Abstract
Foxtail millet is one of the commonly cultivated, nutritionally competitive source of protein, fibre, phytochemicals and other micronutrients, as compared to major cereals like wheat and rice. Considering the potential of these grains, the high pressure processed flours of germinated (GFMF) and non-germinated foxtail millet (NGFMF) grains were studied for its functional, moisture sorption and thermodynamic properties. Germination and high-pressure processing of foxtail millet grains significantly improved the functional properties of the flour. Apart from this, the moisture sorption isotherms of both the flours were determined at 10, 25 and 40 °C and the sorption data was fitted to Guggenheim-Anderson-De Boer (GAB) sorption model. The monolayer moisture content for NGFMF and GFMF ranged between 3.235–2.364 and 2.987–2.063 g g−1, respectively. The isosteric heat of sorption ranged between − 76.35 to − 38.23 kJ mol−1 for NGFMF and 172.55 to − 34.02 kJ mol−1 for GFMF at a moisture range of 0 to 36%, whereas, the integral entropy of sorption for NGFMF ranged between − 0.404 and − 0.120 kJ mol−1 K−1 and for GFMF between − 0.667 and − 0.383 kJ mol−1 K−1. Along with the validation of the compensation theory, the values of spreading pressures lied in the range of 0–0.078 J m−2 for NGFMF and 0– 0.124 J m−2 for GFMF, while, the glass transition temperatures ranged between 82.25 and 28.67 °C for NGFMF and from 51.11 to 11.83 °C for GFMF at all three temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water sorption characteristics are necessary for predicting the shelf life and determining the moisture content and critical activity for acceptability of products that deteriorate, mainly due to moisture gain, and also for drying, packaging and storage (Katz and Labuza 1981). A food moisture sorption isotherm describes the relationship between the moisture uptake in food and the relative humidity of the air with which the food is in equilibrium at a constant temperature (Lagoudaki et al. 1993). Thermodynamics has been reported as an approach to understand the properties of water and calculate the energy requirements of heat and mass transfer in biological systems (Rizvi and Benado 1983; Fasina et al. 1997; Fasina et al. 1999). Properties such as isosteric heat of sorption, net integral enthalpy and entropy and spreading pressures are important for understanding the energy requirement during dehydration, the food microstructure, physical phenomena on the food surfaces and sorption kinetic parameters (Rizvi and Benado 1983). Also, the glass transition temperature is an important physical parameter, which serves to explain the physical and chemical behaviour of food systems (Bell and Labuza 2000), and it is defined as the temperature at which the material changes from the glassy to the rubbery state for a given heating rate.
Foxtail millet (Setaria italica (L.) P. Beauv.) is the sixth highest yielding grain amongst all the millet varieties and has been identified as a major millet in terms of worldwide production (Sharma and Niranjan 2017). Foxtail millet ranks second in the total world millet production of around 29 million tonnes (in 2015–16 season). It originates in the Yellow River Basin country, which makes China a leading foxtail millet producer holding 80% of world production, followed by India with 10% of world production (Zhang et al. 2017a, b). Foxtail millet is one of the oldest cultivated cereal grains belonging to the Setaria genus, of Poaceae family and subfamily Panicoideae. Sharma and Niranjan (2017) have discussed the versatility of foxtail millet as a food source and its potential to address food and nutrition security. These authors have reviewed the effects of various processing techniques on the properties of foxtail millet grains.
It has been recognised that non-thermal processing techniques like high hydrostatic pressures (HHP), can potentially inactivate anti-nutritional factors like phytate and tannin content in cereal grains and preserve their constituents and quality (Estrada-Girón et al. 2005; Yu et al. 2016; Zhang et al. 2017a, b). In addition to high pressure processing, germination, is one of the traditional approaches used to supplement the bio-accessibility of nutrients in plant-based diets. The process of germination in cereal grains results in an increase in enzymatic activity, soluble protein content and breakdown of starch into simple sugars, resulting in the formation of typical colours and flavours (Barreiro et al. 2003). Moisture sorption properties have also been studied for various cereals and its products like various cereal grains and legumes (Al-Mahasneh et al. 2014), milk-foxtail millet powder (Simha et al. 2016), two millet grain varieties (Aviara et al. 2016), tef (Eragrostis tef (Zucc.) Trotter) grain flour (Abebe and Ronda 2015), bulgar (Erbaş et al. 2016), Greek durum wheat semolina (Pollatos et al. 2013), RTE breakfast cereal formulation (Sandoval et al. 2009), oat and rice flour (Brett et al. 2009), maize meal and millet flours (Ikhu-Omoregbe and Chen 2005). In general, very limited moisture sorption data is available for germinated cereals, especially for high–pressure-processed and germinated millet grains. This study was therefore conducted to assess the effect of germination on the functional, moisture sorption and thermodynamic properties of high pressure processed foxtail millet grain flour.
Materials and Methods
Sample Collection
Foxtail millet (Setaria italica L.) grains were procured from authorised grain centres. All procured grains were a month old since harvesting and were tested for viability using tetrazolium chloride test (Lakon 1949). The moisture content of the native grains was found to be 8.41 ± 0.45%, following which the grains were stored in polythene bags at 4 °C until further use. The chemicals used for analysis were procured from Sigma-Aldrich Chemical Co., (St. Louis, Mo, USA).
