Abstract
Valorization of wastes has become an unavoidable goal in the olive oil industry. A possible approach is the recovery of leaf phenolic compounds, which have a great interest for food industries, due to their antioxidant and antimicrobial activities. Thus, this study aims at comparing the effects of encapsulated (e-OLE) and free olive leaf extract (f-OLE) on storage stability of salad dressings prepared as single and double emulsion systems. Creaming, rheological properties, double emulsion yield, pH, total phenol content (TPC), antioxidant activity, and peroxide value (PV) of the dressings were monitored over 90 days at 4 °C. Microstructure examination showed a more homogeneous distribution of the droplet size with the inclusion of OLE. No creaming and very little variations in rheological parameters (< 10%) were observed. OLE enrichment and double emulsion systems significantly (P < 0.05) improved the rheological behavior, with a higher effect of e-OLE due to the alginate-pectin beads. OLE enrichment extended the oxidation induction period from 15–20 days to 50 days. In conclusion, the work demonstrated that OLE encapsulation by emulsification-internal gelation technique was effective in gradually releasing polyphenols during salad dressing storage, thus increasing product protection toward oxidation phenomena.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Valorization of wastes has become an unavoidable subject in the olive oil industry owing to the negative environmental and ecological consequences of their improper disposals. Wastes consist of olive leaves—generated during harvesting—as well as pomace and wastewater, produced during oil extraction (Araújo et al. 2015). Approximately 6% leaves usually accompany harvested olives (Difonzo et al. 2017), with a significant economic loss to olive oil producers who are buying waste material together with olives. Considering the amount of olive oil and table olives produced in the world, over one billion kilograms of olive leaves are yearly generated; thus, a valorization for this industrial waste appears beneficial (Romero-García et al. 2016). The recovery of phenolic compounds from olive leaves has a great interest for food, nutraceutical, pharmaceutical, and cosmetic industries, due to their antioxidant and antimicrobial activities. Moreover, polyphenols have been widely studied for their therapeutic properties, such as the ability in delaying cancer cell proliferation, reducing low-density lipoprotein, acting as anti-inflammatories, vasodilators, immune stimulants, and antivirals (Rahmanian et al. 2015).
In food products, the oxidative degradation causes not only a loss of nutritional properties but also a sensory decay due to the development of undesired odors and flavors. Thus, extracts from olive oil wastes have been proposed as effective natural antioxidants for preservation of different kinds of foods, including emulsions (Caporaso et al. 2016a; Caporaso et al. 2016b; Di Mattia et al. 2014; Giacintucci et al. 2016; Mosca et al. 2013; Paradiso et al. 2016; Silva et al. 2013). A more general overview on recent food applications of phenolic extracts obtained from olive by-products can be found in the review by Caporaso et al. (2019).
Olive leaves contain six major polyphenolic compounds, showing an antioxidant activity higher than many other antioxidants, probably due to the synergy among flavonoids, oleuropeosides, and substituted phenols (Benavente-García et al. 2000). The antioxidant and other functional properties of olive leaf extracts (OLE) are affected by sample origin and extraction conditions (Rahmanian et al. 2015). Besides, polyphenols are characterized by a very high sensitivity to several environmental factors, including heat and light, present low water solubility in the free form, and are metabolized and eliminated from the body at a high rate. All these factors contribute to poor stability and bioavailability, drastically reducing the OLE effectiveness. Another limit to food applications is the bitter and unpleasant taste that often accompanies polyphenolic molecules. Thus, many researches focused the attention on the development of new strategies for protecting phenolic compounds from degradation and for improving their bioactivity and pleasantness. In this context, encapsulation technologies seem to be effective strategies, as well as the inclusion in emulsions (Ezhilarasi et al. 2013; Flamminii et al. 2020; Jia et al. 2016; Lamba et al. 2015; Lu et al. 2016; Parisi et al. 2014).
Salad dressings are very popular emulsified products commercialized in a broad range of forms, with a fat content ranging from 20 to 65% and different viscosities (Abdalla and Roozen 2001). They are usually prepared as single O/W emulsions. However, fractional replacement of oil droplets by an internal aqueous phase makes water-in-oil-in-water (W1/O/W2) double emulsion a protective encapsulation method for various bioactive compounds, thereby facilitating controlled release, improving bioavailability, and masking unpleasant sensory properties (Artiga-Artigas et al. 2019; Fang and Bhandari 2010; Souilem et al. 2014).
Only few studies have been carried out on the possibility of improving oxidative stability and nutritional quality of salad dressings by incorporating extracts from olive mill by-products and, to the best of our knowledge, no papers compare their effects on single and double emulsions systems. Considering the importance of filling this gap in order to increase the sustainability of the olive oil system, this work aims at investigating the effects of encapsulated and free olive leaf extract (OLE) on the physical and oxidative stability of salad dressings prepared as single O/W and double W1/O/W2 emulsion systems.
