high cost of production. Additionally, consumers’ demand for chemical-free natural products has limited utilization of chemically modified proteins. To circumvent these, attention has been drawn to alternative non-thermal technologies such as ultrasound, high pressure, pulse electric field, and high voltage cold atmospheric plasma, which have also produced remarkable structural and functional modifications of plant-based proteins (Akharume et al., 2021; Li et al., 2017). However, their low sensitivities have created a major bottleneck for industrial scale-up and commercialization. Hence, a green and sustainable protein modification method with high sensitivity for industrial scale-up is urgently required.
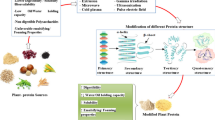
Over the years, enzymatic modification has gained massive attention as an effective, highly sensitive, highly reproducible, green, and cost-effective modification for plant-based proteins (Aluko, 2008; Barać et al., 2011). This approach revolutionized the application of plant-based proteins in the food industry via improved digestibility and techno-functional properties, as well as reduced intrinsic allergenicity (Fig. 1). Additionally, enzymatic modification has been used to produce value-added products and peptides with health-beneficial properties such as antioxidative, antihypertensive, anticancer, antimicrobial, hypocholesterolemic, and immunomodulatory (Aondona et al., 2021; Görgüç et al., 2020; Udenigwe & Aluko, 2012). Thus, this review meets the necessity to assemble the recent information on the process parameters and conditions for enzymatic modification of plant-based proteins and the resultant impact on techno-functional properties, digestibility, allergenicity, and production of bioactive peptides.
Fermentation and Enzymatic Modification
It is worth noting that enzymatic modification of plant-based proteins started from fermentation via the activities of microorganisms, which involves the breakdown of macromolecules into smaller fragments or molecules such as disaccharides, monosaccharides, and peptides, that are easily digested or absorbed in the body (Campbell-Platt, 1994). Depending on the method of preparation, fermentation can be spontaneous (indigenous microorganisms) or non-spontaneous (starter culture) (Fadimu et al., 2022). In the case of plant-based proteins, proteolytic enzymes produced by fermenting organisms (bacteria, yeasts, or molds) break down proteins into amino acids and peptides with varying molecular weights. The resulting products have been associated with improved techno-functional properties, digestibility, and reduced allergenicity (Fadimu et al., 2022; Meinlschmidt et al., 2016). Additionally, fermented plant-based proteins have superior organoleptic properties. For example, the bitterness of pea protein isolates was reduced after 24- and 48-h fermentation with Lactobacillus plantarum DSM-20174, L. perolens DSM-12744, L. fermentum DSM-20391, L. casei DSM-20011, Leuconostoc mesenteroides subsp. Cremoris DSM-20200, and Pediococcus pentosaceus DSM-20336 (García Arteaga et al., 2020b). Schlegel et al. (2019a) reported that lupin fermented by Lactobacillus brevis TMW 1.1326 had superior sensory properties when compared to the unfermented counterpart.
Lactic acid bacteria (LAB) including Lactobacillus, Staphylococcus, Pediococcus, Enterococcus, Staphylococcus, Micrococcus, and Leuconostoc are mostly used for plant-based proteins fermentation (Biscola et al., 2017; El Mecherfi et al., 2021; Lu et al., 2022; Meinlschmidt et al., 2016). Apart from these microorganisms, some fungi, such as Candida spp have also been used for fermenting plant-based proteins (Arte et al., 2015). The proteolytic system of these microorganisms comprises proteases (proteinases and peptidases), that cleave protein into peptides, which are further broken down to release peptides and amino acids (Kieliszek et al., 2021). Biscola et al. (2017) demonstrated that fermentation of soymilk using Enterococcus faecalis VB43 at 37 ℃ for 24 h, led to the reduction in immunoreactivity of allergenic proteins. Likewise, the allergenicity of gluten was reduced after fermentation with Lactococcus lactis LLGKC18 at 37 ℃ for 24 h (El Mecherfi et al., 2021). However, the complexity of biological materials, which might vary depending on the nature or geographical location and the processing conditions, contributes significantly to the limitations of fermentation. Additionally, the long fermentation time and non-uniformity of the final product were drawbacks of this method. Hence, enzymatic hydrolysis was developed to overcome these limitations.
The enzymatic hydrolysis process, which is widely used to modify proteins (Fig. 2), involves the direct addition of proteolytic enzymes for either cleaving the peptide bonds in proteins to produce short-chain peptides or amino acids with lower molecular weights protein or crosslinking polypeptide chains (Eckert et al., 2019; Olatunde & Benjakul, 2018). The type of protein and the high sensitivity of enzymes influence the modification process and, consequently, the size, amino acid sequence, digestibility, techno-functional properties, and in some cases, their bioactivities (Akharume et al., 2021). Proteolytic enzymes, e.g., papain, Alcalase, Neutrase, Flavorzyme, Trypsin, Thermolysin, Pepsin, Alcalase, Pronase, and Ficin are capable of splitting peptide bonds, consequently leading to the release of peptides of varying sizes and free amino acids from proteins (Olatunde & Benjakul, 2018; Zamani et al., 2017). The extent of enzymatic hydrolysis, specificity of enzyme action (Endo, Exo, Carbo and Aminopeptidase activities), ionic strength of the solution, duration of the treatment, and other environmental (pH, temperature, and pressure) conditions alter the mechanical properties (performance and behavior) of the proteins and hence, the technological application in the industry. Depending on the mechanism of action and catalytic site, proteases can be categorized as endopeptidases or exopeptidases, in which the latter cleaves the protein at the N- and C- terminal while the former cleaves within the polypeptide chain at specific or random sites. For example, peptide bonds made by basic residues such as lysine and arginine are cleaved by trypsin, while those formed with aromatic residues including phenylalanine, tyrosine, and tryptophan are cleaved by chymotrypsin (Akharume et al., 2021). The degree of hydrolysis (DH) measured by the ratio of the cleaved peptide bonds to the total number of peptide bonds is an important parameter in determining the efficacy of an enzyme for protein modification, which in turn depends on the yield and characteristics of the produced peptides. Aluko (2018) documented that the potential inactivation of hydrolytic enzymes occasioned by excessive reductions in pH during hydrolysis could be prevented by adding the appropriate base to achieve the optimum pH level. Several factors, such as the type of enzyme, the nature of the protein substrate, the enzyme-to-substrate ratio, process conditions (pH, temperature, and pressure), and presence/absence of proteolytic inhibitors, influence the enzymatic hydrolysis of proteins (Ahmadifard et al., 2016). Table 1 summarizes the commonly used enzymes, optimal conditions, substrates, and intended purpose in plant-based protein enzymatic modification.
In addition to protein hydrolysis, non-proteolytic enzymatic modification with transglutaminases (TG), peroxidases, laccases, sulfhydryl oxidases, and tyrosinase are used for protein build-up via protein–protein crosslinking and/or polymerization (Buchert et al., 2010). Glutamine and lysine residue in protein are linked by TG, the only commercially available food-grade crosslinking enzyme used for crosslinking plant-based proteins. This crosslinking modification has found application in improving the techno-functional properties of plant-based proteins. In the early 2000s, the efficacy of TG in producing tofu from soybean milk protein with improved textural properties and shelf stability was disclosed (Matsuura et al., 2000; Nonaka et al., 1991). Additionally, Baugreet et al. (2018) optimized the development of a novel restructured beef steak using rice protein, pea protein isolate, and lentil flour with TG. The restructured beef steak improved nutritional and chemosensory requirements for older adults. Similarly, Sah and Alisha (2018) demonstrated the efficacy of TG in the production of restructured meat from a mixture of soy proteins, which yielded a meat analog with improved textural properties. The modification of vegetable proteins using TG for improved textural properties in food applications has been extensively documented (Dube et al., 2007).
Several strategies/techniques have been developed and documented for assessing the extent of protein modification via crosslinking and peptide bond breakage. DH is used to measure the extent of hydrolysis during proteolytic enzymatic modification (Zamani et al., 2017). Nevertheless, FTIR, SDS-PAGE, chromatographic separation, and mass spectroscopic analyses of crosslinked protein products have also shown promising potential in assessing and confirming proteolytic and non-proteolytic enzymatic modifications. MALDI-TOF–MS is a very sensitive method for protein analysis. It is robust and relatively tolerant to salts and buffers. Extensive studies have been conducted on applying MALDI-TOF–MS in the analysis of crosslinked protein products (Pearson et al., 2002; Trester-Zedlitz et al., 2003; Wine et al., 2002). Li et al. (2021) applied fluorescence spectroscopy to evaluate the tertiary conformational changes in the microenvironment of aromatic amino acid residues following modification of potato protein crosslinking by laccase. The authors reported a reduction in the fluorescence signal and an increase in wavelength of maximum emission intensity (λmax), which correlated with the relocation of tryptophan residues to the hydrophilic solvent environment and the presence of compounds with shielding effect (Jia et al., 2019). Furthermore, SDS-PAGE has also shown potential in showing the patterns of enzymatically modified proteins as compared to the unmodified molecules. Kasera et al. (2015) confirmed the hydrolysis of high molecular weight proteins (> 29 kDa) in kidney beans, peanuts, and black gram by sequential treatment with Alcalase and Flavourzyme for a minimum of 30 min, suggesting the cleavage of most of the proteins into smaller peptides. Similarly, the reduction of Ara h 1 and Ara h 2 in peanut protein subjected to combined ultrasonic and enzymatic (α-chymotrypsin and trypsin) treatments were further confirmed by SDS–PAGE, via the disappearance of these bands after treatments (Li et al., 2013). Meinlschmidt et al. (2015) reported effective enzymatic hydrolysis using SDS-PAGE when soybean protein isolate was treated with Alcalase, pepsin, and papain as a panacea to degrade its major allergens.
