Abstract
In the present study, the antimicrobial efficiency of thymol and thymol-nanoemulsion (NE) was investigated against S. aureus, E. coli, and C. perfringens on a sausage product during 4 weeks. The droplets size of the thymol-NE was 86.39 nm with the zeta potential of −0.86 mV. The MIC and MBC values for thymol were approximately twice that of NE, indicating that NE was more effective in inhibiting the growth of three tested bacteria. According to agar well diffusion test results, the antimicrobial activity of thymol and the NE was not different against three tested bacteria. In inoculated sausages with E. coli, C. perfringens, and S. aureus at 105.5 log CFU/g, the control had the highest bacterial counts, followed by 120 mg/kg nitrite treated samples, both of which had an increasing trend over time. In contrast, the growth trend of all three bacteria, in the samples treated with thymol (600 mg/kg), NE (containing 600 mg/kg thymol), thymol (600 mg/kg) + nitrite (60 mg/kg), and NE (containing 600 mg/kg thymol) + nitrite (60 mg/kg) decreased significantly (5.68–6.33 log CFU/g reduction in comparison with control), with no significant difference among them against each bacterium. The color quality of NE + nitrite and thymol + nitrite was quite similar to 120 mg/kg nitrite-containing samples. The bacteria growth followed a first-order kinetic. The results showed the great potential of thymol and its NE for being used as an antimicrobial agent in the meat products, and NE + nitrite and thymol + nitrite can be a good choice to maintain the microbial and color quality of the sausage products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hot meat products analogous to sausages are considered as the most-consumed and desirable meat products in Iran. To prolong the shelf life and maintain the nutritional value of such meat products, the additives such as nitrites and nitrates have been long used to stabilize the red color of the meat, enhance flavor, and prevent the growth of certain microorganisms like Clostridium spp. (Mortazavi & Motamedzadegan, 2004). Nitrate entering the human body through food is deadly at 330 mg/kg body weight, with nitrite being ten times more poisonous than nitrate (Sadeghi et al., 2015a, 2015b). These compounds react with amines and amides to form the carcinogenic N-nitrogen compounds (Ding et al., 2018). The permissible nitrite and nitrate levels in meat products at the time of production are set at 120 and 500 mg/kg, respectively, in Iran (Sadeghi et al., 2015a, 2015b).
Many efforts have been made to find a suitable substitute for nitrate in sausages that offers the same commercial benefits with fewer risks to human health. One possible alternative would be the use of essential oils (EOs) as natural antimicrobial compounds. Essential oils are good sources of natural antimicrobial agents that are active against organisms such as insects, bacteria, fungi, and viruses (Pavela & Benelli, 2016). Due to their antimicrobial, antioxidant, anti-inflammatory, and anti-cancer properties, EOs can be a good substitute for the nitrates used in sausages and other processed meats (Sharifi-Rad et al., 2017).
Among the great variety of EOs, thyme EOs (Thymus vulgaris) have attracted the attention of many food researchers due to their strong antimicrobial properties (Zarzuelo & Crespo, 2003). The strong antimicrobial activity of thyme EO is mainly related to its high amount of thymol (10–64%) (Almasi et al., 2020a). Thymol (2-isopropyl-5-methyl phenol) is a monoterpene phenol component (Xue et al., 2015), which is well known for its antimicrobial activity against Gram-positive (G+) and Gram-negative (G−) bacteria and is listed as GRAS (generally recognized as safe) (Marchese et al., 2015). However, the direct use of thymol in food systems is limited due to its unstable nature and volatility, decomposition phenomenon under environmental and chemical conditions (light, oxygen, dampness, etc.), and the sensory properties change of the EO and the career products. To decrease thymol instability and volatility and increase the possibility of its use in the aqueous food systems, it can be encapsulated and in fact, entrapped within different coating materials (Almasi et al., 2020a). Various colloidal delivery systems including microemulsions (MEs) (Xu et al., 2008), nanoemulsions (NEs) (Almasi et al., 2020a), solid lipid nanoparticles (SLNs) (Bagheri et al., 2019), nanogels (Cinay et al., 2017), and nanoliposomes (Bae et al., 2009) have been used for encapsulating the EOs. Using nanoscale technology to encapsulate thymol is a viable solution to the problem of EO degradation Robledo (2018b).
Nanoemulsions (NEs) efficiently increase the dispersibility of EOs in foods, enhance their antimicrobial properties, and decrease their adverse effects on the quality parameters of the food stuffs (Silva et al., 2012; Galvão et al., 2018). Nanoemulsions are thermodynamically unstable colloidal dispersions made up of two immiscible liquids, with one of the liquids being dispersed as small spherical droplets smaller than 100 nm in radius (Radi et al., 2018). Many attempts have been performed to encapsulate bioactive compounds by nanoemulsification. In this regard, Robledo et al. (2018b) observed higher antifungal activity for thymol-NE against tomato fungi in comparison with unprotected-thymol. Also, in another experiment, Chaudhari et al. (2020) declared that anethole-based chitosan NE has a good potential for being used on stored maize to reduce aflatoxin and fungal contamination. Xu et al. (2020) used cinnamon EO-NE with ascorbic acid to reduce the enzymatic browning of cloudy apple juice. These findings suggest that NEs can be used as an effective technique to improve the antimicrobial effects of EOs against pathogenic microorganisms. However, no study was found by the authors to investigate the effect of a NE system containing EO on the shelf life of a sausage product. In a study performed by Saggiorato et al. (2012), the antifungal activity of basil EO was evaluated on an Italian-type sausage, but the effect of basil EO-NE was not investigated. In another study, eugenol was microencapsulated in gelatin-based emulgel to prolong the shelf life of refrigerated meat (Wan et al., 2020). Accordingly, this investigation was carried out to assess the antibacterial effect of NE containing thymol (as a strong antimicrobial agent) against Escherichia coli, Staphylococcus aureus, and Clostridium perfringens in sausage. To the best of our knowledge, the efficiency of thymol and its NE as a nitrite substitute has not been investigated in sausage. As the sausage medium is a strong emulsifying medium, this study was conducted to determine whether NE can enhance the antimicrobial effect of an antimicrobial agent like thymol in a strong emulsion system like sausage.