Materials and Methods
Materials
A single batch of refined corn oil (Carrefour, Boulogne-Billancourt, France) was purchased in a local supermarket and used to produce all the salad dressing samples. Xanthan gum (Comprital, Settala, Italy) was used as polysaccharide stabilizer. The OLE Oleafit Antiox Complex 40 (Gricar Chemical srl, Brugherio, Italy) was kindly provided by Panakeia (Teramo, Italy); its total phenol content (TPC) expressed in gallic acid equivalents (GAE) was 207 ± 4 mg/g dry matter (dm). The extract was used free (f-OLE) and after calcium alginate-pectin microencapsulation (e-OLE) (Flamminii et al. 2020). Briefly, a solution of alginate, pectin, and calcium citrate was emulsified with a vegetable oil to form a water-in-oil dispersion. Gelation of water droplets was promoted by the release of calcium due to pH reduction. The e-OLE contained 78 ± 1 mg GAE/g dm.
Sodium chloride, citric acid, PTSA (1,3,6,8-pyrenetetrasulfonic acid tetrasodium salt hydrate), Oil Red O pigment, and Folin-Ciocalteu phenol reagent were purchased from Merck (Darmstadt, Germany), whereas all the other reagents were obtained from Sigma-Aldrich (Steinheim, Germany).
Preparation of Salad Dressings
Six pourable salad dressing samples were produced (800 mL each) following the procedure shown in Fig. 1. Three samples were single O/W emulsions (coded as 1e) and three were W1/O/W2 double emulsions (coded as 2e). For each type of emulsion, one sample was enriched with f-OLE (samples 1eA and 2eA), one with the e-OLE (samples 1eB and 2eB), and the third sample was not enriched and used as reference (samples 1Ref and 2Ref). Dressing formulation was studied in order to simulate commercial products. All the samples had 25% oil phase mass and 75% total water phase mass; the amount of f-OLE or e-OLE to be added in polyphenol-enriched samples was calculated to obtain a final TPC of 160 mg GAE/kg.
The aqueous phase was prepared by dispersing xanthan gum (0.8 g/100 mL) and sodium chloride (0.4 g/100 mL) in deionized water containing citric acid (0.5 g/100 mL) and by stirring overnight at room temperature for complete dissolution. The given amount of OLE, when present, was dispersed in a portion of the aqueous phase and stirred for 10 min before addition into the remaining water phase.
O/W emulsion dressings were produced in a two-step homogenization process, using a heavy duty blender (Waring Blendor, Torrington, CT). The mixture, 25% oil and 75% water phase, was firstly homogenized at 18,000 rpm for 30 s, allowed to rest for 30 s, and finally homogenized at 20,800 rpm for the same time.
For W1/O/W2 dressings, OLE, when present, was dispersed only in the inner water phase of the primary emulsion (W1/O) that was prepared similarly to O/W emulsion, combining 20% water phase and 80% corn oil. Then, 31.3% W1/O and 68.7% water phase were homogenized with the same two-step procedure previously described.
All the dressing samples were poured into 50-mL glass jars with screw caps, flushing the air space with nitrogen before closing. Jars were stored at 4 °C for a maximum of 90 days. At least, eight sampling points were analyzed during storage for every sample, using one jar for each sampling time.
Optical Imaging of Salad Dressings
Microstructure of freshly prepared salad dressings was observed by a digital imaging microscope (Nikon Eclipse ME600, Nikon Instruments SpA, Campi Bisenzio, Italy) managed by the NIS Elements software (Nikon Corporation, Tokyo, Japan). A drop of sample was placed on a glass slide, covered with lid and observed under ×20 magnifying objective lens.
Creaming Stability
Creaming stability of dressings during storage was determined in triplicate as reported by Moriano and Alamprese (2020), according to a method adapted from Karaca et al. (2011). Briefly, each type of dressing (50 g) was prepared as previously described, but using as oil phase the corn oil previously stained Oil Red O pigment (0.015 g/100 mL), in order to make clearer the oil layer separation during storage.
Rheological Behavior
Rheological properties of dressing samples were analyzed using a rheometer Physica MCR 102 (Anton Paar, Graz, Austria) equipped with coaxial cylinders (CC27). Flow curves were measured in triplicate at 4 °C, ranging shear rate from 20 to 500 s-1. Experimental data were fitted with the power law model in order to calculate the consistency coefficient (K) and flow behavior index (n) (Steffe 1996).
Double Emulsion Yield
The percentage amount of inner aqueous phase remaining inside the oil droplets of double emulsions was expressed as yield and determined following the method by Perez-Moral et al. (2014), adapted as reported by Moriano and Alamprese (2020). Briefly, PTSA (0.2 g/100 mL) was added as a tracer to the inner water phase of purposely prepared W1/O/W2 samples (100 g). At every sampling point, the concentration of PTSA appeared in the external water phase was determined spectrophotometrically (V-650 spectrophotometer, Jasco Europe, Cremella, Italy) at 374 nm by using a calibration curve. The measurement was performed in quadruplicate and yield was expressed in percentage.
Measurement of pH
Dressing pH was determined in triplicate during storage using a SevenEasy pH-meter (Mettler Toledo, Columbus, OH) equipped with an electrode for liquid samples.