Applications of Enzymatic Modification in Plant-Based Protein Utilization
Improving Techno-functional Properties
The techno-functional properties such as gelation, emulsification, foaming, water holding capacity, and others are mostly dependent on the degree of the protein’s secondary, tertiary, and quaternary structures (Singh et al., 2021) as well as the amino acid composition, molecular weight, and protein charge. The variations in these intrinsic protein structures generally influence hydrophobicity, flexibility, and other functionalities. Generally, plant-based proteins has been associated with poor techno-functional properties. However, enzymatic hydrolysis has proven to be an environmentally friendly method to circumvent this limitation (Görgüç et al., 2020). For instance, the emulsifying property of soy protein isolates (SPI) was enhanced by transforming the SPI into nanoparticles (80–170 nm) through enzymatic hydrolysis (Shen et al., 2020). Furthermore, Meinlschmidt et al. (2016) documented superior techno-functional properties for SPI hydrolysate prepared using Alcalase, Papain, and the enzyme combination (Papain and Flavourzyme) subjected to controlled liquid state fermentation using Actinomucor elegans DSM 1174, Rhizopus oryzae DSM 2200, and Lactobacillus perolens DSM 12,744.
Different enzyme actions affect the treated proteins' peptide chain length, electrostatic charges, and rigidity (α-helix to β-sheet and lower random coils in secondary structures). Such modifications can simultaneously influence the stability, surface hydrophobicity, and interfacial activity of protein formulations. The changes in the structural sub-units (α-helix, β-sheet, random coils) can also systematically be correlated with the change in extruded protein functional groups like -NH2, -COOH, and -CO-NH2, -OH, -C–O–C-, hydrocarbon (-CH2) chains/ units, etc. The application of enzymatic modification for improving the techno-functional properties of plant-based proteins has been summarized in Table 2.
Protein Solubility
Protein solubility has been documented as a prerequisite for other techno-functional properties. The solubility of any protein is influenced by the molecular weight, charge, amino acid composition, and conformational structure. For plant-based proteins, applications in the food industry are limited mainly by poor solubility. To circumvent this deficiency, enzymatic modification has been applied. The major mechanism for improved solubility has been associated with decreased molecular weight and protein structure changes. In Meinlschmidt et al. (2015), soybean protein hydrolysates were produced with Alcalase, Flavourzyme, Corolase 2TS, Pepsin, Corolase 7089, and Papain, and the solubility of the hydrolysate was evaluated at pH 4 and 7. The authors reported a high solubility for the hydrolysate compared to the unhydrolyzed protein regardless of the enzymes used, which was attributed to the lower molecular weight of peptides produced from enzymatic hydrolysis. However, solubility at pH 4 was highest in hydrolysates prepared with pepsin, while those prepared with Corolase 7089 showed the highest solubility at pH 7 (Meinlschmidt et al., 2015). Furthermore, Beaubier et al. (2021) attribuited the two fold increase in the solubility of sunflower protein hydrolysate prepared by Alcalase and Prolyve to the induced structural changes, via the decreased surface hydrophobicity, which exposed the polar groups initially buried in the native protein to the aqueous solvent.
Emulsifying Properties
The observed emulsifying property of proteins is due to their amphiphilic nature, which reduces the interfacial tension at the oil–water interface in oil-in-water (O/W) or water-in-oil (W/O) emulsion systems. The conformational changes in protein molecules after exposure to certain enzymatic actions could improve or sometimes reduce the emulsifying properties of plant-based proteins. The higher DH of protein molecules tends to produce lower molecular weight peptides, which might increase protein solubility but could have a less stearic effect at the oil–water interface. Hence, the enzymatic hydrolysis parameters must be carefully selected to produce polypeptides with optimum emulsifying properties. Furthermore, the efficacy of enzymatic treatment can also be increased by treatment with combined processing techniques. For instance, the extrusion pretreatment of SPI improved the emulsifying properties of subsequent protein hydrolysates obtained by pancreatin hydrolysis. The emulsion prepared with and without the pre-extrusion phenomenon showed lower emulsion stability due to the bridging flocculation and creaming phenomenon. Whereas higher emulsification ability was achieved with SPI pretreatment followed by enzymatic activity, which was associated with lower surface hydrophobicity, molecular chain length, and higher unfolding flexibility at the O/W interface (Akharume et al., 2021; Chen et al., 2011; Shekarforoush et al., 2022). Therefore, the DH, solubility, molecular weight, and composition of a protein hydrolysate, which are all influenced by the parameters of enzymatic hydrolysis have a direct impact on the emulsification properties of enzymatically treated plant-based proteins.
Foaming Properties
The use of plant-based proteins as foaming agents over synthetic agents in the food industry could provide health and economic benefits without compromising the desirable texture properties of foods (ice cream, coffee, whipped cream, cakes, beers, etc.). Being thermodynamically unstable, foams are affected by diffusion and coalescence, which are influenced by the interfacial properties of proteins. The optimum charge distribution, ionic strength, and interface distribution ability of protein isolates results in improved foaming ability of the hydrolysates. The foaming ability of protein isolates is relatively lowered in most protein isolates/ hydrolysate at pH near or at the isoelectric point due to reduced solubility. Nevertheless, the specificity of different enzymes used for hydrolysis primarily results in the differences in the foaming capability and stability of the produced hydrolysate. For example, the treatment of oat protein isolates using trypsin, and Alcalase resulted in faster and higher foaming ability when compared to the untreated protein isolates. However, these properties were higher in the hydrolysate prepared with trypsin even at pH 4, which is close to the isoelectric point of oat protein when compared to hydrolysis prepared with Alcalase (Brückner-Gühmann et al., 2018). Many authors have reported that DH has a lower impact on the foaming ability of plant-based proteins; however, extensive hydrolysis should be avoided to ensure that the resulting molecular weights of the peptides favor the formation of cohesive or viscous films at the water and air interfaces (lamella). Partial enzymatic hydrolysis of soy globulin (β-conglycinin, fraction 7S) using endopeptidase improved the interfacial adsorption (air–water), surface dilatational, and foaming properties of the protein hydrolysate (Ruíz-Henestrosa et al., 2007). Although higher enzymatic activity is reported to lower the mechanical strength of the released peptide units, interestingly, high concentrations of such protein hydrolysates (with low molecular weight peptide chains) may compensate for the poor foam stability of the individual small-size peptides and can find application in the food industry (Ivanova et al., 2018; Ruíz-Henestrosa et al., 2007; Urbizo-Reyes et al., 2019). To circumvent low foaming properties in protein hydrolysate, enzymatic hydrolysis has been combined with non-thermal technologies. For example, the forming properties of chia seed protein subjected to enzymatic combined with microwave treatment were higher than that treated with the conventional hydrolysis methods (Urbizo-Reyes et al., 2019).
Gelation
The gelling property, defined by the ability of the protein to form a three-dimensional continuous network that can immobilize or trap liquids, is one of the most important properties of proteins in promoting their application as a structuring agent in the food industry. Enzymatic modification via hydrolysis often may decrease plant-based proteins ' ability to make or form a gel with good mechanical strength (Galante et al., 2020). This is mostly associated with the lower chain length of the peptides, which reduces the strength of the 3-dimensional network structure. Recently, Chen and Campanella (2022) reported improved gelation properties for pea protein hydrolysate prepared using Alcalase, which was associated with the augmented surface hydrophobicity of peptides. This promoted hydrophobic-hydrophobic interaction resulting in stronger gel structure. However, enzymatic modification via crosslinking has improved the gelling properties of plant-based proteins. Zhang et al. (2022) demonstrated the gel formation with improved mechanical properties prepared from heat-denatured pea protein (10%, w/w) and different concentrations of pectin (0–1%, w/w) using transglutaminase (2%, w/w). Similarly, Djoullah et al. (2018) demonstrated the efficacy of transglutaminase in producing gel from denaturated pea albumin and globulin fractions. The authors reported that only the globulin fraction in a native or denatured state could make gels, whereas no gel was formed when the albumin fraction was used. The enzymatic crosslinking reaction was attributed to the polypeptides composition and protein conformation. The gelation properties of the canola protein isolates were enhanced by enzymatic crosslinking using transglutaminase. However, the gel strength was dependent on the protein concentration, enzyme concentration, and treatment temperature (Pinterits & Arntfield, 2008).
Enhanced Digestibility
Despite the increasing demand for plant-based proteins, researchers are working assiduously to confirm that the high protein content of most plant sources is available to humans in terms of protein digestibility and bio-accessibility of essential amino acids (Joshi & Varma, 2016; Pal et al., 2016). Proteins need to be digested into amino acids and small peptides to be useful for the growth and maintenance of the human body. However, the poor digestibility of plant-based proteins, which is mediated by complex molecular weight, amino acid sequence, and the presence of antinutritional factors, has contributed to the limited food applications. Enzymatic modification has shown promising potential in influencing some properties, such as improved amino acid profile, conformation, molecular weights, and antinutritional factors, thus enhancing the digestibility of plant-based proteins (Tapal & Tiku, 2019).