Materials and Methods
Materials
Thymol was purchased from Gol-Ghatreh (Fars, Iran). Strains of S. aureus (ATCC 1337), E. coli (ATCC 1276), and C. perfringens (ATCC) were purchased from the Persian Type Culture Collection (PTCC). The materials used, including Tween 80 (T80), triphenyl tetrazolium chloride, nutrient broth, Sulfadiazine Polymyxin Sulfite agar (SPS agar), Violet Red Bile Agar (VRBA), Baird Parker agar (BPA), and Mueller-Hinton agar (MHA), were purchased from Merck Chemical Co. (Darmstadt, Germany).
Formulation of NE
One gram of thymol was added to 100 mL distilled water containing 2 g T80, and then stirred for 30 min at 1200 rpm to form a coarse emulsion. A nanoemulsion was then produced by sonicating (Q700, Q-Sonica, USA) the coarse emulsion for 4 min at an intensity of 100 W cm−2. A jacketed glass container and a cold water circulation device (TC502D, Brookfield Engineering, MA, USA) were used to prevent temperature rise during sonication (Almasi et al., 2020b).
Determination of Particle Size Distribution and Zeta Potential
The particles size of the thymol nanocapsules in the NE system was measured. The measuring instrument was equipped with a 4-mW He–Ne laser. Particle size measurement was performed at 633 nm with a dynamic viscosity of 8.76 mPa-s (at 25 °C) and detection angles of 70 and 90° (Nano ZS, Malvern Instruments Ltd., UK). A DTS software (5.02 version, Malvern Instruments Ltd., UK) was used to analyze the z-average, hydrodynamic droplet size, and polydispersity index (Almasi et al., 2020a).
Minimum Inhibitory Concentration
Minimum inhibitory concentration was measured using the microdilution broth method (Almasi et al., 2020a). This method was used in the cases where the well diffusion method showed a significant effect. A bacterial suspension was prepared from an overnight culture which was diluted to 0.1 and then 0.01 of its initial concentration in order to determine the approximate number of 106 log CFU microorganisms per mL of suspension. To perform the experiment, Müeller-Hinton broth medium and 96-well microplates were used. Only the culture medium and a bacterial suspension (10 μL, S. aureus, E. coli, and C. perfringens) were added to the first row of wells. In the next rows, 100 μL of the Müeller-Hinton medium was added to the plates and then 6.25, 12.5, 25, 50, 100, and, 200 μg/mL thymol or NEs containing the equal amounts of thymol were added to the wells. Ten microliter of the bacterial suspension was added to each well, separately. The microplates were then incubated at 37 °C for 24 h. The turbidity or non-turbidity of the wells was first visually evaluated. Then the lowest concentration of bacterial growth inhibitor (MIC) was determined using a 5-mg/mL solution of triphenyl tetrazolium chloride (a growth color change reagent that is colorless in the oxide state but when is reduced by microorganisms, turns to red due to the formation of formazan). Thereby, the reagent (50 μL) was poured into all plate wells before re-incubation for 3 h. After the required time, the microplates were taken out and the results were evaluated. The concentration higher than the last concentration that turned to a red color was considered as the MIC.
Minimum Bactericidal Concentration
To determine the MBC of thymol and the NE containing thymol against S. aureus, E. coli, and C. perfringens, 100 μL of the non-turbid wells were transferred into Müeller-Hinton agar and incubated at 37 ± 2 °C for 24 h. The lowest concentration at which no growth was observed was considered as MBC (Almasi et al., 2020a).
Agar Diffusion Method
The antimicrobial activity of thymol in plain or nano-emulsified form was assessed using the agar well diffusion method (Almasi et al., 2020a). For this purpose, Mueller-Hinton Agar plates with three wells were inoculated with relevant microbial suspension (0.1 mL) containing 106 CFU/mL. Afterward, 50 μL of thymol or NEs containing equal amounts of thymol was added into the wells. For this purpose, the concentration of 6.25, 12.5, 25, 50, 100, and, 200 μg/mL of thymol were used for both free and nano-emulsified thymol in the wells. Lastly, the plates were incubated at 37 ± 2 °C for 24 to 48 h, and the inhibition zones were measured. At the same time, the antimicrobial activity of thymol and its NE was compared with the penicillin antibiotic. For this purpose, a penicillin solution containing 10 µg/mL penicillin was used in the wells and the diameter of the inhibition zones was reported for each bacterium.