Polyphenol Extraction
Extraction of polyphenolic compounds from corn oil and salad dressings was carried out according to the liquid-liquid method proposed by Pirisi et al. (2000), modified as follows: 3-g sample was mixed with 15-mL methanol/deionized water solution (70:30) and 3-mL hexane. The mixture was sheared with an UltraTurrax T25 (IKA, Staufen, Germany) at 20,000 rpm for 60 s, sonicated for 30 min at 25 °C, and then centrifuged (LISA 2 L centrifuge, AFI, Château-Gontier-sur-Mayenne, France) at 4000 g (6000 rpm) for 10 min at 25 °C. The methanol/water phase was collected and centrifuged again at 9000 g (9000 rpm) for 5 min at 25 °C. This step was repeated twice and then the methanol/water phase was filtered through Whatman nylon filters 0.20 μm (GE Healthcare, Amersham, UK). For each sample, three extracts were prepared and used for TPC and antioxidant activity determination.
Total Phenol Content
TPC of corn oil and salad dressings was determined in duplicate for each sample extract, for a total of six determinations for each sample. The Folin-Ciocalteu method was applied, according to Singleton and Rossi (1965). Results are expressed as mg GAE/kg of dressing or oil.
Antioxidant Activity
Antioxidant activity of the phenolic extracts obtained from salad dressings was measured by ABTS• (2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)) and DPPH• (2,2-diphenyl-1-picrylhydrazyl hydrate) assays. ABTS• assay was carried out as described by Re et al. (1999). DPPH• test was performed according to Brand-Williams et al. (1995). In both cases, the results are expressed as μmol Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) equivalents (TE)/g of dressing, as the average of six determinations (two for each sample extract).
Peroxide Value
Peroxide value (PV) of corn oil and salad dressings was determined following the official method for olive oil (Commission Regulation (EEC) No. 2568/91 1991). For dressing samples, the analysis was carried out on the oil phase previously separated as reported by Alamprese et al. (2017), modifying the method as follows: 16-g salad dressing was mixed with 32-mL chloroform. Before solvent evaporation, the mixture was sheared with an UltraTurrax T25 (IKA, Staufen, Germany) at 20,000 rpm for 1 min and centrifuged at 9000 g (9000 rpm) for 5 min at 25 °C (LISA 2 L centrifuge, AFI, Château-Gontier-sur-Mayenne, France) in order to collect the oil/chloroform layer. Results are expressed as meqO2/kg of oil or oil phase, as the average of two measurements.
Statistical Analyses
Statgraphics Centurion 18 statistical package (Statgraphics Technologies Inc., The Plains, VA, USA) was used to perform one-way analysis of variance (ANOVA) and least significant difference (LSD) test (P < 0.05) to determine significant differences among samples and during storage.
Overall pattern recognition of the OLE-enriched salad dressing samples during storage was evaluated by principal component analysis (PCA) using The Unscrambler X software (v. 10.4, Camo ASA, Oslo, Norway). Prior to PCA analysis, data were mean centered and standardized to unit variance in order to avoid misinterpretations arising from the different orders of magnitude of both mean value and variance of the parameters analyzed.
Results
Microstructural Properties of Salad Dressings
At the optical microscope examination, a visible compartmentalized inner water phase appeared in the oil droplets of W1/O/W2 dressings (Fig. 2b and d), confirming the effectiveness of the double emulsion preparation procedure. Both single and double emulsions showed polydispersity, with a more homogeneous droplet size distribution in the OLE-enriched samples (Fig. 2c and d) than in references (Fig. 2a and b). In the OLE-enriched O/W sample (Fig. 2c), most of the oil droplets have a mean diameter of 10–20 μm, with the occasional presence of larger droplets (about 40-μm mean diameter). A higher number of large droplets appeared in the OLE-enriched double emulsion samples, especially when e-OLE was used (sample 2eB; Fig. 2d).
Physical Stability of Salad Dressings
Over the whole storage period, there were no observable creaming phenomena; all the dressing samples showed a constant CS of 100%. Similarly, the rheological behavior of samples was stable during storage. All dressings exhibited a non-Newtonian, shear-thinning behavior (Fig. 3) with differences in K and n values due to formulations (Table 1). Only in few cases, after 90 days, K and n values were significantly (P < 0.05) different from those at time 0 (Table 1), but with little variations (< 10%). The double emulsion systems showed K and n values significantly (P < 0.05) higher than those of the corresponding single emulsion samples. The OLE-enriched dressings exhibited significantly (P < 0.05) higher K values and lower n values than the reference samples. The salad dressings enriched with e-OLE (1eB and 2eB) showed viscosity and K values significantly higher (P < 0.05) than those of samples enriched with f-OLE.
In fresh double emulsion samples, yield was very high (ranging from 94.0 to 95.1%). During storage, the reference sample (2Ref) experienced no significant reduction of yield, with a final value of 94.7 ± 0.3%, representing over 99% retention rate (Fig. 4). Conversely, the OLE-enriched dressings at the end of storage reached significantly (P < 0.05) lower yield values (88.7 ± 0.4 and 88.1 ± 0.1% for 2eA and 2eB, respectively) with respect to fresh samples, but in any case, the retention rate was high (> 93%).
Chemical Stability of Salad Dressings
Fresh dressing samples had on average a pH of 2.61 ± 0.04, which remained stable over storage, with an average of 2.65 ± 0.06 after 90 days at 4 °C.