The increased digestibility of plant-based proteins is based on the enzymatic breakdown of peptide bonds to produce peptides with lower molecular weight. Venuste et al. (2013) investigated the efficacy of enzymatic hydrolysis using Protamex, Alcalase, Neutrase, or Flavourzyme to improve pumpkin meal's protein digestibility. The authors reported significant increases in the amino acid score, biological value, essential amino acid/total amino acid ratio (EAA/TAA), and protein efficiency ratio (PER) of pumpkin meal when Alcalase was used for hydrolysis. Dias et al. (2010) reported that enzyme hydrolysis of bean powder with trypsin increased protein digestibility from 54.4% for the untreated flour to 81.6%, which favored absorption of amino acids and short-chain peptides.
The improvement of plant-based protein digestibility via reduced antinutritional factors caused by enzymatic modification has also been investigated. Condensed tannins in 19 traditional Italian legumes belonging to Lathyrus sativus, Pisum sativum, Cicer arietinum, Phaseolus vulgaris, and Lens culinaris species were reduced via fermentation at 30 °C for 24 h with Lactobacillus plantarum C48 and Lactobacillus brevis AM7 when compared to the unfermented counterpart (Curiel et al., 2015). Furthermore, the fermentation of favabean with Lactobacillus plantarum VTT E-133328 reduced the contents of vicine and convicine, trypsin inhibitor activity, and condensed tannins and increased the amount of free fatty acids, essential amino acids, and γ-aminobutyric acid in the fermented product (Coda et al., 2015). Pontonio et al. (2020) documented increased protein digestibility for hemp seed subjected to fermentation using Lactobacillus plantarum 18S9 and Leuconostoc mesenteroides 12MM1. Similarly, phytic acid and trypsin inhibitory activity levels were lower in grass pea flour fermented with lactobacillus plantarum DSM 20,174 than in the unfermented counterpart (Starzynska-Janiszewska & Stodolak, 2011). Furthermore, raffinose, phytic acid, trypsin inhibitory activity, and condensed tannins were decreased in sprouted wheat, barley, chickpea, lentil, and quinoa grains fermented with Lactobacillus sanfranciscensis DE9, Lactobacillus plantarum 1A7, and Lactobacillus rossiae LB5 (Montemurro et al., 2019).
Apart from fermentation, proteolysis has also significantly reduced antinutritional factors in plant-based proteins. In the work of Pontonio et al. (2020), food-grade protease and xylanase were used to hydrolyze hemp seed proteins. The authors reported the release of protein fractions with high biological value and increased digestibility. Phytic acid, raffinose, and condensed tannins were decreased while digestibility was increased in hemp, chickpea, and milling by-products after enzymatic treatment using xylanase and proteases (Schettino et al., 2019). Similarly, higher protein digestibility was documented for wheat bran subjected to the combined treatment of starter cultures (Lactobacillus brevis E-95612 and Candida humilis E-96250) and cell wall degrading enzymes (Depol 761P and Viscoferm) (Arte et al., 2015). Increased protein digestibility and absorption were documented for hydrolyzed cowpea proteins using Alcalase or Flavourzyme, which was comparable to hydrolyzed casein (Segura-Campos et al., 2012).
Alleviating Allergenicity
Plant-based protein allergens, which are mostly more life-threatening when compared to other food allergens, affecting at least 1 in 100 adults and 1 in 10 children (Mills & Shewry, 2004), are one of the major limitations in plant-based proteins applications, particularly in the food and pharmaceutical industries. Undoubtfully, several interventions, including thermal (boiling, roasting, microwave, and ohmic heating) and non-thermal (enzymatic modification, cold plasma, high-pressure processing, ultrasound, radiation) treatments have been implemented to circumvent this limitation, which yielded plant-based protein products with reduced levels or no allergens (Fadimu et al., 2022). In addition, the enzymatic modification process, either by natural fermentation or enzymatic hydrolysis alleviates plant-based protein allergens via the degradation of allergenic epitopic sequences.
Natural/non-spontaneous fermentation has been used for alleviating allergens in plant-based proteins. In the study by Yang et al. (2018), soybean meal was fermented with a mixture of Bacillus subtilis CICC No. 20641, Lactobacillus casei CGMCC No. 1. 539, and yeast at a ratio of 2:1:1 (v/v/v) using solid-state fermentation at 30 °C for 3 days. The authors reported a significant reduction in the allergen of soybean meal after fermentation, which was attributed to the breakdown of allergenic epitopic sequences of β-conglycinin and glycinin into smaller molecular-weight polypeptides. IgE-binding proteins (albumins/globulins and gliadins mainly) in wheat and rye dough were degraded after fermentation with several species of LAB, including Lactobacillus brevis 14G, L. sanfranciscensis 7A, L. alimentarius 15 M, and L. hilgardii 51B (Rizzello et al., 2006). In addition, Enterococcus faecalis isolated from Tunisian fermented wheat was effective in hydrolyzing gliadin, an allergen in wheat (M’hir et al., 2008). Furthermore, gliadins and glutenins in wheat were decreased after fermentation with 9 strains of Enterococcus faecalis and Lactococcus lactis. However, L. lactis LLGKC18 showed the highest degradation of the main gluten allergenic proteins (El Mecherfi et al., 2021). The immunoreactivity of β-conglycinin (7S) and glycinin (11S) in soymilk was reduced after fermentation with Enterococcus faecalis VB43 (Biscola et al., 2017). Soymilk fermented with a cocktail of L. brevis CICC 23,474 and L. brevis CICC 23,470 had lower antigenicity and potential allergenicity than the unfermented counterpart (Lu et al., 2022).
The impact of enzymatic hydrolysis on the allergenicity of plant-based proteins has also been elucidated. Two major peanut allergens (Ara h 1 and Ara h 2) in roasted peanut kernels and peanut butter were subjected to enzymatic hydrolysis using chymotrypsin and trypsin either individually or in combination significantly reduced, particularly when the combined enzymes treatment was used (Yu et al., 2011, 2013). Additionally, Cabanillas et al. (2010) documented proteolytic destruction of IgE-binding epitopes in lentil protein hydrolyzed with Alcalase and Flavourzyme. Shi et al. (2013) documented the efficacy of Alcalase, pepsin, and Flavourzyme under different conditions in reducing allergens in peanut flour. The authors reported a lower IgE binding capacity regardless of the peptidase used for hydrolysis compared to non-hydrolyzed counterparts. IgE binding fractions in the insoluble protein extract from kidney beans, black gram, and peanuts were degraded to varying degrees after hydrolysis with Alcalase and Flavourzyme (Kasera et al., 2015).
The enzyme used for hydrolysis influences its efficacy in reducing the allergenicity of plant-based proteins. Allergenic gliadins in wheat flour hydrolyzed with Alcalase from Bacillus licheniformisas, and papain from the latex of papaya fruit was degraded more effectively when compared to the use of Flavourzyme, pepsin, trypsin, or chymotrypsin for hydrolysis, which is associated to the differences in enzymes proteolytic specificity and hence nature of the peptide products (Li et al., 2016). Different enzymes, including alkaline protease, GC106, papain, Bromelain, Collupulin, Flavourzyme, and Protamex showed promising potential in reducing allergenicity in buckwheat proteins, in which alkaline protease, classified as serine peptidase, displayed the highest efficacy (Sung et al., 2014). Cabanillas et al. (2012) documented a higher reduction in IgE reactivity for roasted peanut soluble protein hydrolyzed with Alcalase than those hydrolyzed with Flavourzyme, which was related to the breakdown of peptide bonds at nonterminal amino acids by Alcalase.
To further increase the efficacy of enzymatic hydrolysis in reducing allergenicity in plant-based proteins, combined treatments with other non-thermal technologies have been documented. These treatments are effective because they induce exposure to enzyme cleavage sites. To loosen the peanut kernel structure, roasted peanut kernels were pretreated with ultrasound for 1 h at 50 Hz before hydrolysis with Alcalase using different concentrations (Yu et al., 2015). The authors reported decreased Ara h 1 and Ara h 2 concentrations in both insoluble and soluble peanut portions compared to the control (untreated peanuts). Furthermore, Li et al. (2013) investigated the efficacy of ultrasonic-assisted enzymatic treatment in reducing allergens in peanuts. The authors reported a significant reduction in Ara h 1 and Ara h 2, as well as lowered IgE binding for roasted peanut kernel when subjected to the combined treatment of sonication (1–5 h) and hydrolysis using trypsin or chymotrypsin at different enzyme/peanut ratios (0.0- 0.3% w/w).
Production of Bioactive Peptides with Health-Beneficial Properties
A broad range of health benefits or bioactivities such as antimicrobial, antioxidant, anticancer, antihypertensive, hypocholesterolemic, immunomodulatory, and opioid-like properties have been studied and reported for plant-based proteins derived bioactive peptides. However, these biological activities are significantly influenced by the kind of peptide, especially the nature of the amino acid, their sequence, and molecular weight, which are all determined by the enzymes (types and concentration) as well as other processing parameters, including pH, temperature, and time used during enzymatic hydrolysis. A summary of the bioactive peptides derived from plant-based proteins using enzymatic hydrolysis is presented in Table 3.