Sausage Preparation
A mixture of 70% beef, soy protein isolate (20%), sodium chloride (1%), corn starch (1%), sodium phosphates (0.2%), water (5%), oil (2%), and dry milk (0.8%) was used for the sausage preparation. Spices were removed from the sausage formula to remove any interference effect with the antimicrobial effect of thymol or its nanoemulsion. The raw materials were all mixed (Robokit 2154, BEKO, Istanbul,Turkey) well to produce a batter. The produced batter was then divided into six parts, and each part was well homogenized (Robokit 2154, BEKO, Istanbul,Turkey) with thymol (600 mg/kg), NE containing 600 mg/kg thymol, nitrite (120 mg/kg), thymol (600 mg/kg) + nitrite (60 mg/kg), and NE (containing 600 mg/kg thymol) + nitrite (60 mg/kg), separately. The concentration of thymol used in the sausage formulation did not adversely affect the sausage taste and was consistent with the taste of this product. A sample without nitrite, thymol, or NE was considered as control. Afterward, each treatment was divided into three parts, for being inoculated with 105.5 log CFU/g E. coli, S. aureus, and C. perfringens, separately. The inoculated batter sausages were packaged in plastic wraps using a Bush filler (MFW68640, Stuttgart, Germany). Then, baking was carried out at a temperature of 70 °C for 40 min using a water bath (SHZ-82, Aria teb, Tehran, Iran). After baking, the samples were transferred into a refrigerator (4 °C), and the effect of treatments on the bacterial growth was assessed every week for 30 days.
A completely randomized design was used for the experiment. The sausages were packaged in 100 g plastic wraps, and 5 plastic wraps containing 100 g sausage were placed in a tray to be used as a replicate for each measurement time. Regarding the presence of 5 measurement times (throughout the storage time), 25 plastic wraps were considered for the total measurement time of 30 days for one replicate and a total of three replicates were considered for each treatment.
Sausages Microbial Counts
Ten grams of each sample (sausage) was aseptically weighed and diluted with 50 mL of peptone water (BPW, Biokar diagnostics, France). After performing the serial dilution, a 0.1 mL of the desired dilutions was cultured on the surface of BPA (for treatments inoculated with S. aureus), SPS agar (for treatments inoculated with C. perfringens), and VRBA (for treatments inoculated with E. coli) using the surface culture method (surface plate count method). For S. aureus, the Petri dishes were kept at 37 °C for 48 h and glossy black colonies with thin white edges and a clear and colorless halo were counted. In regard to E. coli, the plates were incubated at 37 °C for 24 h, and the numbers of purple colonies were counted (Almasi et al., 2020b). For C. perfringens, SPS agar was the medium of choice and the plates were incubated at 37 °C for 48 h under anaerobic conditions. The differentiation of microorganisms grown on SPS agar is based on the reduction of sulfate to sulfite, which turns the colony and sometimes the surrounding environment black. Sulfadiazine inhibits coliforms, Proteus, and Pseudomonas growth, and polymyxin inhibits the growth of G− bacteria. Black colonies, with or without a halo, were selected phenotypically and counted as suspected C. perfringens (Ghorchian et al., 2019).
Mathematical Modeling
The growth rate of E. coli, S. aureus, and C. perfringens in the sausages was calculated by the first order model: ln(C) = ln(C0)−kt, where C is the bacterium population at time t ((log CFU/g)), C0 (the bacterium population at time zero (log CFU/g)), k (S. aureus, E. coli, and C. perfringens growth rate (per week)), and t (the storage time (in week)) (Amiri & Niakousari, 2008; Parsa et al., 2020). The validity of model was evaluated by the calculation of Pearson R2 and the lack of fit test by using IBM SPSS version 22 (IBM Armonk, NY, USA).
Statistical Analysis
A completely randomized design was used. All experiments were performed in triplicate, and the average values were recorded. IBM SPSS version 22 (IBM Armonk, NY, USA) was used for the statistical analyses, and an alpha level of P < 0.05 was set as a threshold for differentiating the means by using Duncan’s multiple range test (after ANOVA) or t-test.
Result and Discussion
Zeta Potential and Dynamic Light Scattering
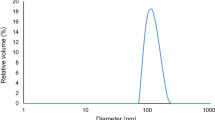
The zeta potential was measured using a nanoPartica SZ-100 instrument (Horiba Ltd., Japan), and it was −0.86 mV. It has been reported that zeta potentials lower than −30 mV indicates the presence of a strong electrical charge for droplets which keeps the droplets stable due to the repulsive forces between them. Therefore, NEs have higher stability than macro emulsions (Salvia-Trujillo et al., 2015). The mean droplet size of thymol-NE and its polydispersity index (PDI) were 86.39 nm and 0.28, respectively. The polydispersity index shows the particle homogeneity and ranges between 0 and 1. When the index moves to zero, the system is more homogenous (Amiri et al., 2013). Moghimi et al. (2016) also obtained a diameter of 143 nm for their Thymus daenensis NE. Liu et al. (2020) and Salvia-Trujillo et al. (2013) reported particle sizes of 97.1 and 5.12–34.95-nm diameters for their star anise EO, polylysine, and nisin NE and lemongrass EO-alginate NEs, respectively.
The In Vitro Antimicrobial Activity of Thymol and the NE Containing Thymol
The MIC and MBC values of plain thymol and the NE containing thymol against S. aureus, E.coli, and C. perfringens are presented in Table 1. According to Table 1, the MIC values of 103, 60, and 93.3 mg/L and MBC values of 406, 200, and 406 mg/L were obtained for thymol against E. coli, S. aureus, and C. perfringens, respectively. The MIC value of 1.2 mmol/L for thymol against E. coli was reported before. Meanwhile, it was reported that the MIC values of thymol ranged from 0.03 to 0.06% v/v against 8 strains of S. aureus (Marchese et al., 2015).