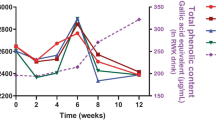
The corn oil used for dressing production and the fresh reference samples (1Ref and 2Ref) were analyzed for TPC in order to exclude a possible ingredient contribution to the phenol content of the emulsion systems. Indeed, a non-detectable level was confirmed; thus, 1Ref and 2Ref were not further analyzed for TPC during storage. Preliminary trials were performed to assess the phenol recovery efficiency of the extraction procedure applied to salad dressings. Samples containing f-OLE (1eA and 2eA) generated 158 ± 4 mg GAE/kg TPC, with approximately 100% recovery efficiency (Fig. 5a). On the contrary, when e-OLE was added (samples 1eB and 2eB), only 109 ± 1 mg GAE/kg TPC was obtained, corresponding to 69% recovery efficiency. Therefore, a factor of +31% was used to correct TPC data of the salad dressings enriched with e-OLE, accounting for the phenolic extraction loss. At the end of storage, an average TPC reduction of 8% was observed for the f-OLE-enriched dressings, whereas the addition of e-OLE resulted in a TPC increase of 10 and 2% for samples 1eB and 2eB, respectively (Fig. 5b).
Salad dressing samples enriched with olive leaf extract: a initial recoverable total phenol content; b total phenol content during storage; c ABTS radical scavenging capacity during storage; d DPPH radical scavenging capacity during storage. Samples: 1eA, single emulsion with free olive leaf extract (black square broken line); 1eB, single emulsion with encapsulated olive leaf extract (black triangle broken line); 2eA, double emulsion with free olive leaf extract (gray square solid line); 2eB, double emulsion with encapsulated olive leaf extract (gray triangle solid line)
Similarly to TPC, antioxidant capacity of the reference samples (1Ref and 2Ref) was tested only immediately after production because they did not show any detectable results. Interestingly, the same correction factor calculated for TPC data (+31%) was obtained in the preliminary ABTS• and DPPH• assays performed on e-OLE-enriched samples. During storage, antioxidant capacity of all the dressings followed the same pattern observed for TPC (Fig. 5c and d). In the ABTS• assay, the O/W salad dressings (1eA and 1eB) exhibited an initial radical scavenging ability significantly (P < 0.05) higher than that of W1/O/W2 systems. However, after 7 days of storage, all the samples settled at an average value of 0.70 ± 0.02 μmol TE/g. At the end of storage, the samples containing f-OLE (1eA and 2eA) showed an antioxidant activity nonsignificantly different from that after 7 days of storage, whereas the e-OLE-enriched samples (1eB and 2eB) revealed a significant (P < 0.05) 10% increase. Considering the DPPH• assay results, all the fresh dressings had almost the same activity (mean value of 0.50 ± 0.02 μmol TE/g), but at the end of storage, significantly (P < 0.05) higher values were measured, with a total average of 0.62 ± 0.07 μmol TE/g. The highest increase was found in single emulsion systems (about +40%).
The corn oil used in dressing preparation had a low PV (1.23 ± 0.08 meqO2/kg), according to its refined state. Considering the O/W emulsions at time 0 (Fig. 6a), PV significantly (P < 0.05) decreased from 1Ref to 1eB and 1eA. The same effect was measured also in fresh double emulsion samples, although to a lower extent (Fig. 6b). Apart from 2Ref, all the dressings at the end of storage showed a PV significantly (P < 0.05) higher than that of the corresponding fresh samples. By fitting data with a sigmoid function (Fig. 6), it was possible to notice that the reference samples had an oxidation induction time of 15–20 days, whereas in the OLE-enriched samples, the induction period lasted up to 50 days. After the induction period, the samples with f-OLE (1eA and 2eA) showed an oxidation rate higher than that of dressings with e-OLE (1eB and 2eB).
Effect of storage time on hydroperoxide formation in salad dressing samples: a single emulsions; b double emulsions. Samples: 1Ref and 2Ref, single and double emulsion references (diamond); 1eA and 2eA, single and double emulsion with free olive leaf extract (square); 1eB and 2eB, single and double emulsion with encapsulated olive leaf extract (triangle)
PCA Data Exploration
A PCA was carried out with the analytical data of all samples, with the exception of 1Ref and 2Ref because of the absence of polyphenols. Score and loading plots (Fig. 7) were reported only for the first two principal components (PC), because they explained 65% of the total data variance. The main observable sample pattern (Fig. 7a) was the separation of salad dressings based on the OLE nature; the right positive plane of PC1 was occupied by the samples containing e-OLE (1eB and 2eB), while in the left negative plane of PC1, there were the f-OLE-enriched samples (1eA and 2eA). No clear pattern of the samples based on storage time (indicated by the progressive sampling number in the sample code) was evidenced, thus confirming the intrinsic physical and chemical stability of the OLE-enriched salad dressings. Similarly, it was not possible to highlight a sample distribution linked to the type of emulsion. However, within the two groups of dressings containing f-OLE (negative values of PC1) or e-OLE (positive values of PC1), the double emulsion systems were closer with respect to the more scattered single emulsion samples.