Germination of Foxtail Millet Grains
The foxtail millet grains were washed using 1.5% formaldehyde solution to prevent any contamination. To remove the traces formaldehyde, the grains were then washed thoroughly with distilled water and tested for any residues using the ferric chloride method described by VICH Steering Committee (2002). Prior to germination, the foxtail millet grains were first soaked in deionised water for 15 h and then were subjected to germination in a controlled-environment growth cabinet. The temperature was maintained at 25 ± 2 °C and the relative humidity was controlled at 50%; these conditions were based on the work reported by Sharma et al. (2015). Following the method described by Elkhalifa and Bernhardt (2010), the activities of alpha amylase and protease were monitored at regular intervals of 12 h, and the germination period of 72 h represented the time taken for their activities to reach a maximum value. The maximum alpha amylase and protease activity was found to be attained after 72 h (Fig. 1). The germinated foxtail millet grains were dried to a final moisture content of 7–8%, packed and stored in air-tight containers at 4 °C until further analysis. Non-germinated foxtail millet grains were used as a control.
High Pressure Soaking
Both germinated and non-germinated foxtail millet grains (25 g each) were subjected to high hydrostatic pressures in a temperature-controlled pressure vessel system (vessel dimensions 37 mm diameter and 246 mm length, Food LAB 900, Stansted Fluid Power Ltd., Stansted, UK). A blend of castor oil and ethanol in a ratio of 80:20 v/v was used as a medium to transmit pressure to the packed samples in the pressure vessel chamber. The foxtail millet grains were treated at a pressure of 400 MPa for 60 min at a temperature of 60 °C. For this, 25 g foxtail millet grain samples were suspended in 150 ml of deionised water and sealed with virtually no headspace polyethylene pouches of dimensions 3 cm × 20 cm by employing Cryovac™. All the experiments were triplicated.
After high-pressure processing under specified conditions of pressure and temperature for a stipulated time, the water was completely drained from the pouches, and the water occluding to the grains separated, was then blotted using a tissue paper. The processed grains were then subjected to freeze drying and were finally milled into fine flour passing through sieves of mesh sizes ranging between 100 and 200 μm, using vibratory sieve shaker (Fritsch Analysette 3, Germany). The resulting flour was packed and stored in air-tight containers at 4 °C until further analysis.
Determination of Functional Properties
The bulk density, porosity and flowability of the optimised foxtail millet flour, as the angle of repose (as a static measure for flowability), were determined by the method of Sjollema (1963). The occluded air content was determined by the methods used by Jha et al. (2002). The wettability was determined by the method given by Muers and House (1962). The dispersibility of the optimised baby food was determined by the method described by the American Dry Milk Institute (ADMI 1965). The insolubility index was determined by the modified ADMI method described by Jha et al. (2002). The water, oil absorption capacity and swelling capacity of the product were determined by the method described by Sodipo and Fashakin (2011). The emulsion activity, emulsion stability, foaming capacity and foaming stability were determined using the methods described by Elkhalifa and Bernhardt (2010).
Finally, the gelatinization temperatures were determined using Differential Scanning Calorimetry, where a suspension of the resulting flour in deionised water was prepared in a ratio of 1:3; equilibrated for 15–18 h at ambient temperature and presented to the differential scanning calorimeter, equipped with a built-in software (Model Q 2000a TA Instruments, New Castle, USA). A 10–12 mg suspension sample was weighed precisely in an aluminium sample pan and hermetically sealed. A similar empty pan was used as a reference for the analysis. A temperature range of 10 to 140 °C was used for scanning the sample in the pan at a heating rate of 10 °C min−1. The resulting thermogram gave the values of onset (T o), peak (T p) and conclusion temperatures (T c) in oC.
Sorption Studies
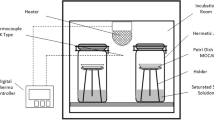
Gravimetric method described by Lang et al. (1981), was used to determine the sorption isotherms for processed foxtail millet flour. The saturated salt solutions of eight reagent-grade salts, namely lithium chloride, magnesium chloride, potassium carbonate, magnesium nitrate, sodium chloride, potassium chloride, potassium nitrate and potassium sulphate, were chosen as mentioned by Greenspan (1977) to cover the water activity range from 0.11 to 0.97. It is important to note that the water activity values for these salts can show slight variations at different temperatures. Therefore, different water activity values of these salts at temperatures 10, 25 and 40 °C were used in this study. These saturated solutions were placed in air tight, wide mouth glass jars. Approximately 1 g of sample was weighed into a glass beaker placed on glass bead support inside the glass jar. The glass beakers were equilibrated for 3 days with the humid atmosphere before placing the sample. Then the moisture sorption characteristics were studied at 10, 25 and 40 °C (± 2 °C) by placing the sample containing glass jars in incubators for equilibration. These conditions were chosen in this study, as they represent the possible storage conditions for cereals like rice (Ondier et al. 2012) The samples in bottles were weighed at a regular interval of 3 days till a constant weight was attained. Equilibrium was considered to have accomplished when the weight difference between two successive weighing was less than 1 mg. Experiment was conducted in triplicates for all the samples at each relative humidity and temperature conditions. After the equilibrium has been attained, the moisture content of the samples was determined and was expressed in gram per litre (g/L). The average standard deviation between the replicates was calculated to be less than 1% of the mean of the three values. These average values of equilibrium moisture content were then plotted against relative humidities or water activities to obtain moisture sorption isotherms.