Antioxidant and Anti-inflammatory Activity
Several clinical investigations have evaluated the relationship between the oxidative stress triggered by the reactive oxygen species (ROS) and the development of different chronic diseases, including diabetes, inflammation, rheumatoid arthritis, and cancer (Ibrahim et al., 2018). Therefore, dietitians, clinicians, and health organizations highly recommend the consumption of an antioxidant-rich diet. Currently, the food industry is also interested in natural ingredients with antioxidant properties due to their ability to delay and/or inhibit oxidative degeneration of macromolecules in food components, like lipids and proteins, in addition to enhanced shelf-life and quality of the food (Nwachukwu & Aluko, 2019). Bioactive peptides show varying antioxidant properties, which are dependent on their conformational structure, amino acid composition, and hydrophobicity. For instance, among the 20 amino acids studied by Xu et al. (2017), histidine, tryptophan, methionine, lysine, arginine, cysteine, and tyrosine exhibited stronger antioxidant activities than others. Some of the works carried out on the antioxidant properties of bioactive peptides are shown in Table 3. The protein hydrolysate produced from defatted peanut kernel protein was demonstrated to exhibit antioxidant properties. The study found that the pea protein hydrolysate derived after treatment with Esperase showed the strongest antioxidant capacity regarding linolenic acid peroxidation when compared to other proteases, including protease A, protease N, pepsin, and Neutrase (Samtiya et al., 2021). Recent work by Idowu et al. (2021) evaluated the sesame seed hydrolysate generated using pepsin and pancreatic enzymes for their antioxidant properties. The results established that the highest hydroxyl radical scavenging and metal-binding activity was found with the sesame seed protein hydrolysate in contrast with the unhydrolyzed protein. IQDKEGIPPDQQR, a bioactive peptide derived from lupin, was evaluated using a macrophage inflammatory cytokine production assay. The outcome revealed that the peptide suppressed monocyte chemoattractant protein-1, IL-1, IL-6, and TNF-α synthesis by 40.43, 44.70, 38.52., and 51.20%, respectively (Gao et al., 2020).
Antihypertensive Activity
Hypertension is one of the major public health concerns in the world. This disease is diagnosed when systolic and diastolic blood pressure rise beyond > 140 and 190 mmHg, respectively (Oparil et al., 2018). Diabetes, obesity, and kidney diseases are well-established risk factors for hypertension. Health conditions involving disorders of the renin-angiotensin system (RAS) are also contributing factors in the development of hypertension. Within the RAS, the angiotensin-converting enzyme (ACE) plays a crucial role in converting inactive angiotensin I to vasoactive angiotensin II (Fig. 3). Consequently, extreme activities of ACE can result in strong vasoconstrictions with weak vasodilation, thus developing high blood pressure. The suppression of ACE activity by drugs has proven to be an effective pharmacological strategy for decreasing blood pressure. Nonetheless, the side effects linked to the present-day antihypertensive ACE-inhibitory drugs could bring about compromised health conditions for the patient with significant negative effects on life expectancy or quality in addition to the associated high cost of healthcare (Samtiya et al., 2021). Therefore, there is a rising interest in applying natural ACE inhibitors such as plant-based protein derived bioactive peptides in replacing or complementing drugs as antihypertensive agents (Aluko, 2015). Ma et al. (2019) studied the exceptional ACE-inhibiting peptides produced from Ginko Biloba seed. Using LC–MS/MS, they detected three unique ACE-inhibitory peptides, including TNLDWY (IC50 = 1.932 mM), RADFY (IC50 = mM), and RVFDGAV (IC50 = 1.006 mM). Aondona et al. (2021) evaluated the in vitro antihypertensive properties of sesame seed protein hydrolysate and its fractionated peptides using pepsin and pancreatin. The results demonstrated that the < 1 kDa peptide fraction was the most effective (75–85%) renin inhibitor, suggesting sesame seed protein hydrolysate food products as potential antihypertensive agents. Other antihypertensive evaluations of peptides from plant sources have been carried out on peas (Li & Aluko, 2010), hemp seed (Girgih et al., 2014), and wheat bran (Zou et al., 2020) protein hydrolysates.
A diagrammatic representation of the blood pressure regulating renin-angiotensin system (RAS) mechanism with the possible molecular targets (renin and angiotensin-converting enzyme, ACE) for bioactive peptides. The inhibition of renin decreases the rate of ACE-dependent synthesis of angiotensin-II (Samtiya et al., 2021; Udenigwe & Aluko, 2012)
Anticancer Activity
Bioactive peptides possess cytotoxic activity in various cancer cell lines and could be applied as anticancer agents with minimum or no adverse effects. Additionally, these peptides could function as carriers of cytotoxic agents in targeting cancer cells directly without harming normal cells (Boohaker et al., 2012). Peptides can inhibit specific molecular signaling pathways of cancer cells associated with the mechanism of oncogenesis, cancer stem cell self-revitalization, and pathways of differentiation. Previous reports have established the inhibitory activities of adzuki bean, mung bean, black soybean, and soybean meal proteins against cancer cells (ovarian cancer cell line SKOV3 and hepatocellular carcinoma cells SMMC-7721) within the range of 200—600 µg/mL (Chen et al., 2017). Another study assessed and reported the anticancer properties of quinoa peptides, such as IFQEYI, DVYSPEAG, RELGEWGI (F-3), DKDYPK (F-2), and LWREGM (F-1), using human colorectal cancer cell lines (Vilcacundo et al., 2018). Taniya et al. (2020) also investigated the anticancer activity of an amaranth seed protein hydrolysate. The hydrolysate was produced through simulated gastrointestinal tract digestion and was further studied for anticancer activity using an in vitro breast cancer cell line. The outcome established that the hydrolyzed amaranth sample suppressed the growth of cells with a 50% growth inhibition concentration of 48.3 µg/mL. A previous work reported on the antitumor properties of an olive seed-derived peptide (LLPSY) confirmed that this peptide exhibited antiproliferative activity in a dose-dependent manner, using MDA-MB-468 and PC-3 cancer cells (Vásquez-Villanueva et al., 2018).
Antidiabetic Activity
Various strategies have been successfully applied in managing diabetes in the last few years. Bioactive protein hydrolysates from different edible seeds have demonstrated antidiabetic effects with promising potential as an alternative therapy to synthetic drugs. Peptides can impart hypoglycemic effects via one or more pathways, including the inhibition of dipeptidyl peptidase IV (DPP-IV), leading to a lowered blood glucose level and a peak in the physiological insulin level (Samtiya et al., 2021). Aside from the inhibition of DPP-IV, the consumption of natural ingredients embedded with compounds that inhibit α-glucosidase and α-amylase is another important approach to controlling diabetes. This is because α-glucosidase and α-amylase enzymes mainly take part in the digestion of dietary carbohydrates. Olagunju et al. (2021) reported pigeon pea peptides' in vitro antidiabetic activity. In their study, pigeon pea protein was hydrolyzed with Thermoase and the hydrolysate was divided into different peptide fractions (< 1, 1–3, 3–5, 5–10, and > 10 kDa) by ultrafiltration. The in vitro results observed that all the peptide fractions suppressed α-amylase and α-glucosidase activities, with the 3–5 and > 10 kDa peptides being the most effective. Lammi et al. (2016) observed that IAVPTGVA (soybean) and LTFPGSAED) (lupin) are the most potent inhibitors of dipeptidyl-peptidase IV with IC50 values of 106 and 228 μM, respectively. Other bioactive peptides and protein hydrolysates with antidiabetic activities are included in Table 3.
Mineral-Binding Activity
Minerals, including iron, zinc, and calcium, are significant inorganic micronutrients that play important roles in the management of human health (Silva et al., 2019). Therefore, mineral deficiencies are a serious public health issue globally. For example, iron is a crucial part of erythrocyte hemoglobin, muscle myoglobin, and liver ferritin and functions as a cofactor for various cellular enzymes. Ion-binding peptides, especially from plant seeds, can be used as potential carriers of mineral supplements to prevent mineral deficiencies (Caetano-Silva et al., 2021). Different organic compounds, such as amino acid binders, that form complexes with metal ions have decreased the interactions of minerals with food matrices. Currently, a wide range of metal-chelating peptides has been produced and identified from several edible seed proteins (Cui et al., 2018). Wang et al. (2018) demonstrated that a pentapeptide FVDVT from wheat germ protein hydrolysate exhibited significantly (86%) better calcium-binding activity than crude hydrolysate. Lv et al. (2017) also reported the separation of iron-binding peptides VEDELVAVV and LAGNPDDEFRPQ from defatted walnut flakes. Another study by Chunkao et al. (2020) on mung beans confirmed the iron-chelating ability of mung bean protein-derived peptides.
Limitations of Enzymatic Modification
The bitterness caused by the exposure to the hydrophobic amino acid residues such as isoleucine, tyrosine, phenylalanine, and tryptophan via the alteration of the structural integrity of proteins by proteolytic enzymes is one of the major limitations of enzymatic modification (Akharume et al., 2021; Idowu & Benjakul, 2019). Several factors, including hydrophobicity, DH, molecular weight, the position of proline residues, the type of enzymes used, and an amino acid sequence, influence the bitterness of protein hydrolysates (Yarnpakdee et al., 2015). The bitterness of plant-based protein has been reported. Großmann et al. (2020) documented varying degree of bitterness for hydrolysate prepared from soy, canola protein, and pea protein using Flavourzyme 1000L, DeltazymAPS-M-FG, Protease P, Promod278, Peptidase R ProteAX-K, and “Amano” 6SD. Similarly, pea protein hydrolysate prepared using Flavourzyme, α-Chymotrypsin, papain, Trypsin and Alcalase show different intensities of bitterness (Humiski & Aluko, 2007).