Score (a) and loading (b) plot of the principal component analysis applied to analytical data of olive leaf-enriched samples during storage for 90 days at 4 °C. Samples: 1eA and 2eA, single and double emulsion with free olive leaf extract (square); 1eB and 2eB, single and double emulsion with encapsulated olive leaf extract (triangle); figures in the sample codes after the underscore indicate the progressive sampling number
PCA results seem to indicate a higher physical and oxidative stability of samples enriched with e-OLE, as already highlighted by the chemical evaluations; actually, these samples were in the area of the score plot (Fig. 7a) associated with the highest values of K and TPC, which had the highest loading values on PC1 (Fig. 7b). The separation of dressings based on the type of OLE used was mainly related to the rheological parameters, being dressings with e-OLE the most viscous (higher values of K) and pseudoplastic (lower values of n) samples, due to the biopolymers used for encapsulation. K and n had indeed the highest and lowest loading values on PC1, respectively (0.525 for K and −0.510 for n). The loading plot also revealed an inverse correlation of K and n, meaning that the higher consistency of samples is related to a more pronounced shear-thinning behavior. No other variable correlations were evidenced. In particular, as already discussed, ABTS and DPPH results were not correlated, nor directly linked to TPC. Negative values of PC2 were mainly associated with samples with longer storage time (from 28 days on). The most important variable on PC2 was the antioxidant activity tested by DPPH• assay (loading value on PC2 = −0.740), thus confirming the controlled release of antioxidants observed for samples enriched with e-OLE.
Discussion
Microstructural properties of the salad dressings, particularly droplet size, are important because they influence phase stability as well as rheological and sensory features (McClements 2016). In general, the microstructure examination showed that the OLE addition resulted in a more homogeneous distribution of droplet size, which can be ascribed to the emulsifying activity of polyphenols, which have an amphiphilic nature (Flamminii et al. 2019; Mosca et al. 2013). A higher number of large droplets was observed in the double emulsion samples, probably due to a higher expansion resistance of the interfacial layer conferred by the inclusion of the OLE solution (i.e., inner water phase) in the oil droplets. Actually, Souilem et al. (2014) and Di Mattia et al. (2011) demonstrated that the surface activity of oleuropein can lead to higher particle sizes in emulsions probably related to a compositional change of the O/W interface. When e-OLE was used (sample 2eB), the increase of the droplet size was even higher, due to the swelling of alginate and pectin microparticles, which have a high water holding capacity due to the nature of the polymers used (Rubio-Senent et al. 2015).
The complete absence of phase separation in conjunction with the stable rheological behavior of all the dressing samples and the high yields of double emulsions throughout the storage indicated a high physical stability of the salad dressings. This result was even more important considering that no emulsifiers were used in formulations. Only a texture modifier (xanthan gum) was added, in order to slow down possible creaming phenomena. Indeed, according to McClements (2016), creaming is typical of both O/W and W1/O/W2 emulsions, due to the lower density of the dispersed phase with respect to the continuous water phase. Moreover, creaming is one of the physical mechanisms responsible for food emulsion instability, affecting texture and shelf life of dressings. In this work, the use of xanthan gum in the salad dressing water phase resulted in a shear-thinning behavior, with high values of apparent viscosity (> 1000 mPa⋅s) at low shear rates (20 s-1), which slowed down any gravitational movement and avoided the occurrence of creaming phenomena. The thickening effect was even higher in samples enriched with e-OLE, due to the presence, besides xanthan gum, of the hydrocolloids constituting the beads (i.e., alginate and pectin). The higher values of K and n observed in double emulsions were due to the higher particle concentration generated by the inner water phase entrapped in the oil droplets. Indeed, as already reported by Moriano and Alamprese (2020) and Oppermann et al. (2016), with the increase of the volume fraction of primary W1/O emulsion, the apparent viscosity of W1/O/W2 increases.
The high yield values measured for fresh double emulsions confirmed the efficacy of the double emulsion preparation procedure, allowing the entrapment of almost all the primary water phase in the oil droplets. Yield values during storage were higher than those reported by Souilem et al. (2014) for food-grade monodisperse W1/O/W2 emulsions loaded with a hydrophilic bioactive oleuropein and similar to those reported by Perez-Moral et al. (2014) for double emulsions stabilized by polyglycerol polyricinoleate and whey protein or Tween 20.
The preliminary trials carried out to assess the suitability of the phenolic substances’ extraction demonstrated that the alginate-pectin network stabilized by calcium ions impaired a complete recovery of phenols from e-OLE. Indeed, in order to evaluate the encapsulation efficiency of alginate-pectin microparticles, a sodium citrate solution is commonly used to completely dissolve the hydrogel matrix (Belščak-Cvitanović et al. 2015; Flamminii et al. 2020). However, this approach was not adequate for phenol extraction in complex food systems like dressings; therefore, according to the obtained results, a correction factor was calculated and used for TPC and antioxidant activity data of the e-OLE-enriched salad dressings, in order to account for the phenolic extraction loss.