Sorption Model
Various approaches have been used to describe sorption isotherms of foods. Amongst which, Guggenheim-Anderson-De Boer (GAB) (Pérez-Alonso et al. 2006) is mostly used to study the correlation between the equilibrium moisture content (M) and water activity (a w ) for various foods. The following linearized form of GAB model were used for evaluating the best fitted values of model constants using linear regression:
The GAB model could be rearranged into a second-degree polynomial equation as given below:
where, a, b and c are model constants and significantly depend on type of regression analysis. A nonlinear regression analysis of a w /W v/s a w yields a polynomial equation of the second order. The coefficients a, b and c were thus obtained from this polynomial equation and then substituted to obtain GAB constants W o, G and k. W o is the moisture content corresponding to saturation of all primary adsorption sites by one water molecule, G is the Guggenheim constant and k is the factor correcting for properties of multilayer molecule with respect to the bulk liquid.
Thermodynamic Properties
The integral enthalpy, also known as net isosteric heat of sorption, was determined using Clausius-Clayperon equation (Rizvi 1986; Bell and Labuza 2000):
where, T is the absolute temperature (K), Q st is the net isosteric heat of sorption (kJ mol−1) and R is the universal gas constant (8.314 × 10−3 kJ mol−1 K−1).
The calculation procedure assumed that net heat of sorption was independent of temperature change. The slope of the plot of ln (a w ) versus 1/T at constant moisture content gave the net isosteric heat of sorption (Eq. 3). Adding the latent heat of vaporisation of pure water at 10, 25 and 40 °C to the net isosteric heat of sorption (Q st), gave the values of isosteric heat of sorption. A cubic spline interpolation was used to determine a w values at various temperatures for a given moisture content (M).
Substituting free Gibbs energy in the Gibbs-Helmholtz equation (McMinn and Magee 2003; Kaya and Kahyaoglu 2005), the differential entropy was calculated using the following equation:
where, λ is the latent heat of vaporisation of pure water (44.045 kJ mol−1 at 25 °C), and ΔS is the integral entropy of sorption (kJ mol−1 K−1). The maximum storage stability point is when the water activity and temperature gives the minimum value of integral entropy (Alpizar-Reyes et al. 2017).
From the intercept of the plot of ln (a w ) versus 1/T at specified moisture levels of eq. (4), the integral entropy was evaluated.
Isokinetic Theory
A linear relationship between the isosteric heat of sorption (differential enthalpy) and differential entropy established the enthalpy-entropy theory (McMinn and Magee 2003; Arslan and Togrul 2006):
where, Q st is the isosteric heat of sorption (kJ mol−1), T β is the isokinetic temperature (K), and α is the constant or Gibb’s free energy (kJ mol−1).
The isokinetic temperature (T β ) is a characteristic property of the material surface (Aguerre et al. 1986), and it represents the slope of the enthalpy-entropy linear relationship (Eq. 5). Linear regression of Q st with ΔS d evaluated T β . A statistical test proposed by Krug et al. (1976) verified the enthalpy-entropy compensation theory by defining the harmonic mean temperature (T hm) as:
where, T hm is the harmonic mean temperature (K), and n is the number of temperature levels. Linear enthalpy-entropy compensation theory applies only when T β ≠ T hm; the process is enthalpy driven if T β > T hm and entropy driven if T β < T hm (Leffler and Grunwald 1963). Eq. (6) shows that T hm is a function of temperature levels selected for the experiment and is independent of the material considered. Analyses were conducted with α = 0 to evaluate the effect of temperature on the sorption behaviour by introducing a temperature correction factor (Aguerre et al. 1986) as:
Spreading Pressures
Spreading pressure was evaluated based on the analytical procedure described by Iglesias et al. (1976) and Fasina et al. (1999). Spreading pressure is given by the following equation:
where, π is the spreading pressure (J m−2), K is the Boltzman constant (1.380 × 10−23 J K−1) and A m is the surface area of a water molecule (1.06 × 10−19 m2).
This integral becomes indeterminate at a w = 0.0. Therefore, spreading pressure was evaluated by dividing the total limit (0.0 to a w ) into a very small interval of 0.0 to 0.05 and the remaining 0.05 to a w . The integral in the first infinitesimal a w range (0.0 to 0.05) was evaluated assuming a linear relationship (Henry’s law) between m and a w with usual notations (Fasina et al. 1999) as:
The integral in the second a w range (0.05 to a w ) was evaluated after fitting isotherm data (m, a w ) to the GAB model equation, and the spreading pressure was obtained by integrating the equation in the second interval of 0.05 to a w (Iglesias et al. 1976):
Adding the results of the two selected a w intervals produced the spreading pressures at any studied temperature and water activity combination.