Bitterness in hydrolysate is mostly evaluated by trained sensory panelists using the hedonic scale sensory evaluation. Sensory panelist are trained with different intensities of standard solution such as caffeine and quinine HCl (Idowu & Benjakul, 2019). In addition, taste dilution analysis (TDA) based on the determination of the relative taste thresholds of compounds in serial dilutions of samples has also proven to be an effective method for the determination of bitterness in plant-based protein hydrolysate (Seo et al., 2008).
Most reports have shown that the DH of protein enzymatic hydrolysis process is directly proportional to the bitternesss of the resulting hydrolysate. However, the enzymatic hydrolysis with a DH < 10% produces peptides that exhibit improved techno-functional properties and low or no bitterness (Akharume et al., 2021; Eckert et al., 2019). In addition, several interventions, such as treatment with alcohol, activated carbon, conjugation via Maillard reaction, chromatographic separation, and plastein reaction, have been developed to mitigate the bitterness of protein hydrolysates (Idowu & Benjakul, 2019).
Conclusions
To meet the demand for protein with improved quality and functionalities, it is imperative to employ chemical, physical, or biological modification methods, which can alter the structure, chemical, surface-active, and biophysical properties of plant-based proteins as a panacea for improving digestibility, functionalities, sensory property, and allergenicity. Simply put, modification of plant-based proteins is crucial in augmenting their industrial applications. Enzymatic modification has shown promising potential in addressing the low digestibility, poor techno-functional properties, and inherent allergenicity of plant-based proteins. In addition, the enzymatic modification of plant-based proteins has been exploited in developing functional ingredients with beneficial health properties, including antioxidants, antimicrobial, anticancer, antiproliferative, antidiabetic properties, etc., which also serve as a value-added approach. The high sensitivity, reproducibility, low cost of production, and environmental friendliness of enzymatic hydrolysis give this method leverage when compared to other protein modification methods. Although this is true for most plant-based proteins, commercialization and optimization of the process conditions (type of enzyme, concentration, hydrolysis time, protein source) are imperative to achieve an improved protein ingredient. Otherwise, enzymatic modification may unintentionally cause undesirable changes such as suppressed techno-functionalities, reduced nutritional quality, and loss of desirable organoleptic properties.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Ahmadifard, N., Murueta, J. H. C., Abedian-Kenari, A., Motamedzadegan, A., & Jamali, H. (2016). Comparison the effect of three commercial enzymes for enzymatic hydrolysis of two substrates (rice bran protein concentrate and soy-been protein) with SDS-PAGE. Journal of Food Science and Technology, 53(2), 1279–1284.
Akharume, F. U., Aluko, R. E., & Adedeji, A. A. (2021). Modification of plant proteins for improved functionality: A review. Comprehensive Reviews in Food Science and Food Safety, 20(1), 198–224.
Al-Ruwaih, N., Ahmed, J., Mulla, M. F., & Arfat, Y. A. (2019). High-pressure assisted enzymatic proteolysis of kidney beans protein isolates and characterization of hydrolysates by functional, structural, rheological and antioxidant properties. LWT-Food Science and Technology, 100, 231–236.
Aluko, R. E. (2008). Determination of nutritional and bioactive properties of peptides in enzymatic pea, chickpea, and mung bean protein hydrolysates. Journal of AOAC International, 91(4), 947–956.
Aluko, R. E. (2015). Structure and function of plant protein-derived antihypertensive peptides. Current Opinion in Food Science, 4, 44–50.
Aluko, R. E. (2018). Food protein-derived peptides: Production, isolation, and purification. In R. Y. Yada (Ed.), Proteins in Food Processing (Second Edition) (pp. 389–412). Woodhead Publishing.
Aondona, M. M., Ikya, J. K., Ukeyima, M. T., Gborigo, T. W. J., Aluko, R. E., & Girgih, A. T. (2021). In vitro antioxidant and antihypertensive properties of sesame seed enzymatic protein hydrolysate and ultrafiltration peptide fractions. Journal of Food Biochemistry, 45(1), e13587.
Arte, E., Huang, X., Nordlund, E., & Katina, K. (2019). Biochemical characterization and technofunctional properties of bioprocessed wheat bran protein isolates. Food Chemistry, 289, 103–111.
Arte, E., Rizzello, C. G., Verni, M., Nordlund, E., Katina, K., & Coda, R. (2015). Impact of enzymatic and microbial bioprocessing on protein modification and nutritional properties of wheat bran. Journal of Agricultural and Food Chemistry, 63(39), 8685–8693.
Bandara, N., Akbari, A., Esparza, Y., & Wu, J. (2018). Canola protein: A promising protein source for delivery, adhesive, and material applications. Journal of the American Oil Chemists’ Society, 95(8), 1075–1090.
Barać, M., Čabrilo, S., Pešić, M., Stanojević, S., Pavlićević, M., Maćej, O., & Ristić, N. (2011). Functional properties of pea (Pisum sativum, L.) protein isolates modified with chymosin. International Journal of Molecular Sciences, 12(12), 8372–8387.
Baugreet, S., Kerry, J. P., Brodkorb, A., Gomez, C., Auty, M., Allen, P., & Hamill, R. M. (2018). Optimisation of plant protein and transglutaminase content in novel beef restructured steaks for older adults by central composite design. Meat Science, 142, 65–77.
Beaubier, S., Albe-Slabi, S., Aymes, A., Bianeis, M., Galet, O., & Kapel, R. (2021). A rational approach for the production of highly soluble and functional sunflower protein hydrolysates. Foods, 10(3), 664.
Biscola, V., de Olmos, A. R., Choiset, Y., Rabesona, H., Garro, M. S., Mozzi, F., Chobert, J.-M., Drouet, M., Haertlé, T., & Franco, B. (2017). Soymilk fermentation by Enterococcus faecalis VB43 leads to reduction in the immunoreactivity of allergenic proteins β-conglycinin (7S) and glycinin (11S). Beneficial Microbes, 8(4), 635–643.
Boohaker, R. J., Lee, M. W., Vishnubhotla, P., Perez, J. M., & Khaled, A. R. (2012). The use of therapeutic peptides to target and to kill cancer cells. Current Medicinal Chemistry, 19(22), 3794–3804.
Boutureira, O., & Bernardesa. J., G. (2015). Advances in chemical protein modification. Chemical Reviews, 115(5), 2174–2195.
Brückner-Gühmann, M., Heiden-Hecht, T., Sözer, N., & Drusch, S. (2018). Foaming characteristics of oat protein and modification by partial hydrolysis. European Food Research and Technology, 244(12), 2095–2106.
Buchert, J., Ercili Cura, D., Ma, H., Gasparetti, C., Monogioudi, E., Faccio, G., Mattinen, M., Boer, H., Partanen, R., & Selinheimo, E. (2010). Crosslinking food proteins for improved functionality. Annual Review of Food Science and Technology, 1, 113–138.
Budseekoad, S., Yupanqui, C. T., Sirinupong, N., Alashi, A. M., Aluko, R. E., & Youravong, W. (2018). Structural and functional characterization of calcium and iron-binding peptides from mung bean protein hydrolysate. Journal of Functional Foods, 49, 333–341.
Cabanillas, B., Pedrosa, M. M., Rodríguez, J., Gonzalez, A., Muzquiz, M., Cuadrado, C., Crespo, J. F., & Burbano, C. (2010). Effects of enzymatic hydrolysis on lentil allergenicity. Molecular Nutrition and Food Research, 54(9), 1266–1272.
Cabanillas, B., Pedrosa, M. M., Rodriguez, J., Muzquiz, M., Maleki, S. J., Cuadrado, C., Burbano, C., & Crespo, J. F. (2012). Influence of enzymatic hydrolysis on the allergenicity of roasted peanut protein extract. International Archives of Allergy and Immunology, 157(1), 41–50.
Caetano-Silva, M. E., Netto, F. M., Bertoldo-Pacheco, M. T., Alegría, A., & Cilla, A. (2021). Peptide-metal complexes: Obtention and role in increasing bioavailability and decreasing the pro-oxidant effect of minerals. Critical Reviews in Food Science and Nutrition, 61(9), 1470–1489.
Calderón-chiu, C., Calderón-santoyo, M., Damasceno-gomes, S., & Ragazzo-Sánchez, J. A. (2021). Use of jackfruit leaf (Artocarpus heterophyllus L.) protein hydrolysates as a stabilizer of the nanoemulsions loaded with extract-rich in pentacyclic triterpenes obtained from Coccoloba uvifera L. leaf. Food Chemistry: X, 12, 100138.
Campbell-Platt, G. (1994). Fermented foods—a world perspective. Food Research International, 27(3), 253–257.
Chen, D., & Campanella, O. H. (2022). Limited enzymatic hydrolysis induced pea protein gelation at low protein concentration with less heat requirement. Food Hydrocolloids, 128, 107547.
Chen, L., Chen, J., Ren, J., & Zhao, M. (2011). Modifications of soy protein isolates using combined extrusion pre-treatment and controlled enzymatic hydrolysis for improved emulsifying properties. Food Hydrocolloids, 25(5), 887–897.
Chen, Z., Wang, J., Liu, W., & Chen, H. (2017). Physicochemical characterization, antioxidant and anticancer activities of proteins from four legume species. Journal of Food Science and Technology, 54(4), 964–972.