The polyphenols added in salad dressings were stable and retained their free radical scavenging ability during storage at 4 °C up to 90 days. Considering the acidic pH of the salad dressings, it is reasonable to presume that OLE, whether free or encapsulated, could retain antioxidant activity even in digestive physiological conditions. The significant (P < 0.05) TPC increase registered in sample 1eB could be attributed to a leakage of phenolic substances from the alginate-pectin beads of the e-OLE. The phenomenon was more evident in O/W emulsion rather than in W1/O/W2 system, probably due to the protective effect of the primary emulsion toward the inner water phase containing the e-OLE, impairing the polyphenol leakage from the oil droplets. Moreover, other studies confirmed the ability of multiple emulsions to protect hydrophilic polyphenols while controlling their release (Lu et al. 2016). The controlled release was also demonstrated by the significant (P < 0.05) increase of the free radical scavenging activity observed in samples 1eB and 2eB. However, the results of ABTS• and DPPH• tests were not completely consistent, maybe due to differences in the reaction kinetics involved, complex interfacial relationships between emulsion phases, and different antioxidant capacities of phenolic species contained in the extracts.
To complete the chemical stability investigation, PV of oil phase was determined in order to understand the role of OLE in dressing oxidation phenomena. The protecting role of OLE against oxidation was evident even during dressing production, when, as reported by Waraho et al. (2011), aeration and increased temperature could favor lipid oxidation. In fact, the OLE-enriched samples showed a significantly lower PV with respect to the reference samples. The inhibiting effect of OLE toward hydroperoxide formation in enriched emulsions confirmed the previous findings (Laguerre et al. 2015; Mohammadi et al. 2016). A higher protective effect on fresh samples was measured when the f-OLE was used, probably due to the entrapment of polyphenols in the alginate-pectin beads that slowed down the antioxidant activity. Indeed, the e-OLE-enriched samples showed a lower oxidation rate during storage, due to the controlled release of polyphenols from the alginate-pectin beads, prolonging their antioxidant activity. Similar results were found by Mohammadi et al. (2016), who demonstrated that encapsulation of phenolic compounds and their dispersion in double emulsion systems can increase antioxidant capacity thanks to a controlled release.
Different behaviors were registered for the two types of emulsions, but it must be considered that in W1/O/W2 dressings, OLE was incorporated only in the inner water phase; moreover, the oxidation phenomena in emulsions are mechanistically different from those occurring in bulk oils. For instance, the organization of fat molecules within the system (single or multiple emulsions) and their interactions with other food components, in addition to oxygen incorporated during homogenization, can influence emulsion vulnerability to oxidation (Laguerre et al. 2015).
PCA results did not evidence a clear pattern of the samples based on storage time (Fig. 7a). This is contrary to the observation of Biller et al. (2018), who observed significant impacts of storage time on the distribution of whey-enriched mayonnaise-like emulsions using PCA. On the contrary, the importance of rheological parameters in differentiating dressings with free or encapsulated OLE was observed also by Yesiltas et al. (2017) on sodium caseinate and sodium alginate stabilized emulsions. The PV values for all the samples were low and hence did not significantly influence the trends in the sample distribution.
The near center position of ABTS and DPPH in the loading plot (Fig. 7b) indicates their stability and consistency throughout the storage period and thus accounts for the low oxidation rate. pH is one of the factors that determines the effectiveness of added antioxidant in emulsion, in which acidic pH has been linked to lower rate of lipid oxidation (Mancuso et al. 1999). This explains why PV and pH are located on the same side of loading, hence suggesting more stable e-OLE-enriched dressings.
The effectiveness of OLE in stabilizing salad dressings toward physical and chemical instability, coupled with the easy and economical way of salad dressing preparation and the absence of emulsifiers, paves the way for an industrial scale-up of the production, which can valorize one of the olive oil by-products produced in the highest amounts (i.e., olive leaves), thus increasing sustainability of the olive oil system. No clear benefits were observed when OLE was entrapped in a double emulsion, even if a slightly high stability was evidenced by the multivariate analysis of the results. This type of system should be further investigated for possible beneficial effects on sensory properties (e.g., masking of bitterness) and for the development of low-fat high-quality dressings.
Conclusions
The study demonstrated the effectiveness of OLE, especially when encapsulated, in protecting salad dressings toward oxidation phenomena and physical instability. The greater effect of e-OLE is due to a gradual release of polyphenols during dressing storage, favored by the rheological properties imparted to the final product.
References
Abdalla, A. E., & Roozen, J. P. (2001). The effects of stabilised extracts of sage and oregano on the oxidation of salad dressings. European Food Research and Technology, 212(5), 551–560.
Alamprese, C., Cappa, C., Ratti, S., Limbo, S., Signorelli, M., Fessas, D., & Lucisano, M. (2017). Shelf life extension of whole-wheat breadsticks: formulation and packaging strategies. Food Chemistry, 230, 532–539.
Araújo, M., Pimentel, F. B., Alves, R. C., & Oliveira, M. B. P. P. (2015). Phenolic compounds from olive mill wastes: health effects, analytical approach and application as food antioxidants. Trends in Food Science and Technology, 45(2), 200–211.
Artiga-Artigas, M., Molet-Rodríguez, A., Salvia-Trujillo, L., & Martín-Belloso, O. (2019). Formation of double (W1/O/W2) emulsions as carriers of hydrophilic and lipophilic active compounds. Food and Bioprocess Technology, 12(3), 422–435.