Results showed that the MIC and MBC of NE were significantly lower than that of thymol against all examined bacteria (Table 1). In this regard, the MIC and MBC values for thymol were approximately double the values of NE, indicating that NE was more effective in inhibiting the growth of S. aureus, E. coli, and C. perfringens. The antimicrobial activity of thymol may relate to the ability of this compound to perturb the lipid fraction of bacterial membrane (Marchese et al., 2015). Thymol has a phenolic hydroxyl on its phenolic ring, which increases thymol hydrophilic ability and helps it dissolve in microbial membrane and damage the membrane (Xu et al., 2008).
Thereby, thymol increases membrane permeability and decreases the stability of bilayer, resulting in the intracellular materials leakage (Marchese et al., 2015). Membrane potential is another important mechanism of thymol (and other EO components) to inactive bacteria. When thymol disturbs the membrane integrity and increases membrane permeability, the leakage of protons and potassium is induced which in turn leads to the loss of membrane potential. The presence of the hydroxyl group on thymol plays an important role to depolarize membrane potential and decreases the membrane potential (Xu et al., 2008). Besides, thymol may cause some changes in the secondary structure of DNA, resulting in the alteration of DNA morphology and the creation of a minor groove in DNA (Liu & Liu, 2020). The antimicrobial activity of thymol has demonstrated by Ma et al. (2016), Robledo et al. (2018a), and Wattanasatcha et al. (2012).
The results of this study showed that thymol nanoemulsification improved its antimicrobial activity. This is due to the uniform and homogeneous distribution of the antimicrobial agent in the microbial broth medium, which enabled thymol to act well against the three examined bacteria according to the above mechanisms. However, free thymol certainly did not have a good distribution in the microbial broth environment due to its oily nature, and a significant part of it accumulated on the surface of the broth medium. It has been reported that the conversion of thymol to nano-scale particles enhances the antibacterial activity of the antimicrobial agent (Moghimi et al., 2016), due to the particle size reduction of the EO, which increases the affinity with the bacteria cell wall as well as enhances the stability and solubility of the EO (Liu et al., 2020). Similar results were also obtained by Zhang et al. (2014) on D-limonene NE and Bhargava et al. (2015) on oregano oil NE, who reported improved antibacterial activity for their EOs (or flavor) NE systems. However, Ma et al. (2016) and Almasi et al. (2020a) reported higher MIC and MBC values for their ME systems containing thyme EO than that of free EO. Wattanasatcha et al. (2012) found the same MIC and MBC values for free thymol and the encapsulated one against E. coli and S. aureus.
Among the three pathogenic bacteria, S. aureus showed the lowest MIC and MBC values (MIC (60 mg/L) and MBC (100 mg/L) for NE and MIC (110 mg/L) and MBC (200 mg/L) for thymol), indicating the higher sensitivity of this bacterium to thymol and the relevant NE. E. coli and C. perfringens showed almost the same sensitivity against thymol and the NE. These results were also confirmed by the agar well diffusion test, which indicated higher inhibition zones and therefore lower resistance for S. aureus against thymol and its NE in comparison with E. coli and C. perfringens. In this regard, E. coli and C. perfringens showed smaller halo diameters than S. aureus but equal to each other.
Gram-negative bacteria are slightly less sensitive to EOs than G+ bacteria (Seow et al., 2014) which is due to the structural difference in their cell walls. In G+ bacteria, hydrophobic molecules easily penetrate through the thick peptidoglycan layer but in G− bacteria, the outer membrane is almost impermeable to these molecules, which limits their access to the cell membrane (Nazzaro et al., 2013). Nanoemulsion droplets can efficiently penetrate through the porin proteins of the outer membrane due to their smaller size, enabling effective delivery of the EO to the G− bacteria cell membrane (Moghimi et al., 2016).
According to the agar well diffusion test results, the halo diameters ranged between 6.6 and 38.6 mm depending on the antimicrobial agent and the kind of bacterium. The inhibition zones of the penicillin antibiotic were significantly higher than those of thymol and the corresponding NE. In this regard, there was no significant difference in the antimicrobial activity of thymol and its NE against three tested bacteria. In the well diffusion test, the antimicrobial agent must diffuse in the medium to be effective, and it seems that thymol could diffuse into the medium in the same way as its NE form, resulting in the same performance of thymol and its NE against S. aureus, E. coli, and C. perfringens.
Meanwhile, thymol concentration (in the plain or nanocapsulated form) had a significant positive effect on the antimicrobial activity, so that by increasing the concentration of thymol in free and nanoencapsulated forms, the halo diameter increased significantly against all three tested bacteria. Liu and Liu (2020) declared that the higher concentration of thymol increases the permeability of the bacterial cell membranes, leading to leakage of intracellular materials and change in the morphology of cells. However, the maximum inhibition zone diameters were observed against S. aureus for penicillin, and then thymol and thymol-NE. E. coli and C. perfringens showed the same resistance to the studied antimicrobial agents and, of course, their resistance was higher than that of S. aureus, as they formed smaller halo diameters than S. aureus. In line with our findings, Liu and Liu (2020) found out that chitosan NE loading thyme EO or thymol inhibited S. aureus and E. coli. The inhibitory activities of the thyme EO-chitosan and thymol-chitosan NEs on S. aureus were better than E. coli, which was due to the differences in the cell-wall structure of the bacteria. In Trombetta et al. (2005) studies, S. aureus appeared to be more sensitive than E. coli toward thymol. In a study conducted by Almadiy et al. (2016), four Achillea species EOs and their NEs were investigated for their antibacterial activity against two G+ foodborne bacteria (S. aureus and Listeria monocytogenes) and three G− species (E. coli, Pseudomonas aeruginosa, and Salmonella enteritidis). These researchers reported that the G+ bacteria were more susceptible than the G− ones, where P. aeruginosa was the most resistant one. Besides, the activity of EOs increased significantly in the NE forms.