Chunkao, S., Youravong, W., Yupanqui, C. T., Alashi, A. M., & Aluko, R. E. (2020). Structure and function of mung bean protein-derived iron-binding antioxidant peptides. Foods, 9(10), 1406.
Coda, R., Melama, L., Rizzello, C. G., Curiel, J. A., Sibakov, J., Holopainen, U., Pulkkinen, M., & Sozer, N. (2015). Effect of air classification and fermentation by Lactobacillus plantarum VTT E-133328 on faba bean (Vicia faba L.) flour nutritional properties. International Journal of Food Microbiology, 193, 34–42.
Cui, P., Lin, S., Jin, Z., Zhu, B., Song, L., & Sun, N. (2018). In vitro digestion profile and calcium absorption studies of a sea cucumber ovum derived heptapeptide–calcium complex. Food and Function, 9(9), 4582–4592.
Curiel, J. A., Coda, R., Centomani, I., Summo, C., Gobbetti, M., & Rizzello, C. G. (2015). Exploitation of the nutritional and functional characteristics of traditional Italian legumes: The potential of sourdough fermentation. International Journal of Food Microbiology, 196, 51–61.
del Mar Yust, M., Pedroche, J., del Carmen Millán-Linares, M., Alcaide-Hidalgo, J. M., & Millán, F. (2010). Improvement of functional properties of chickpea proteins by hydrolysis with immobilised Alcalase. Food Chemistry, 122(4), 1212–1217.
Dias, D. R., Abreu, C. M. P. D., Silvestre, M. P. C., & Schwan, R. F. (2010). In vitro protein digestibility of enzymatically pre-treated bean (Phaseolus vulgaris L.) flour using commercial protease and Bacillus sp. protease. Food Science and Technology, 30(1), 94–99.
Djoullah, A., Husson, F., & Saurel, R. (2018). Gelation behaviors of denaturated pea albumin and globulin fractions during transglutaminase treatment. Food Hydrocolloids, 77, 636–645.
Dube, M., Schäfer, C., Neidhart, S., & Carle, R. (2007). Texturisation and modification of vegetable proteins for food applications using microbial transglutaminase. European Food Research and Technology, 225(2), 287–299.
Eckert, E., Han, J., Swallow, K., Tian, Z., Jarpa-Parra, M., & Chen, L. (2019). Effects of enzymatic hydrolysis and ultrafiltration on physicochemical and functional properties of faba bean protein. Cereal Chemistry, 96(4), 725–741.
El Mecherfi, K.-E., Lupi, R., Cherkaoui, M., Albuquerque, M. A., Todorov, S. D., Tranquet, O., Klingebiel, C., Rogniaux, H., Denery-Papini, S., & Onno, B. (2021). Fermentation of gluten by Lactococcus lactis LLGKC18 reduces its antigenicity and allergenicity. Probiotics and Antimicrobial Proteins, 1–13.
Fadimu, G. J., Olatunde, O. O., Bandara, N., & Truong, T. (2022). Reducing allergenicity in plant-based proteins. In B. B. S. Prakash, & C. Gaiani (Ed.), Engineering Plant-based Food Systems.).
Galante, M., De Flaviis, R., Boeris, V., & Spelzini, D. (2020). Effects of the enzymatic hydrolysis treatment on functional and antioxidant properties of quinoa protein acid-induced gels. LWT-Food Science and Technology, 118, 108845.
Gao, Y., Zhang, X., Ren, G., Wu, C., Qin, P., & Yao, Y. (2020). Peptides from extruded lupin (Lupinus albus L.) regulate inflammatory activity via the p38 MAPK signal transduction pathway in RAW 264.7 cells. Journal of Agricultural and Food Chemistry, 68(42), 11702–11709.
Garcia-Mora, P., Penas, E., Frias, J., Zielinski, H., Wiczkowski, W., Zielinska, D., & Martinez-Villaluenga, C. (2016). High-pressure-assisted enzymatic release of peptides and phenolics increases angiotensin converting enzyme I inhibitory and antioxidant activities of pinto bean hydrolysates. Journal of Agricultural and Food Chemistry, 64(8), 1730–1740.
García Arteaga, V., Apéstegui Guardia, M., Muranyi, I., Eisner, P., & Schweiggert-Weisz, U. (2020a). Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innovative Food Science and Emerging Technologies, 65, 102449.
García Arteaga, V., Leffler, S., Muranyi, I., Eisner, P., & Schweiggert-Weisz, U. (2020b). Sensory profile, functional properties and molecular weight distribution of fermented pea protein isolate. Current Research in Food Science, 4, 1–10.
Gençdağ, E., Görgüç, A., & Yılmaz, F. M. (2021). Recent advances in the recovery techniques of plant-based proteins from agro-industrial by-products. Food Reviews International, 37(4), 447–468.
Girgih, A. T., Alashi, A. M., He, R., Malomo, S. A., Raj, P., Netticadan, T., & Aluko, R. E. (2014). A novel hemp seed meal protein hydrolysate reduces oxidative stress factors in spontaneously hypertensive rats. Nutrients, 6(12), 5652–5666.
Görgüç, A., Gençdağ, E., & Yılmaz, F. M. (2020). Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments – A review. Food Research International, 136, 109504.
Großmann, K. K., Merz, M., Appel, D., Thaler, T., & Fischer, L. (2020). Impact of peptidase activities on plant protein hydrolysates regarding bitter and umami taste. Journal of Agricultural and Food Chemistry, 69(1), 368–376.
Guan, H., Diao, X., Jiang, F., Han, J., & Kong, B. (2018). The enzymatic hydrolysis of soy protein isolate by Corolase PP under high hydrostatic pressure and its effect on bioactivity and characteristics of hydrolysates. Food Chemistry, 245, 89–96.
Hewage, A., Olatunde, O. O., Nimalaratne, C., Malalgoda, M., Aluko, R. E., & Bandara, N. (2022). Novel Extraction technologies for developing plant protein ingredients with improved functionality. Trends in Food Science and Technology, 129, 492–511.
Humiski, L., & Aluko, R. (2007). Physicochemical and bitterness properties of enzymatic pea protein hydrolysates. Journal of Food Science, 72(8), S605–S611.
Ibrahim, H. R., Isono, H., & Miyata, T. (2018). Potential antioxidant bioactive peptides from camel milk proteins. Animal Nutrition, 4(3), 273–280.
Idowu, A. O., Famuwagun, A. A., Fagbemi, T., & N, & Aluko, R. E. (2021). Antioxidant and enzyme-inhibitory properties of sesame seed protein fractions and their isolate and hydrolyzate. International Journal of Food Properties, 24(1), 780–795.
Idowu, A. T., & Benjakul, S. (2019). Bitterness of fish protein hydrolysate and its debittering prospects. Journal of Food Biochemistry, 43(9), e12978.
Ismail, B. P., Senaratne-Lenagala, L., Stube, A., & Brackenridge, A. (2020). Protein demand: Review of plant and animal proteins used in alternative protein product development and production. Animal Frontiers, 10(4), 53–63.
Ivanova, P., Kalaydzhiev, H., Dessev, T. T., Silva, C. L. M., Rustad, T., & Chalova, V. I. (2018). Foaming properties of acid-soluble protein-rich ingredient obtained from industrial rapeseed meal. Journal of Food Science and Technology, 55(9), 3792–3798.
Jia, N., Zhang, F., Liu, Q., Wang, L., Lin, S., & Liu, D. (2019). The beneficial effects of rutin on myofibrillar protein gel properties and related changes in protein conformation. Food Chemistry, 301, 125206.
Joshi, P., & Varma, K. (2016). Effect of germination and dehulling on the nutritive value of soybean. Nutrition and Food Science.
Kasera, R., Singh, A., Lavasa, S., Prasad, K. N., & Arora, N. (2015). Enzymatic hydrolysis: A method in alleviating legume allergenicity. Food and Chemical Toxicology, 76, 54–60.
Kieliszek, M., Pobiega, K., Piwowarek, K., & Kot, A. M. (2021). Characteristics of the proteolytic enzymes produced by lactic acid bacteria. Molecules, 26(7), 1858.
Lammi, C., Zanoni, C., Arnoldi, A., & Vistoli, G. (2016). Peptides derived from soy and lupin protein as dipeptidyl-peptidase IV inhibitors: In vitro biochemical screening and in silico molecular modeling study. Journal of Agricultural and Food Chemistry, 64(51), 9601–9606.
Li, H., & Aluko, R. E. (2010). Identification and inhibitory properties of multifunctional peptides from pea protein hydrolysate. Journal of Agricultural and Food Chemistry, 58(21), 11471–11476.
Li, H., Yu, J., Ahmedna, M., & Goktepe, I. (2013). Reduction of major peanut allergens Ara h 1 and Ara h 2, in roasted peanuts by ultrasound assisted enzymatic treatment. Food Chemistry, 141(2), 762–768.
Li, J., Xiang, Q., Liu, X., Ding, T., Zhang, X., Zhai, Y., & Bai, Y. (2017). Inactivation of soybean trypsin inhibitor by dielectric-barrier discharge (DBD) plasma. Food Chemistry, 232, 515–522.
Li, M., Blecker, C., & Karboune, S. (2021). Molecular and air-water interfacial properties of potato protein upon modification via laccase-catalyzed cross-linking and conjugation with sugar beet pectin. Food Hydrocolloids, 112, 106236.