Glass Transition Temperature
Differential scanning calorimetry was used to determine the glass transition temperature. A suspension of processed foxtail millet flours and deionised water was prepared in a ratio of 1:3 and equilibrated for 15–18 h at ambient temperatures and were analysed using a TA Instruments model Q-2000 differential scanning calorimeter, equipped with a built-in software (TA Instruments, Newcastle, USA). For this, 10–12 mg of flour, equilibrated at a range of water activities (0.11–0.85) and temperatures (10, 25 and 40 °C) were weighed in an aluminium sample pan and hermetically sealed. A similar empty pan was used as a reference for the analysis. A temperature range of − 50 to 150 °C was used for scanning the sample in the pan with a heating rate of 2°°C min−1 (Alpizar-Reyes et al. 2017). The resulting thermogram gave the values of onset (T o), peak (T p) and conclusion temperatures (T c) in oC and gelatinisation enthalpies (ΔH) in kJ kg−1. The final values obtained were an average of three replicates for each sample, with a maximum deviation of less than 1.5%. The midpoint of the baseline shift obtained using DSC was taken as the glass transition temperature (T g). The measurements were made in triplicates. Further, to deduce the plasticizing effect of water on T g, the DSC data was put in the Gordon-Taylor equation:
where, W 1 and W 2 are the mass fractions of the flours and of water, respectively, T g1 is the T g value of flour at zero moisture content and T g2 is the T g value of water (− 135 °C), and K is a constant.
Statistical Analysis
The differences amongst the samples were determined using one-way analysis of variance (ANOVA) using Minitab 17.0 software. Using the Duncan’s test the differences were considered statistically significant at p value ≤ 0.05. The degree of fitness of the model was evaluated using determination coefficient (R 2).
Results and Discussion
Functional Properties of the Flours
The functional properties of the processed germinated foxtail millet flour (GFMF) as well as non-germinated foxtail millet flour (NGFMF) are shown in Table 1. Germination of foxtail millet increased the values of porosity, occluded air content, water and oil absorption capacity, dispersibility, swelling capacity, wettability, flowability, gelatinization enthalpy, emulsification activity and stability; while it decreased the values of bulk density, insolubility index, foaming capacity and stability. Albarracín et al. (2016) reported that the water absorption and water solubility values were higher for germinated brown rice samples as compared to the control brown rice samples. Similar results were also stated by Chinma et al. (2009) for varieties of germinated tigernut flour; Devisetti et al. (2014) for millet flours and Elkhalifa and Bernhardt (2010) for germinated sorghum flour.
Moisture Sorption Characteristics
The effect of germination was studied for the stability of the high pressure processed foxtail millet flours.
Equilibrium Moisture Content- Water Activity
The relationship between the EMC and water activity (a w ) at a constant temperature forms the MSI. Therefore, foods with different moisture content have different water activity, thus they form their own sorption isotherm (Alpizar-Reyes et al. 2017). Figure 2a, b shows the experimental isotherms for NGFMF and GFMF at 10, 25 and 40 °C, respectively, with which the thermodynamic functions related to moisture sorption in porridge flours were determined. Both figures depict that the EMC increased with increasing water activity ranging from 0.11 to 0.97 at a constant temperature. A constant linear increase in EMC at lower water activities ranging from 0.11 to 0.55 was observed for NGFMF and from 0.11 to 0.76 for GFMF, which could be due to the ability of the flour to lower the vapour pressure, with the decrease in the relative humidity. While, a rapid increase in EMC at higher water activities from 0.55 to 0.97 for NGFMF (Fig. 2a) and an impulsive increase (Fig. 2b) in the EMC from 0.76 to 0.97 for GFPF could be attributed to the high sorptive capacity of hydrophilic starch in foxtail millet flours consisting of a large number of free hydroxyl sites (Pollatos et al. 2013). This impulsive increase in the case of GFMF could be because of elevated enzyme activity and breakdown of complex sugars into simple sugars during germination (Wu et al. 2013). Barreiro et al. (2003) also found similar results for malted barley flours. Therefore, it could be concluded from the results that GFMF would require improved storage conditions with relative humidity not exceeding 76% and NGFMF not exceeding 55%.
The increase in temperatures did not have a significant effect on the moisture sorption behaviour of both the flours. Though it was observed that for NGFMF, the EMC decreased with the increase in temperatures for a given water activity, showing a less hygroscopic behaviour of NGFMF at higher temperatures. This is because high temperatures increase the kinetic energy of the water molecules, thus increasing the distance between them and causing easy binding of these water molecules to the suface of the flours (Pollatos et al. 2013; Alpizar-Reyes et al. 2017). On the other hand, an opposite trend was depicted by GFMF, which could be attributed to the higher content of sugars due to germination, thus increasing the active sites for the sorption of water by proteins (Barreiro et al. 2003; Pollatos et al. 2013). In addition to this, the MSI’s of both NGFMF and GFMF were of the characteristic sigmoidal shape (BET II type), which is likely found in all types of dry foods rich in starch and sugars.