The In Vivo Antimicrobial Activity of Thymol and the NE Containing Thymol
For in vivo investigation, a meat product (sausage) was inoculated with E. coli, C. perfringens, and S. aureus to evaluate the effect of thymol and thymol containing NE on the microbial count of sausages. Figure 1 shows the antimicrobial activity of thymol and thymol containing NEs on the inoculated sausages. The starting population of S. aureus, C. perfringens, and E. coli on the sausages was about 105.5 log CFU/g. In the control samples, this population increased from 5.5 log CFU/g to 8.0 (for S. aureus and E. coli) and 8.3 log CFU/g (for C. perfringens) after 4 weeks of storage.
For three examined bacteria, the sausage samples containing 120 mg/kg nitrite had the highest bacterial growth after control, and this growth, like control, had an increasing trend over time. In these samples, nitrite did not affect the bacterial population until about the third week of storage but after this time, nitrite began to slow down the bacterial growth trend significantly, so that in the third and fourth weeks, the bacterial populations reduced by about one logarithmic cycle.
The growth trend of all three bacteria, in the samples treated with plain thymol, NE, thymol + nitrite, and NE + nitrite followed a different trend and decreased significantly (Fig. 1). However, no significant difference was observed in the performance of thymol, NE, thymol + nitrite, and, NE + nitrite against each tested bacterium (P > 0.05).
The samples treated with thymol, NE, thymol + nitrite, and, NE + nitrite reduced the population of S. aureus by 1.60, 2.62, 5.02, and 5.68 logarithmic cycles after 1, 2, 3, and 4 weeks of storage, respectively (P < 0.05). This reduction was about 1.60, 3.11, 4.23, and 6.33 logarithmic cycles after 1, 2, 3, and 4 weeks of storage for E. coli, respectively (P < 0.05). Meanwhile, the bacterial count reduction was estimated about 1.03, 3.46, 4.03, and 6.16 logarithmic cycles after 1, 2, 3, and 4 weeks of storage for C. perfringens, respectively (P < 0.05). Thereby, the effect of thymol or its NE on the reduction of the bacterial count was remarkably great in the sausage product.
In sausages, as in the well diffusion test, no statistically significant difference was observed between the performance of thymol and its NE against the three tested bacteria. For manufacturing of the sausages, thymol was dissolved in the oily phase of the sausage and then was added into the product batter. As sausage batter is a very strong emulsion, thymol was evenly and homogeneously distributed in sausage tissue. In this case, it can be said that thymol was encapsulated in the oil droplets. On the other hand, NEs are susceptible to the Ostwald ripening phenomenon over time. Ostwald ripening is the main degradation mechanism of NEs. It is noteworthy to point that the experimental rates of Ostwald ripening are much higher than those of the theoretical values, indicating that other phenomena like flocculation, coalescence, and subsequent creaming influence the breakdown of NEs (Koroleva et al., 2018). Thereby, the NE system might have been affected by the Ostwald ripening phenomenon, and thus, its thymol content, like free thymol, was integrated into the oil droplets of the sausage and thereby, was emulsified in the sausage medium. Therefore, no difference was observed between the performance of free thymol and the nanoemulsified one.
Liu and Liu (2020) reported that the total viable counts (TVC) of refrigerated pork treated with the thyme-chitosan and thymol-chitosan NEs were 5.93 and 5.77 log CFU/g, respectively, on the 12th day, which were much lower than the control samples. Jayari et al. (2018) reported that Thymus capitatus and Thymus algeriensis EOs exerted a bacteriostatic effect against E. coli and S. typhimurium at low concentrations (0.01 and 0.05% (v/w)), which increased significantly at higher concentrations of 1 and 3% (v/w). Liu et al. (2020) reported the longest shelf life (16 days) for ready-to-eat Yao meat products due to the use of NE-based active coatings with a composite mixture of nisin, star anise EO, and polylysine. Robledo et al. (2018a) observed higher antifungal activity for thymol NE/quinoa protein/chitosan coating on strawberries than that of the controls, increasing the shelf life of these samples in 4 days.
In addition, the microbial growth reduction obtained in this study was in accordance with the antimicrobial effects of orange peel EO NE and microemulsion on fresh-cut orange (Radi et al., 2018), carnauba wax-based NE containing lemongrass oil on fresh-cut apples (Salvia-Trujillo et al., 2015) and grape berry (Kim et al., 2012), carnauba-shellac wax-based NE containing lemongrass oil on apple fruit (Jo et al., 2014), and oregano oil NE on fresh lettuce (Bhargava et al., 2015).
Kinetic study of S. aureus, E. coli, and C. perfringens growth in sausages
Microbial population is an important quality parameter of sausages, in which its increase seriously limits the product shelf life. When the logarithms of S. aureus, E. coli, and C. perfringens growth were plotted versus time, straight lines were obtained (with determination coefficients between 0.82 and 0.99; Fig. 2; Table 2). Table 2 shows the growth rate (k) of S. aureus, E. coli, and C. perfringens in the sausages during 4 weeks of storage. Results showed a first-order reaction for all three bacteria growth during 4 weeks of storage. However, the bacteria growth increased with storage time in the control and nitrite containing samples, as a positive k values were obtained for these samples. In contrast, negative k values were achieved for treated samples with thymol, NE, thymol + nitrite, and NE + nitrite, indicating a decrease in the bacterial growth of these samples. It seems that different mechanisms or some new factors are involved in the bacterial growth of these samples, mainly referring to the antimicrobial agents added to the sausages.