Li, Y., Yu, J., Goktepe, I., & Ahmedna, M. (2016). The potential of papain and alcalase enzymes and process optimizations to reduce allergenic gliadins in wheat flour. Food Chemistry, 196, 1338–1345.
Lu, Q., Zuo, L., Wu, Z., Li, X., Tong, P., Wu, Y., Fan, Q., Chen, H., & Yang, A. (2022). Characterization of the protein structure of soymilk fermented by Lactobacillus and evaluation of its potential allergenicity based on the sensitized-cell model. Food Chemistry, 366, 130569.
Lv, Y., Wei, K., Meng, X., Huang, Y., Zhang, T., & Li, Z. (2017). Separation and identification of iron-chelating peptides from defatted walnut flake by nanoLC-ESI–MS/MS and de novo sequencing. Process Biochemistry, 59, 223–228.
M’hir, S., Aldric, J.-M., El-Mejdoub, T., Destain, J., Mejri, M., Hamdi, M., & Thonart, P. J. (2008). Proteolytic breakdown of gliadin by Enterococcus faecalis isolated from Tunisian fermented dough. World Journal of Microbiology and Biotechnology, 24(12), 2775–2781.
Ma, F.-F., Wang, H., Wei, C.-K., Thakur, K., Wei, Z.-J., & Jiang, L. (2019). Three novel ACE inhibitory peptides isolated from Ginkgo biloba seeds: Purification, inhibitory kinetic and mechanism. Frontiers in Pharmacology, 9, 1579.
Matsuura, M., Sasaki, M., Sasaki, A., & Takeuchi, T. J. P. U. (2000). Production for producing packed tofu. 6042851.
Meinlschmidt, P., Schweiggert-Weisz, U., & Eisner, P. (2016). Soy protein hydrolysates fermentation: Effect of debittering and degradation of major soy allergens. LWT-Food Science and Technology, 71, 202–212.
Meinlschmidt, P., Sussmann, D., Schweiggert-Weisz, U., & Eisner, P. (2015). Enzymatic treatment of soy protein isolates: Effects on the potential allergenicity, technofunctionality, and sensory properties. Food Science and Nutrition, 4(1), 11–23.
Mills, E. C., & Shewry, P. R. (2004). Plant food allergens: Wiley Online Library.
Mojica, L., Luna-Vital, D. A., & de Mejia, E. G. (2018). Black bean peptides inhibit glucose uptake in Caco-2 adenocarcinoma cells by blocking the expression and translocation pathway of glucose transporters. Toxicology Reports, 5, 552–560.
Montemurro, M., Pontonio, E., Gobbetti, M., & Rizzello, C. G. (2019). Investigation of the nutritional, functional and technological effects of the sourdough fermentation of sprouted flours. International Journal of Food Microbiology, 302, 47–58.
Nadeeshani, H., Senevirathne, N., Somaratne, G., & Bandara, N. (2022). Recent trends in the utilization of pulse protein in food and industrial applications. ACS Food Science & Technology, 2(5), 722–737.
Nasrabadi, M. N., Doost, A. S., & Mezzenga, R. (2021). Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocolloids, 118, 106789.
Nisov, A., Ercili-Cura, D., & Nordlund, E. (2020). Limited hydrolysis of rice endosperm protein for improved techno-functional properties. Food Chemistry, 302, 125274.
Nonaka, M., Soeda, T., Yamagiwa, K., Kowata, H., Motogi, M., & Toiguchi, S. (1991). Process of preparing shelf-stable" tofu" at normal temperature for long term. In: Google Patents.
Nwachukwu, I. D., & Aluko, R. E. (2019). Structural and functional properties of food protein-derived antioxidant peptides. Journal of Food Biochemistry, 43(1), e12761.
Olagunju, A. I., Omoba, O. S., Enujiugha, V. N., Alashi, A. M., & Aluko, R. E. (2021). Thermoase-hydrolysed pigeon pea protein and its membrane fractions possess in vitro bioactive properties (antioxidative, antihypertensive, and antidiabetic). Journal of Food Biochemistry, 45(3), e13429.
Olatunde, O. O., & Benjakul, S. J. (2018). Natural preservatives for extending the shelf-life of seafood: A revisit. Comprehensive Reviews in Food Science and Food Safety, 17(6), 1595–1612.
Oparil, S., Acelajado, M., Bakris, G., Berlowitz, D., Cífková, R., Dominiczak, A., Grassi, G., Jordan, J., Poulter, N., & Rodgers, A. (2018). Hypertension. Nature reviews. Disease primers, 4, 18014. In).
Pal, R., Bhartiya, A., ArunKumar, R., Kant, L., Aditya, J., & Bisht, J. (2016). Impact of dehulling and germination on nutrients, antinutrients, and antioxidant properties in horsegram. Journal of Food Science and Technology, 53(1), 337–347.
Pan, X., Fang, Y., Wang, L., Xie, M., Hu, B., Zhu, Y., Zhao, E., Pei, F., Shen, F., & Li, P. (2019). Effect of enzyme types on the stability of oil-in-water emulsions formed with rice protein hydrolysates. Journal of the Science of Food and Agriculture, 99(15), 6731–6740.
Pearson, K. M., Pannell, L. K., & Fales, H. M. (2002). Intramolecular cross-linking experiments on cytochrome c and ribonuclease A using an isotope multiplet method. Rapid Communications in Mass Spectrometry, 16(3), 149–159.
Perreault, V., Hénaux, L., Bazinet, L., & Doyen, A. (2017). Pretreatment of flaxseed protein isolate by high hydrostatic pressure: Impacts on protein structure, enzymatic hydrolysis and final hydrolysate antioxidant capacities. Food Chemistry, 221, 1805–1812.
Pinterits, A., & Arntfield, S. D. (2008). Improvement of canola protein gelation properties through enzymatic modification with transglutaminase. LWT - Food Science and Technology, 41(1), 128–138.
Pontonio, E., Verni, M., Dingeo, C., Diaz-de-Cerio, E., Pinto, D., & Rizzello, C. G. (2020). Impact of enzymatic and microbial bioprocessing on antioxidant properties of Hemp (Cannabis sativa L.). Antioxidants, 9(12), 1258.
Rizzello, C. G., De Angelis, M., Coda, R., & Gobbetti, M. (2006). Use of selected sourdough lactic acid bacteria to hydrolyze wheat and rye proteins responsible for cereal allergy. European Food Research and Technology, 223(3), 405–411.
Ruíz-Henestrosa, V. P., Carrera Sánchez, C., del Mar Yust, M., Pedroche, J., Millán, F., & Rodríguez Patino, J. M. (2007). Limited enzymatic hydrolysis can improve the interfacial and foaming characteristics of β-conglycinin. Journal of Agricultural and Food Chemistry, 55(4), 1536–1545.
Sah, W. I., & Alisha, N. (2018). The Influences of transglutaminase enzyme dosage on the meat characteristic from restructuring the animal and vegetable protein sources. In E3S Web of Conferences, vol. 67 (pp. 03043): EDP Sciences.
Samtiya, M., Acharya, S., Pandey, K. K., Aluko, R. E., Udenigwe, C. C., & Dhewa, T. (2021). Production, purification, and potential health applications of edible seeds’ bioactive peptides: A concise review. Foods, 10(11), 2696.
Sandoval-Sicairos, E. S., Milán-Noris, A. K., Luna-Vital, D. A., Milán-Carrillo, J., & Montoya-Rodríguez, A. (2021). Anti-inflammatory and antioxidant effects of peptides released from germinated amaranth during in vitro simulated gastrointestinal digestion. Food Chemistry, 343, 128394.
Schettino, R., Pontonio, E., & Rizzello, C. G. (2019). Use of fermented hemp, chickpea and milling by-products to improve the nutritional value of semolina pasta. Foods, 8(12), 604.
Schlegel, K., Leidigkeit, A., Eisner, P., & Schweiggert-Weisz, U. (2019a). Technofunctional and sensory properties of fermented lupin protein isolates. Foods, 8(12), 678.
Schlegel, K., Sontheimer, K., Hickisch, A., Wani, A. A., Eisner, P., & Schweiggert-Weisz, U. (2019b). Enzymatic hydrolysis of lupin protein isolates-Changes in the molecular weight distribution, technofunctional characteristics, and sensory attributes. Food Science and Nutrition, 7(8), 2747–2759.
Segura-Campos, M., Espinosa-García, L., Chel-Guerrero, L., & Betancur-Ancona, D. (2012). Effect of enzymatic hydrolysis on solubility, hydrophobicity, and in vivo digestibility in cowpea (Vigna unguiculata). International Journal of Food Properties, 15(4), 770–780.
Seo, W. H., Lee, H. G., & Baek, H. H. (2008). Evaluation of bitterness in enzymatic hydrolysates of soy protein isolate by taste dilution analysis. Journal of Food Science, 73(1), S41–S46.
Shekarforoush, E., Jiang, X., Kedir Muhammed, M., Whitehead, K. A., Arneborg, N., & Risbo, J. (2022). Enzymatic modification and adsorption of hydrophobic zein proteins on lactic acid bacteria stabilize pickering emulsions. Food Research International, 161, 111783.
Shen, P., Zhou, F., Zhang, Y., Yuan, D., Zhao, Q., & Zhao, M. (2020). Formation and characterization of soy protein nanoparticles by controlled partial enzymatic hydrolysis. Food Hydrocolloids, 105, 105844.