Belščak-Cvitanović, A., Komes, D., Karlović, S., Djaković, S., Špoljarić, I., Mršić, G., & Ježek, D. (2015). Improving the controlled delivery formulations of caffeine in alginate hydrogel beads combined with pectin, carrageenan, chitosan and psyllium. Food Chemistry, 167, 378–386.
Benavente-García, O., Castillo, J., Lorente, J., Ortuño, A., & Del-Rio, J. A. (2000). Antioxidant activity of phenolics from Olea europaea L. leaves. Food Chemistry, 49, 2480–2485.
Biller, E., Waszkiewicz-Robak, B., Longo, E., Boselli, E., Obiedzinski, M., Siwek, A., & Stachelska, M. A. (2018). Effects of the addition of spray-dried whey on the stability of fat-reduced mayonnaise-type emulsions during storage. Journal of the American Oil Chemists' Society, 95(3), 337–348.
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology, 28(1), 25–30.
Caporaso, N., Genovese, A., Burke, R., Barry-Ryan, C., & Sacchi, R. (2016a). Effect of olive mill wastewater phenolic extract, whey protein isolate and xanthan gum on the behaviour of olive O/W emulsions using response surface methodology. Food Hydrocolloids, 61, 66–76.
Caporaso, N., Genovese, A., Burke, R., Barry-Ryan, C., & Sacchi, R. (2016b). Physical and oxidative stability of functional olive oil-in-water emulsions formulated using olive mill wastewater biophenols and whey proteins. Food & Function, 7(1), 227–238.
Caporaso, N., Formisano, D., & Genovese, A. (2019). Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Critical Reviews in Food Science and Nutrition, 58, 2829–2841.
Commission Regulation (EEC) No. 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis, (1991). Official Journal of the European Communities, L248, 1–83.
Di Mattia, C. D., Sacchetti, G., & Pittia, P. (2011). Interfacial behavior and antioxidant efficiency of olive phenolic compounds in O/W olive oil emulsions as affected by surface active agent type. Food Biophysics, 6(2), 295–302.
Di Mattia, C., Paradiso, V., Andrich, L., Giarnetti, M., Caponio, F., & Pittia, P. (2014). Effect of olive oil phenolic compounds and maltodextrins on the physical properties and oxidative stability of olive oil O/W emulsions. Food Biophysics, 9(4), 396–405.
Difonzo, G., Russo, A., Trani, A., Paradiso, V. M., Ranieri, M., Pasqualone, A., Summo, C., Tamma, G., Silletti, R., & Caponio, F. (2017). Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. Journal of Functional Foods, 31, 63–70.
Ezhilarasi, P. N., Karthik, P., Chhanwal, N., & Anandharamakrishnan, C. (2013). Nanoencapsulation techniques for food bioactive components: a review. Food and Bioprocess Technology, 6(3), 628–647.
Fang, Z., & Bhandari, B. (2010). Encapsulation of polyphenols - a review. Trends in Food Science and Technology, 21(10), 510–523.
Flamminii, F., Di Mattia, C. D., Difonzo, G., Neri, L., Faieta, M., Caponio, F., & Pittia, P. (2019). From by-product to food ingredient: evaluation of compositional and technological properties of olive-leaf phenolic extracts. Journal of the Science of Food and Agriculture, 99(14), 6620–6627.
Flamminii, F., Di Mattia, C. D., Nardella, M., Chiarini, M., Valbonetti, L., Neri, L., Difonzo, G., & Pittia, P. (2020). Structuring alginate beads with different biopolymers for the development of functional ingredients loaded with olive leaves phenolic extract. Food Hydrocolloids, 105849.
Giacintucci, V., Di Mattia, C., Sacchetti, G., Neri, L., & Pittia, P. (2016). Role of olive oil phenolics in physical properties and stability of mayonnaise-like emulsions. Food Chemistry, 213, 369–377.
Jia, Z., Dumont, M. J., & Orsat, V. (2016). Encapsulation of phenolic compounds present in plants using protein matrices. Food Bioscience, 15, 87–104.
Karaca, A. C., Low, N., & Nickerson, M. (2011). Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Research International, 44(9), 2742–2750.
Laguerre, M., Bayrasy, C., Panya, A., Weiss, J., McClements, D. J., Lecomte, J., Decker, E. A., & Villeneuve, P. (2015). What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Critical Reviews in Food Science and Nutrition, 55(2), 183–201.
Lamba, H., Sathish, K., & Sabikhi, L. (2015). Double emulsions: emerging delivery system for plant bioactives. Food and Bioprocess Technology, 8(4), 709–728.
Lu, W., Kelly, A. L., & Miao, S. (2016). Emulsion-based encapsulation and delivery systems for polyphenols. Trends in Food Science and Technology, 47, 1–9.
Mancuso, J. R., Mcclements, D. J., & Decker, E. A. (1999). The effects of surfactant type, pH, and chelators on the oxidation of salmon oil-in-water emulsions. Journal of Agriculture and Food Chemistry, 47(10), 4112–4116.
McClements, D. J. (2016). Food emulsions. Principles, practices, and techniques (3rd ed.). Boca Raton: CRC Press.