Comparison of the k factors in all treatments showed that this factor was the highest in the control, followed by nitrite containing samples. This indicates a higher rate of bacterial growth in the control and then nitrite containing sausages. Meanwhile, no significant difference was observed among the k values of thymol, NE, thymol + nitrite, and NE + nitrite samples, indicating the same trend in bacterial growth in these samples. It should be noted that the numerical value of k in the samples containing thymol, NE, thymol + nitrite, and NE + nitrite was higher than those of nitrite containing samples, which indicates that the rate of bacterial count reduction in these samples was faster than the growth rate of bacteria in the samples containing nitrite. The comparison of k values of different bacteria indicated that the growth rate of C. perfringens was higher than that of S. aureus, which in turn was higher than that of E. coli.
Conclusion
In this study, thymol-NE was successfully prepared and its particles diameter was measured which was 89.39 nm. The MIC and MBC values of thymol and thymol-NE showed that thymol-NE could significantly improve the antimicrobial performance of thymol. But a comparison of their antimicrobial activity in the sausage product showed no significant difference among them. This result might be due to the occurrence of the Ostwald phenomenon in NEs over time as well as the good emulsification of thymol in the sausage medium. The results of this study were contrary to our previous results on the application of thyme NE on strawberry and cucumber (Almasi et al., 2020a) and ground meat (Almasi et al., 2021) which showed that thyme NE was able to reduce the microbial load of these products effectively and was better than the plain EO. It seems that NEs can act well in food mediums that are not emulsions, and thereby, the nanoemulsification of the EO makes it act better than the plain EO. But in foodstuffs that have their own emulsion systems such as sausages, NE may not lead to a better EO performance.
According to the color analysis of sausage samples, the reduction of nitrite concentration to about half of the initial value (60 mg/kg) could maintain the quality parameters of color as well as 120 mg/kg. On the other hand, the use of thymol or its NE could reduce the bacterial population to 5.68–6.33 log CFU/g. Therefore, it can be concluded that the use of thymol or its NE in combination with 60 mg/kg nitrite can be very effective in maintaining the microbial and color quality of the sausage products.
Availability of Data and Material
Data will be available if requested.
References
Almadiy, A. A., Nenaah, G. E., Al Assiuty, B. A., Moussa, E. A., & Mira, N. M. (2016). Chemical composition and antibacterial activity of essential oils and major fractions of four Achillea species and their nanoemulsions against foodborne bacteria. LWT - Food Science and Technology, 69, 529–537. https://doi.org/10.1016/j.lwt.2016.02.009
Almasi, L., Radi, M., Amiri, S., & Torri, L. (2020a). Fully dilutable Thymus vulgaris essential oil:Acetic or propionic acid microemulsions are potent fruit disinfecting solutions. Food Chemistry, 343, 128411. https://doi.org/10.1016/j.foodchem.2020.128411
Almasi, L., Radi, M., & Amiri, S. (2020b). The release rate and antimicrobial activity of calcium-alginate films containing self-microemulsifying Thymus vulgaris essential oil against Escherichia coli and Staphylococcus aureus. Journal of Food Safety, 40(5), 1–8. https://doi.org/10.1111/jfs.12828
Almasi, L., Radi, M., McClementsbc, D. J., & Amiri, S. (2021). Fabrication and characterization of antimicrobial biopolymer films containing essential oil-loaded microemulsions or nanoemulsions. Food Hydrocolloids, 117, 106733. https://doi.org/10.1016/j.foodhyd.2021.106733
Amiri, S., Abbasi, S., Ezzatpanah, H., & Hosseini, E. (2013). Nanocapsulation of orange peel oil using microemulsion technique. Agro Food Industry Hi-Tech, 24(2), 72–75.
Amiri, S., & Niakousari, M. (2008). Shelf life of unpasteurized sour orange juice in Iran. Fruits, 63(1), 11–18. https://doi.org/10.1051/fruits:2007040
Bae, D. H., Shin, J. S., Shin, G. S., Jin, F. L., & Park, S. J. (2009). Effect of lecithin on dermal safety of nanoemulsion prepared from hydrogenated lecithin and silicone oil. Bulletin of the Korean Chemical Society, 30(4), 821–824. https://doi.org/10.5012/bkcs.2009.30.4.821
Bagheri, F., Radi, M., & Amiri, S. (2019). Drying conditions highly influence the characteristics of glycerol-plasticized alginate films. Food Hydrocolloids, 90, 162–171. https://doi.org/10.1016/j.foodhyd.2018.12.001
Bhargava, K., Conti, D. S., da Rocha, S. R. P., & Zhang, Y. (2015). Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiology, 47, 69–73. https://doi.org/10.1016/j.fm.2014.11.007
Chaudhari, A. K., Singh, V. K., Deepika, S. D., Singh, B. K., & Dubey, N. K. (2020). Antimicrobial, aflatoxin B1 inhibitory and lipid oxidation suppressing potential of anethole-based chitosan nanoemulsion as novel preservative for protection of stored maize. Food and Bioprocess Technology, 13, 1462–1477. https://doi.org/10.1007/s11947-020-02479-w
Cinay, G. E., Erkoc, P., Alipour, M., Hashimoto, Y., Sasaki, Y., Akiyoshi, K., & Kizilel, S. (2017). Nanogel-integrated pH-responsive composite hydrogels for controlled drug delivery. ACS Biomaterials Science and Engineering, 3(3), 370–380. https://doi.org/10.1021/acsbiomaterials.6b00670
Ding, Z., Johanningsmeier, S. D., Price, R., Reynolds, R., Truong, V. D., Payton, S. C., & Breidt, F. (2018). Evaluation of nitrate and nitrite contents in pickled fruit and vegetable products. Food Control, 90, 304–311. https://doi.org/10.1016/j.foodcont.2018.03.005
Galvão, K. C. S., Vicente, A. A., & Sobral, P. J. A. (2018). Development, characterization, and stability of o/w pepper nanoemulsions produced by high-pressure homogenization. Food and Bioprocess Technology, 11, 355–367. https://doi.org/10.1007/s11947-017-2016-y
Ghorchian, S., Douraghi, M., Rahimiforoushani, A., & S. D. M. . (2019). Isolation and identification of cpe-positive Clostridium perfringens in bulk and packed dehydrated vegetables. Razi Journal of Medical Sciences, 26(8), 23–30.