Sorption Models
The experimental sorption data for both the flours were fitted using GAB model, with R 2 value of more than 0.998 at all temperatures and RMSE value of less than 0.051. The model parameters were determined using non-linear regression method. Tables 2 and 3 shows the model parameters for NGFMF and GFMF, respectively. MSIs of both the flours can be divided into an (1) initial monolayer region (a w less than 0.2), where the water molecules are strongly absorbed as critical moisture content that starts the chemical reactions in foods. It is also a measure of the availability of active sorption sites; (2) an intermediate multilayer region (a w in the range of 0.2–0.6), that causes lipid oxidation and physical changes in foods and (3) a final condensed water region (a w greater than 0.6), which ascertains the microbial growth (Labuza and Altunakar 2007). The values of monolayer moisture content (W o) for both NGFMF and GFMF decreased with the increase in temperatures from 10 to 40 °C, as expected by the theory of physical sorption (Pollatos et al. 2013). The values for W o for NGFMF and GFMF ranged between 3.235–2.364 and 2.987–2.063 g g−1, respectively. The composition and nature of the product was responsible for the difference in the values of the monolayer moisture content in germinated and non-germinated flours. Thus, NGFMF being a starch rich flour was found to be more hygroscopic and GFMF being a sugar rich flour due to germination, was found to be less hygroscopic.
The constant G is related to the monolayer sorption heat which associates the chemical potential difference with the superior layers of moisture in the flour structure. It has been established that low temperatures assist the formation of strong adsorbent and adsorbate interactions, thus causing an increase in the G values with the increase in temperature (Alpizar-Reyes et al. 2017). While, this study reported a decrease in the G values on increasing the temperature from 10 to 25 °C and then an increase in the G values on further increasing the temperature from 25 to 40 °C, for both NGFMF and GFMF. Thus, it suggested that the flour-water interactions in both NGFMF and GFMF decreased as follows: 10 °C > 40 °C > 25 °C. These results agreed with Pollatos et al. (2013) and Simha et al. (2016).
Another constant k is related to the enthalpy of water multilayer sorption indicating a measure of the interactions between multilayer molecules and adsorbent. When, k values are in the range of 0.24 to 1.0, GAB model best describes the sigmoid-shaped isotherms (Lewicki 1997). For NGFMF and GFMF, the k values were in the range of 0.780–0.881 and 0.852–0.944, respectively. Since the values k < < 1, so it was inferred that the flours comprised of a structured state of adsorbate in the adjacent layers to the monolyer (Alpizar-Reyes et al. 2017).
Thermodynamic Properties
Dried foods like the flours that have been studied here, undergo various changes during storage due to the processes like crystallisation, dissolution, diffusion, swelling, etc. Therefore, various thermodynamic properties like integral enthalpy and entropy determines the condition of maximum food stability.
Net Isosteric Heat of Sorption
The degree of bound water in the flour estimates the variation in the integral enthalpy which is also known as net isosteric heat of sorption (Q st). It indicates the interaction of water vapour with solid substrates in the flours, thus explaining the endothermic or exothermic nature of the interaction (Pushpadass et al. 2014). Fig. 3a, b) shows the relationship between Q st and the moisture content at constant temperature for both NGFMF and GFMF. The negative values of Q st in the graph indicated the exothermic nature of sorption for both flours. Strong water-substrate interactions were suggested at low moisture contents, as the water binding capacity of the flours increased with the increase in the negative values of Q st. The values of Q st ranged between − 76.35 to − 38.23 kJ mol−1 for NGFMF and 172.55 to −34.02 kJ mol−1 for GFMF at a moisture range of 0 to 36%. At the higher moisture contents, the value of Q st became close to the values of heat of sorption of pure water, thus indicating the excess moisture bounded to the flours or condensation in the capillaries of flour particulates. The difference in the Q st of both flours at low moisture contents indicated that the degree of binding of water molecules was higher for GFMF as compared to the NGFMF. This could be because of the availability of the higher energy polar sites on the surface in the initial stages of sorption of GFMF, causing water molecules to form a monomolecular layer. In addition to this, as the moisture content further increased for both the flours, the availability of these sites decreased, and sorption occurred at less active sites, yielding lower heats of sorption (Lagoudaki and Demertzis 1994). Similar findings were observed for chestnut and wheat flours (Moreira et al. 2010); Japanese durum wheat flour (Chuma et al. 2012); whole wheat flour (Martín-Santos et al. 2012) and Greek durum wheat semolina (Pollatos et al. 2013).
Integral Entropy
The integral entropy of sorption (ΔS) for NGFMF ranged between − 0.404 and − 0.120 kJ mol−1 K−1, with a minimum value at 4% moisture content and 40 °C temperature and for GFMF between − 0.667 and − 0.383 kJ mol−1 K−1, with a minimum value at 8% moisture content and 10 °C temperature. Figure 4a, b shows the integral entropy as a function of moisture content at 10, 25 and 40 °C for both flours. These findings were in accordance with Simha et al. (2016). The minimum ΔS value was observed for GFMF as compared to NGFMF, thus interpreting that GFMF is more stable than NGFMF at a temperature range of 10 to 40 °C. This stability is because the water molecules in the flour forms more ordered arrangement, bond between adsorbate and adsorbent being the strongest. Therefore, less moisture is available for spoilage reactions. The subsequent increase in the values of ΔS with the increase in moisture content for both the flours, reflected the formation of multi-layers and freely held molecules of water (Rizvi 1986). The increasing temperatures also had a great impact on the integral entropy of both the flours. With the increase in temperature from 10 to 40 °C, the values of ΔS decreased for NGFMF and increased for GFMF, suggesting that NGFMF was more stable at higher temperatures, while GFMF was more stable at lower temperatures. This could be attributed to the composition of the flours.