Mohammadi, A., Jafari, S. M., Esfanjani, A. F., & Akhavan, S. (2016). Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chemistry, 190, 513–519.
Moriano, M. E., & Alamprese, C. (2020). Whey protein concentrate and egg white powder as structuring agents of double emulsions for food applications. Food and Bioprocess Technology, 13(7), 1154–1165.
Mosca, M., Diantom, A., Lopez, F., Ambrosone, L., & Ceglie, A. (2013). Impact of antioxidants dispersions on the stability and oxidation of water-in-olive-oil emulsions. European Food Research and Technology, 236(2), 319–328.
Oppermann, A. K. L., Piqueras-Fiszman, B., de Graaf, C., Scholten, E., & Stieger, M. (2016). Descriptive sensory profiling of double emulsions with gelled and non-gelled inner water phase. Food Research International, 85, 215–223.
Paradiso, V. M., Di Mattia, C., Giarnetti, M., Chiarini, M., Andrich, L., & Caponio, F. (2016). Antioxidant behavior of olive phenolics in oil-in-water emulsions. Journal of Agricultural and Food Chemistry, 64(29), 5877–5886.
Parisi, O. I., Puoci, F., Restuccia, D., Farina, G., Iemma, F., & Picci, N. (2014). Polyphenols and their formulations: different strategies to overcome the drawbacks associated with their poor stability and bioavailability. In R. R. Watson, V. R. Preedy, & S. Zibadi (Eds.), Polyphenols in human health and disease (pp. 29–45). San Diego: Academic Press.
Perez-Moral, N., Watt, S., & Wilde, P. (2014). Comparative study of the stability of multiple emulsions containing a gelled or aqueous internal phase. Food Hydrocolloids, 42, 215–222.
Pirisi, F. M., Cabras, P., Falqui Cao, C., Migliorini, M., & Muggelli, M. (2000). Phenolic compounds in virgin olive oil. Reappraisal of the extraction, HPLC separation and quantification procedures. Journal of Agricultural and Food Chemistry, 48(4), 1191–1196.
Rahmanian, N., Jafari, S. M., & Wani, T. A. (2015). Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends in Food Science and Technology, 42(2), 150–172.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, 26(9-10), 1231–1237.
Romero-García, J. M., Lama-Muñoz, A., Rodríguez-Gutiérrez, G., Moya, M., Ruiz, E., Fernández-Bolaños, J., & Castro, E. (2016). Obtaining sugars and natural antioxidants from olive leaves by steam-explosion. Food Chemistry, 210, 457–465.
Rubio-Senent, F., Rodríguez-Gutiérrez, G., Lama-Muñoz, A., & Fernández-Bolaños, J. (2015). Pectin extracted from thermally treated olive oil by-products: characterization, physico-chemical properties, in vitro bile acid and glucose binding. Food Hydrocolloids, 43, 311–321.
Silva, K. A., Coelho, M. A. Z., Calado, V. M. A., & Rocha-Leão, M. H. M. (2013). Olive oil and lemon salad dressing microencapsulated by freeze-drying. LWT - Food Science and Technology, 50(2), 569–574.
Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158.
Souilem, S., Kobayashi, I., Neves, M. A., Sayadi, S., Ichikawa, S., & Nakajima, M. (2014). Preparation of monodisperse food-grade oleuropein-loaded W/O/W emulsions using microchannel emulsification and evaluation of their storage stability. Food and Bioprocess Technology, 7(7), 2014–2027.
Steffe, J. F. (1996). Rheological methods in food process engineering (2nd ed.). East Lansing: Freeman Press.
Waraho, T., McClements, D. J., & Decker, E. A. (2011). Mechanisms of lipid oxidation in food dispersions. Trends in Food Science & Technology, 22(1), 3–13.
Yesiltas, B., García-Moreno, P. J., Sørensen, A. D. M., & Jacobsen, C. (2017). Physical and oxidative stability of high fat fish oil-in-water emulsions stabilized with combinations of sodium caseinate and sodium alginate. European Journal of Lipid Science and Technology, 119(11), 1–10.
Acknowledgments
The authors wish to thank Prof. Stefano Farris of the Department of Food, Environmental, and Nutritional Sciences (University of Milan, Italy), for his support in microstructure imaging.
Funding
This work has been supported by AGER 2 Project, grant n° 2016-0105.
Author information
Authors and Affiliations
Contributions
Olusola Samuel Jolayemi: investigation, methodology; data curation, formal analysis, writing (original draft). Nicolò Stranges: investigation, methodology, data curation. Federica Flamminii: methodology, resources. Ernestina Casiraghi: funding acquisition, supervision. Cristina Alamprese: conceptualization; methodology; formal analysis; project administration; writing (original draft).
Corresponding author
Ethics declarations
Conflicts of Interest
The authors confirm that they have no conflicts of interest with respect to the work described in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jolayemi, O.S., Stranges, N., Flamminii, F. et al. Influence of Free and Encapsulated Olive Leaf Phenolic Extract on the Storage Stability of Single and Double Emulsion Salad Dressings. Food Bioprocess Technol 14, 93–105 (2021). https://doi.org/10.1007/s11947-020-02574-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02574-y