Jayari, A., El Abed, N., Jouini, A., Abdul-Wahab, M. S., & O., Maaroufi, A., & Ben Hadj Ahmed, S. . (2018). Antibacterial activity of Thymus capitatus and Thymus algeriensis essential oils against four food-borne pathogens inoculated in minced beef meat. Journal of Food Safety, 38(1), 1–10. https://doi.org/10.1111/jfs.12409
Jo, W. S., Song, H. Y., Song, N. B., Lee, J. H., Min, S. C., & Song, K. B. (2014). Quality and microbial safety of “Fuji” apples coated with carnauba-shellac wax containing lemongrass oil. LWT - Food Science and Technology, 55(2), 490–497. https://doi.org/10.1016/j.lwt.2013.10.034
Kim, D. M., Hyun, S. S., Yun, P., Lee, C. H., & Byun, S. Y. (2012). Identification of an emulsifier and conditions for preparing stable nanoemulsions containing the antioxidant astaxanthin. International Journal of Cosmetic Science, 34(1), 64–73. https://doi.org/10.1111/j.1468-2494.2011.00682.x
Koroleva, M., Nagovitsina, T., & Yurtov, E. (2018). Nanoemulsions stabilized by non-ionic surfactants: Stability and degradation mechanisms. Physical Chemistry Chemical Physics, 20(15), 10369–10377. https://doi.org/10.1039/c7cp07626f
Liu, Q., Zhang, M., Bhandari, B., Xu, J., & Yang, C. (2020). Effects of nanoemulsion-based active coatings with composite mixture of star anise essential oil, polylysine, and nisin on the quality and shelf life of ready-to-eat Yao meat products. Food Control, 107, 106771. https://doi.org/10.1016/j.foodcont.2019.106771
Liu, T., & Liu, L. (2020). Fabrication and characterization of chitosan nanoemulsions loading thymol or thyme essential oil for the preservation of refrigerated pork. International Journal of Biological Macromolecules, 162, 1509–1515. https://doi.org/10.1016/j.ijbiomac.2020.07.207
Ma, Q., Davidson, P. M., & Zhong, Q. (2016). Nanoemulsions of thymol and eugenol co-emulsified by lauric arginate and lecithin. Food Chemistry, 206, 167–173. https://doi.org/10.1016/j.foodchem.2016.03.065
Marchese, A., Orhan, I. E., Daglia, M., Barbieri, R., Di Lorenzo, A., Nabavi, G., & O., Izadi, M., & Nabavi, S. M. . (2015). Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chemistry, 210, 402–414. https://doi.org/10.1016/j.foodchem.2016.04.111
Moghimi, R., Ghaderi, L., Rafati, H., Aliahmadi, A., & Mcclements, D. J. (2016). Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chemistry, 194, 410–415. https://doi.org/10.1016/j.foodchem.2015.07.139
Mortazavi, S. A., & Motamedzadegan, A. (2004). Modern food microbiology (6th ed.). Firdausi University.
Nazzaro, F., Fratianni, F., De Martino, L., Coppola, R., & De Feo, V. (2013). Effect of essential oils on pathogenic bacteria. Pharmaceuticals, 6(12), 1451–1474. https://doi.org/10.3390/ph6121451
Parsa, Z., Roozbehi, S., Hosseinifarahi, M., Radi, M., & Amiri, S. (2020). Integration of pomegranate peel extract (PPE) with calcium sulphate (CaSO4): A friendly treatment for extending shelf-life and maintaining postharvest quality of sweet cherry fruit. Journal of Food Processing and Preservation, 45(1), 1–15. https://doi.org/10.1111/jfpp.15089
Pavela, R., & Benelli, G. (2016). Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends in Plant Science, 21(12), 1000–1007. https://doi.org/10.1016/j.tplants.2016.10.005
Radi, M., Akhavan-Darabi, S., Akhavan, H. R., & Amiri, S. (2018). The use of orange peel essential oil microemulsion and nanoemulsion in pectin-based coating to extend the shelf life of fresh-cut orange. Journal of Food Processing and Preservation, 42(2), 1–9. https://doi.org/10.1111/jfpp.13441
Robledo, N., López, L., Bunger, A., Tapia, C., & Abugoch, L. (2018a). Effects of antimicrobial edible coating of thymol nanoemulsion/quinoa protein/chitosan on the safety, sensorial properties, and quality of refrigerated strawberries (Fragaria × ananassa) under commercial storage environment. Food and Bioprocess Technology, 11, 1566–1574. https://doi.org/10.1007/s11947-018-2124-3
Robledo, N., Vera, P., López, L., Yazdani-Pedram, M., Tapia, C., & Abugoch, L. (2018b). Thymol nanoemulsions incorporated in quinoa protein/chitosan edible films; antifungal effect in cherry tomatoes. Food Chemistry, 246, 211–219. https://doi.org/10.1016/j.foodchem.2017.11.032
Sadeghi, E., Hashemian, A. H., Soltanian, M., Soltanian, S., & Mohammadi, M. (2015a). Study of nitrite and nitrate levels in meat products distributed in Kermanshah. Iran Occupational Health, 11, 94–100.