Enthalpy-Entropy Compensation Theory
The isokinetic theory or the enthalpy-entropy compensation theory determines the existence of true compensation by comparing the difference between the harmonic temperature (T hm) and isokinetic temperature (T β ) (T hm ≠ T β ). This theory helps in evaluating the sorption reactions, confirming if the reactions are enthalpy driven or entropy driven. According to Leffler and Grunwald (1963), if T β > T hm, the process is enthalpy driven, while if T β < T hm, the process is considered to be entropy controlled. The T hm values were found to be 297 and 303 K at all three temperatures for NGFMF and GFMF, respectively. The results of this study showed that the T β values were greater than T i at all three temperatures, therefore it was concluded that the sorption reactions for both NGFMF and GFMF were enthalpy-controlled processes in the studied moisture content range. This further confirmed the predominance of mesopores in the interactions between water-flour (Azuara and Beristain 2006). These values were calculated by fitting the data in Eq. 7. Figure 5a, b depicts the enthalpy-entropy compensation obtained by plotting the integral properties of both flours at constant temperatures of 10, 25 and 40 °C. The linear relationship between the two thermodynamic properties confirmed the existence of compensation theory, which has been already justified for various foods by Fontan et al. (1982).
Spreading Pressures
The spreading pressures (π) represents the excess of free energy at the surface of the flours, and provides an indication of the increase in surface tension at free sorption sites due to adsorbed molecules (Fasina et al. 1999). The spreading pressure isotherms for both NGFMF and GFMF were plotted against the water activity at 10, 25 and 40 °C (Fig. 6a, b). The values of π lied in the range of 0–0.078 J m−2 for NGFMF and 0–0.124 J m−2 for GFMF, indicating higher surface tension on the surface of GFMF at high water activities. The graph showed that the value of π increased with increasing water activity and decreased with increasing temperatures. While, at water activities above 0.76, the values of π significantly increased with increasing temperatures. Similar trend was observed for cassava flour (Ayala-Aponte 2016) and milk-foxtail millet powder (Simha et al. 2016).
Glass Transition Temperatures
DSC thermograms obtained for both NGFMF and GFMF at a range of water activities between 0.11 and 0.97 at 10, 25 and 40 °C, gave the values of glass transition temperatures (T g). T g was obtained from the midpoint of the typical heat capacity change in the thermogram line over a range of temperatures (Sandoval et al. 2009). Increasing temperatures did not have any significant effect on the T g values of both flours. Figure 7a, b shows the relationship between T g and water activity at constant temperature. It was observed that as the water activity increased, the values of T g decreased linearly for both the flours, ranging between 82.25 and 28.67 °C for NGFMF and from 51.11 to 11.83 °C for GFMF at all three temperatures. The lower values of GFMF could be attributed to the conversion of starch into sugars and other compounds due to germination of foxtail millet flours. The low molecular weight of sugars and other compounds formed of germination, has been reported to cause plasticizing effect of small molecules on starchy products (Chen and Yeh 2000). While, the decrease in T g values with increasing temperatures and water activities could be explained by the plasticizing effect of water. Increasing temperatures causes decrease in the attractive forces, thereby causing slow motion of water molecules, that gets easily adsorbed on the flour surfaces, thus decreasing the T g values. Whereas, increasing water activities, increases the moisture content in the flours that acts as plasticizers, thus changing the glassy material into rubbery state (Alpizar-Reyes et al. 2017).
The experimental values of T g for NGFMF and GFMF stored at different water activities and temperatures were fitted in Eq. 3.8.12 with a R 2 value of 0.991. The estimated values of T g were found to be 124.36 °C for NGFMF and 101.82 °C for GFMF. This difference in the result could be due to erratic decrease in the experimental T g values with increasing water activity, predominantly in NGFMF as compared to GFMF. Similar justification was provided for lower T g values of dry amorphous materials in comparison to pure starch materials by Sandoval et al. (2009).
Conclusion
The germinated and non-germinated flours obtained from high-pressure-processed foxtail millet grains were studied for their moisture sorption characteristics to understand the interdependence between the water and the foxtail millet grain components. Thus, the moisture sorption isotherms provided information regarding the stability of these flours in any environment. Apart from this, the thermodynamic properties were also studied that gave information on the changes that took place in the flours during storage. Sorption moisture isotherms showed a sigmoidal type II shape. Experimental data for these isotherms at 10, 25 and 40 °C fitted satisfactorily to the GAB model. Sorption properties of processed germinated and non-germinated foxtail millet flour indicated that the percent of bound water decreased for NGFPF and increased for GFPF, as the temperature increased, confirming that the availability of active sites decreased due to a reduction in the total sorption ability for NGFPF and vice-versa for GFPF. This work also suggested that the moisture sorption, thermodynamic properties, spreading pressures and glass transition temperatures can be related to obtain conditions guaranteeing maximum stability for storage of the flours.