Shi, X., Guo, R., White, B. L., Yancey, A., Sanders, T. H., Davis, J. P., Burks, A. W., & Kulis, M. (2013). Allergenic properties of enzymatically hydrolyzed peanut flour extracts. International Archives of Allergy and Immunology, 162(2), 123–130.
Silva, C. S., Moutinho, C., Ferreira da Vinha, A., & Matos, C. (2019). Trace minerals in human health: Iron, zinc, copper, manganese and fluorine. International Journal of Science and Research Methodology, 13(3), 57–80.
Singh, T. P., Siddiqi, R. A., & Sogi, D. S. (2021). Enzymatic modification of rice bran protein: Impact on structural, antioxidant and functional properties. LWT-Food Science and Technology, 138, 110648.
Sonklin, C., Alashi, A. M., Laohakunjit, N., & Aluko, R. E. (2021). Functional characterization of mung bean meal protein-derived antioxidant peptides. Molecules, 26(6), 1515.
Starzynska-Janiszewska, A., & Stodolak, B. (2011). Effect of inoculated lactic acid fermentation on antinutritional and antiradical properties of grass pea (Lathyrus sativus' Krab') flour. Polish Journal of Food and Nutrition Sciences, 61(4).
Sung, D.-E., Lee, J., Han, Y., Shon, D.-H., Ahn, K., Oh, S., & Do, J.-R. (2014). Effects of enzymatic hydrolysis of buckwheat protein on antigenicity and allergenicity. Nutrition Research and Practice, 8(3), 278–283.
Taniya, M., Reshma, M., Shanimol, P., Krishnan, G., & Priya, S. (2020). Bioactive peptides from amaranth seed protein hydrolysates induced apoptosis and antimigratory effects in breast cancer cells. Food Bioscience, 35, 100588.
Tapal, A., & Tiku, P. K. (2019). Nutritional and nutraceutical improvement by enzymatic modification of food proteins. In M. Kuddus (Ed.), Enzymes in food biotechnology (pp. 471–481). Elsevier.
Trester-Zedlitz, M., Kamada, K., Burley, S. K., Fenyö, D., Chait, B. T., & Muir, T. W. (2003). A modular cross-linking approach for exploring protein interactions. Journal of the American Chemical Society, 125(9), 2416–2425.
Trigui, I., Yaich, H., Sila, A., Cheikh-Rouhou, S., Krichen, F., Bougatef, A., Attia, H., & Ayadi, M. (2021). Physical, techno-functional and antioxidant properties of black cumin seeds protein isolate and hydrolysates. Journal of Food Measurement and Characterization, 15(4), 3491–3500.
Udenigwe, C. C., & Aluko, R. E. (2012). Food protein-derived bioactive peptides: Production, processing, and potential health benefits. Journal of Food Science, 77(1), R11–R24.
Urbizo-Reyes, U., San Martin-González, M. F., Garcia-Bravo, J., Vigil, A. L. M., & Liceaga, A. M. (2019). Physicochemical characteristics of chia seed (Salvia hispanica) protein hydrolysates produced using ultrasonication followed by microwave-assisted hydrolysis. Food Hydrocolloids, 97, 105187.
Vásquez-Villanueva, R., Muñoz-Moreno, L., Carmena, M. J., Marina, M. L., & García, M. C. (2018). In vitro antitumor and hypotensive activity of peptides from olive seeds. Journal of Functional Foods, 42, 177–184.
Venuste, M., Zhang, X., Shoemaker, C. F., Karangwa, E., Abbas, S., & Kamdem, P. E. (2013). Influence of enzymatic hydrolysis and enzyme type on the nutritional and antioxidant properties of pumpkin meal hydrolysates. Food and Function, 4(5), 811–820.
Vilcacundo, R., Martínez-Villaluenga, C., & Hernández-Ledesma, B. (2017). Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. Journal of Functional Foods, 35, 531–539.
Vilcacundo, R., Miralles, B., Carrillo, W., & Hernández-Ledesma, B. (2018). In vitro chemopreventive properties of peptides released from quinoa (Chenopodium quinoa Willd.) protein under simulated gastrointestinal digestion. Food Research International, 105, 403–411.
Wang, B., Meng, T., Ma, H., Zhang, Y., Li, Y., Jin, J., & Ye, X. (2016). Mechanism study of dual-frequency ultrasound assisted enzymolysis on rapeseed protein by immobilized Alcalase. Ultrasonics Sonochemistry, 32, 307–313.
Wang, J., Wang, T., Yu, G., Li, X., Liu, H., Liu, T., & Zhu, J. (2022). Effect of enzymatic hydrolysis on the physicochemical and emulsification properties of rice bran albumin and globulin fractions. LWT, 156, 113005.
Wang, L., Ding, Y., Zhang, X., Li, Y., Wang, R., Luo, X., Li, Y., Li, J., & Chen, Z. (2018). Isolation of a novel calcium-binding peptide from wheat germ protein hydrolysates and the prediction for its mechanism of combination. Food Chemistry, 239, 416–426.
Wine, R. N., Dial, J. M., Tomer, K. B., & Borchers, C. H. (2002). Identification of components of protein complexes using a fluorescent photo-cross-linker and mass spectrometry. Analytical Chemistry, 74(9), 1939–1945.
Wu, W., Zhang, M., Sun, C., Brennan, M., Li, H., Wang, G., Lai, F., & Wu, H. (2016). Enzymatic preparation of immunomodulatory hydrolysates from defatted wheat germ (Triticum Vulgare) globulin. International Journal of Food Science and Technology, 51(12), 2556–2566.
Xu, N., Chen, G., & Liu, H. (2017). Antioxidative categorization of twenty amino acids based on experimental evaluation. Molecules, 22(12), 2066.
Yang, A., Zuo, L., Cheng, Y., Wu, Z., Li, X., Tong, P., & Chen, H. (2018). Degradation of major allergens and allergenicity reduction of soybean meal through solid-state fermentation with microorganisms. Food and Function, 9(3), 1899–1909.
Yarnpakdee, S., Benjakul, S., Kristinsson, H. G., & Kishimura, H. J. (2015). Antioxidant and sensory properties of protein hydrolysate derived from Nile tilapia (Oreochromis niloticus) by one-and two-step hydrolysis. Journal of Food Science and Technology, 52(6), 3336–3349.
Yu, J., Ahmedna, M., Goktepe, I., Cheng, H., & Maleki, S. (2011). Enzymatic treatment of peanut kernels to reduce allergen levels. Food Chemistry, 127(3), 1014–1022.
Yu, J., Goktepe, I., & Ahmedna, M. (2013). Enzymatic treatment of peanut butter to reduce the concentration of major peanut allergens. International Journal of Food Science and Technology, 48(6), 1224–1234.
Yu, J., Hernandez, M., Li, H., Goktepe, I., Robinette, C., Auerbach, A., Peden, D., & Ahmedna, M. (2015). Allergenicity of roasted peanuts treated with a non-human digestive protease. Food Research International, 69, 341–347.
Zamani, A., Madani, R., Rezaei, M., & Benjakul, S. (2017). Antioxidative activitiy of protein hydrolysate from the muscle of common kilka (Clupeonella cultriventris caspia) prepared using the purified trypsin from common kilka intestine. Journal of Aquatic Food Product Technology, 26(1), 2–16.
Zhang, M., & Mu, T.-H. (2017). Identification and characterization of antioxidant peptides from sweet potato protein hydrolysates by Alcalase under high hydrostatic pressure. Innovative Food Science and Emerging Technologies, 43, 92–101.
Zhang, Q., Cheng, Z., Wang, Y., Zheng, S., Wang, Y., & Fu, L. (2021). Combining Alcalase hydrolysis and transglutaminase-cross-linking improved bitterness and techno-functional properties of hypoallergenic soybean protein hydrolysates through structural modifications. LWT, 151, 112096.
Zhang, Z., Kobata, K., Pham, H., Kos, D., Tan, Y., Lu, J., & McClements, D. J. (2022). Production of plant-based seafood: Scallop analogs formed by enzymatic gelation of pea protein-pectin mixtures. Foods, 11(6), 851.
Zou, Z., Wang, M., Wang, Z., Aluko, R. E., & He, R. (2020). Antihypertensive and antioxidant activities of enzymatic wheat bran protein hydrolysates. Journal of Food Biochemistry, 44(1), e13090.
Funding
The authors would like to acknowledge funding support from the Canada Research Chairs Program to N. Bandara and R.E. Aluko and the NSERC Discovery Grant Program to N. Bandara (RGPIN 2020–07136) and R.E. Aluko (RGPIN 2018–06019).
Author information
Authors and Affiliations
Contributions
Oladipupo O. Olatunde, Iyiola O. Owolabi, Olamide S. Fadairo, Anujit Ghosal, and Oluwafemi J. Coker collected and compiled the data, wrote the manuscript, and revised it. Olugbenga P. Soladoye, Rotimi E. Aluko, and Nandika Bandara revised the manuscript. Oladipupo O. Olatunde and Nandika Bandara planned, drafted, and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olatunde, O.O., Owolabi, I.O., Fadairo, O.S. et al. Enzymatic Modification of Plant Proteins for Improved Functional and Bioactive Properties. Food Bioprocess Technol 16, 1216–1234 (2023). https://doi.org/10.1007/s11947-022-02971-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02971-5