Sadeghi, E., Hashemian, A. H., Soltanian, M., Soltanian, S., & Mohammadi, M. (2015b). Study of nitrite and nitrate levels in meat products distributed in Kermanshah. Iran Occupational Health, 11(6), 94–100.
Saggiorato, A. G., Gaio, I., Treichel, H., Oliveira, D., Cichoski, A. J., & Cansian, R. L. (2012). Antifungal activity of basil essential oil (Ocimum basilicum L.): Evaluation in vitro and on an Italian-type sausage surface. Food and Bioprocess Technology, 5, 378–384. https://doi.org/10.1007/s11947-009-0310-z
Salvia-Trujillo, L., Rojas-Graü, A., Soliva-Fortuny, R., & Martin-Belloso, O. (2013). Physicochemical characterization of lemongrass essential oil–alginate nanoemulsions: Effect of ultrasound processing parameters. Food and Bioprocess Technology, 6, 2439–2446. https://doi.org/10.1007/s11947-012-0881-y
Salvia-Trujillo, L., Rojas-Graü, A., Soliva-Fortuny, R., & Martín-Belloso, O. (2015). Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocolloids, 43, 547–556. https://doi.org/10.1016/j.foodhyd.2014.07.012
Seow, Y. X., Yeo, C. R., Chung, H. L., & Yuk, H. G. (2014). Plant essential oils as active antimicrobial agents. Critical Reviews in Food Science and Nutrition, 54(5), 625–644. https://doi.org/10.1080/10408398.2011.599504
Sharifi-Rad, J., Salehi, B., Schnitzler, P., Ayatollahi, S. A., Kobarfard, F., Fathi, M., & Sharifi-Rad, M. (2017). Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantia royleana Benth. and Pulicaria vulgaris Gaertn. Cellular and Molecular Biology, 63(8), 42–47. https://doi.org/10.14715/cmb/2017.63.8.10
Silva, H. D., Cerqueira, M. Â., & Vicente, A. A. (2012). Nanoemulsions for food applications: Development and characterization. Food and Bioprocess Technology, 5, 854–867. https://doi.org/10.1007/s11947-011-0683-7
Trombetta, D., Castelli, F., Sarpietro, M. G., Venuti, V., Cristani, M., Daniele, S., & A., Mazzanti, G., & Bisignano, G. . (2005). Mechanisms of antibacterial action of three monoterpenes. Antimicrobial Agents and Chemotherapy, 49(6), 2474–2478. https://doi.org/10.1128/AAC.49.6.2474-2478.2005
Wan, J., Pei, Y., Hu, Y., Ai, T., Sheng, F., Li, J., & Li, B. (2020). Microencapsulation of eugenol through gelatin-based emulgel for preservation of refrigerated meat. Food and Bioprocess Technology, 13, 1621–1632. https://doi.org/10.1007/s11947-020-02502-0
Wattanasatcha, A., Rengpipat, S., & Wanichwecharungruang, S. (2012). Thymol nanospheres as an effective anti-bacterial agent. International Journal of Pharmaceutics, 434(1–2), 360–365. https://doi.org/10.1016/j.ijpharm.2012.06.017
Xu, J., Zhou, F., Ji, B. P., Pei, R. S., & Xu, N. (2008). The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Letters in Applied Microbiology, 47(3), 174–179. https://doi.org/10.1111/j.1472-765X.2008.02407.x
Xu, J., Zhou, L., Miao, J., Yu, W., Zou, L., Zhou, W., Liu, Ch., & Liu, W. (2020). Effect of cinnamon essential oil nanoemulsion combined with ascorbic acid on enzymatic browning of cloudy apple juice. Food and Bioprocess Technology, 13, 860–870. https://doi.org/10.1007/s11947-020-02443-8
Xue, J., Michael Davidson, P., & Zhong, Q. (2015). Antimicrobial activity of thyme oil co-nanoemulsified with sodium caseinate and lecithin. International Journal of Food Microbiology, 210, 1–8. https://doi.org/10.1016/j.ijfoodmicro.2015.06.003
Zarzuelo, A., & Crespo, E. (2003). The medicinal and non-medicinal uses of thyme. Thyme: The Genus Thymus. New York.
Zhang, Z., Vriesekoop, F., Yuan, Q., & Liang, H. (2014). Effects of nisin on the antimicrobial activity of d-limonene and its nanoemulsion. Food Chemistry, 150, 307–312. https://doi.org/10.1016/j.foodchem.2013.10.160
Acknowledgements
This work was a part of a Ph.D. research project carried out at Islamic Azad University (Yasooj branch).
Author information
Authors and Affiliations
Contributions
Somayeh Sepahvand: Methodology, Visualization, and Writing. Sedigheh Amiri: Conceptualization, Methodology, Writing original draft, and editing. Mohsen Radi: Conceptualization, Methodology, Writing original draft, and editing. Hamid-Reza Akhavan: Writing, review, and editing.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sepahvand, S., Amiri, S., Radi, M. et al. Antimicrobial Activity of Thymol and Thymol-Nanoemulsion Against Three Food-Borne Pathogens Inoculated in a Sausage Model. Food Bioprocess Technol 14, 1936–1945 (2021). https://doi.org/10.1007/s11947-021-02689-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-021-02689-w