References
Abebe, W., & Ronda, F. (2015). Flowability, moisture sorption and thermal properties of tef [Eragrostis tef (Zucc.) Trotter] grain flours. Journal of Cereal Science, 63, 14–20.
ADMI. (1965). Standards for grades of dry milk including methods of analysis. USA: American Dry Milk Institute Bulletin.
Aguerre, R. J., Suarez, C., & Viollaz, P. E. (1986). Enthalpy-entropy compensation in sorption phenomena: application to the prediction of the effect of temperature on food isotherms. Journal of Food Science., 51(6), 1547–1549.
Albarracín, M., Talens, P., Martínez-Navarrete, N., González, R. J., & Drago, S. R. (2016). Physicochemical properties and structural characteristics of whole grain Oryza sativa L. with different treatments. Food Science and Technology International, 22(4), 333–342.
Al-Mahasneh, M., Alkoaik, F., Khalil, A., Al-Mahasneh, A., El-Waziry, A., Fulleros, R., & Rababah, T. (2014). A generic method for determining moisture sorption isotherms of cereal grains and legumes using artificial neural networks. Journal of Food Process Engineering, 37, 308–316.
Alpizar-Reyes, E., Carrillo-Navas, H., Romero-Romero, R., Varela-Guerrero, V., Alvarez-Ramírez, J., & Pérez-Alonso, C. (2017). Thermodynamic sorption properties and glass transition temperature of tamarind seed mucilage (Tamarindus indica L.) Food and Bioproducts Processing., 101, 166–176.
Arslan, N., & Togrul, H. (2006). The fitting of various models to water sorption isotherms of tea stored in a chamber under controlled temperature and humidity. Journal of Stored Products Research, 42(2), 112–135.
Aviara, N. A., Ojediran, J. O., Marwan, S. U., & Raji, A. O. (2016). Effect of moisture sorption hysteresis on thermodynamic properties of two millet varieties. Agricultural Engineering International: CIGR Journal, 18(1), 363–383.
Ayala-Aponte, A. A. (2016). Thermodynamic properties of moisture sorption in cassava flour. Dyna, 83(197), 138–144.
Azuara, E., & Beristain, C. I. (2006). Enthalpic and entropic mechanisms related to water sorption of yogurt. Drying Technology, 24(11), 1501–1507.
Barreiro, J. A., Fernández, S., & Sandoval, A. J. (2003). Water sorption characteristics of six row barley malt (Hordeum vulgare). LWT-Food Science and Technology, 36(1), 37–42.
Bell, L. N., & Labuza, T. P. (2000). Moisture sorption: practical aspects of isotherm measurement and use. St. Paul: American Association of Cereal Chemists.
Brett, B., Figueroa, M., Sandoval, A. J., Barreiro, J. A., & Müller, A. J. (2009). Moisture sorption characteristics of starchy products: oat flour and rice flour. Food Biophysics, 4(3), 151–157.
Chen, C. M., & Yeh, A. I. (2000). Expansion of rice pellets: examination of glass transition and expansion temperature. Journal of Cereal Science, 32(2), 137–145.
Chinma, C. E., Adewuyi, O., & Abu, J. O. (2009). Effect of germination on the chemical, functional and pasting properties of flour from brown and yellow varieties of tigernut (Cyperus esculentus). Food Research International, 42(8), 1004–1009.
Chuma, A., Ogawa, T., Kobayashi, T., & Adachi, S. (2012). Moisture sorption isotherm of durum wheat flour. Food Science and Technology Research, 18(5), 617–622.
Devisetti, R., Yadahally, S. N., & Bhattacharya, S. (2014). Nutrients and antinutrients in foxtail and proso millet milled fractions: Evaluation of their flour functionality. LWT-Food Science and Technology, 59(2), 889–895.
Elkhalifa, A. E. O., & Bernhardt, R. (2010). Influence of grain germination on functional properties of sorghum flour. Food Chemistry, 121(2), 387–392.
Erbaş, M., Aykin, E., Arslan, S., & Durak, A. N. (2016). Adsorption behaviour of bulgur. Food Chemistry, 195, 87–90.
Estrada-Girón, Y., Swanson, B. G., & Barbosa-Cánovas, G. V. (2005). Advances in the use of high hydrostatic pressure for processing cereal grains and legumes. Trends in Food Science and Technology, 16, 194–203.
Fasina, O., Sokhansanj, S., & Tyler, R. (1997). Thermodynamics of moisture sorption in alfalfa pellets. Drying Technology, 15(5), 1553–1570.
Fasina, O. O., Ajibola, O. O., & Tyler, R. T. (1999). Thermodynamics of moisture sorption in winged bean seed and gari. Journal of Food Process Engineering, 22(6), 405–418.
Fontan, C. F., Chirife, J., Sancho, E., & Iglesias, H. A. (1982). Analysis of a model for water sorption phenomena in foods. Journal of Food Science, 47(5), 1590–1594.
Greenspan, L. (1977). Humidity fixed points of binary saturated aqueous solutions. Journal of Research of the National Bureau of Standards, 81(1), 89–96.
Iglesias, H. A., Chirife, J., & Viollaz, P. (1976). Thermodynamics of water vapour sorption by sugar beet root. Journal of Food Technology, 11(1), 91–101.
Ikhu-Omoregbe, D. I. O., & Chen, X. D. (2005). Use of sorption isotherms for the estimation of shelf life of two Zimbabwean flours. Developments in Chemical Engineering and Mineral Processing, 13(1–2), 79–90.
Jha, A., Patel, A. A., & Singh, R. R. B. (2002). Physico-chemical properties of instant kheer mix. Le Lait, 82(4), 501–513.
Katz, E. E., & Labuza, T. P. (1981). Effect of water activity on the sensory crispness and mechanical deformation of snack food products. Journal of Food Science, 46(2), 403–409.
Kaya, S., & Kahyaoglu, T. (2005). Thermodynamic properties and sorption equilibrium of pestil (grape leather). Journal of Food Engineering, 71(2), 200–207.
Krug, R. R., Hunter, W. G., & Grieger, R. A. (1976). Enthalpy-entropy compensation. 2. Separation of the chemical from the statistical effect. The Journal of Physical Chemistry, 80(21), 2341–2351.
Labuza, T. P., & Altunakar, B. (2007). Water activity prediction and moisture sorption isotherms. Water activity in foods: fundamentals and applications, John Wiley & Sons, 1, 109–131.
Lagoudaki, M., & Demertzis, P. G. (1994). Equilibrium moisture characteristics of dehydrated food constituents as studied by a modified inverse gas chromatographic method. Journal of the Science of Food and Agriculture, 65(1), 101–109.
Lagoudaki, M., Demertzis, P. G., & Kontominas, M. G. (1993). Moisture adsorption behaviour of pasta products. LWT-Food Science and Technology, 26(6), 512–516.
Lakon, G. (1949). The topographical tetrazolium method for determining the germinating capacity of seeds. Plant Physiology, 24(3), 389.
Lang, K. W., McCune, T. D., & Steinberg, M. P. (1981). A proximity equilibration cell for rapid determination of sorption isotherms. Journal of Food Science, 46(3), 936–938.
Leffler, J. E., & Grunwald, E. (1963). Rates and equilibria of organic reactions: As treated by statistical, thermodynamic, and extrathermodynamic methods. New York: John Wiley & Sons, Dover Publications.
Lewicki, P. P. (1997). The applicability of the GAB model to food water sorption isotherms. International journal of food science & technology, 32(6), 553–557.
Martín-Santos, J., Vioque, M., & Gómez, R. (2012). Thermodynamic properties of moisture adsorption of whole wheat flour. Calculation of net isosteric heat. International Journal of Food Science & Technology, 47(7), 1487–1495.
McMinn, W. A. M., & Magee, T. R. A. (2003). Thermodynamic properties of moisture sorption of potato. Journal of Food Engineering, 60(2), 157–165.
Moreira, R., Chenlo, F., Torres, M. D., & Prieto, D. M. (2010). Water adsorption and desorption isotherms of chestnut and wheat flours. Industrial Crops and Products, 32(3), 252–257.
Muers, M. M., & House, T. U. (1962). A simple method for comparing wettability of instant spray dried separated milk powder. In Copenhagen, Denmark: XVI International Dairy Congress, 8, 299.
Ondier, G. O., Siebenmorgen, T. J., & Mauromoustakos, A. (2012). Equilibrium moisture contents of pureline, hybrid, and parboiled rice kernel fractions. Applied Engineering in Agriculture, 28(2), 237.
Pérez-Alonso, C., Beristain, C. I., Lobato-Calleros, C., Rodríguez-Huezo, M. E., & Vernon-Carter, E. J. (2006). Thermodynamic analysis of the sorption isotherms of pure and blended carbohydrate polymers. Journal of Food Engineering, 77(4), 753–760.
Pollatos, E. P., Riganakos, K. A., & Demertzis, P. G. (2013). Moisture sorption characteristics of Greek durum wheat semolina. Starch-Stärke, 65(11–12), 1051–1060.
Pushpadass, H. A., Emerald, F., Chaturvedi, B., & Rao, K. J. (2014). Moisture sorption behavior and thermodynamic properties of gulabjamun mix. Journal of Food Processing and Preservation, 38(6), 2192–2200.
Rizvi, S. S. H. (1986). Thermodynamic properties of foods in dehydration. In M. A. Rao, S. S. H. Rizvi, & A. K. Datta (Eds.), Engineering properties of food (3rd ed.). Boca Raton: CRC Press.
Rizvi, S. S. H., & Benado, A. L. (1983). Thermodynamic analysis of drying foods. Drying Technology, 2(4), 471–502.
Sandoval, A. J., Nuñez, M., Müller, A. J., Della Valle, G., & Lourdin, D. (2009). Glass transition temperatures of a ready to eat breakfast cereal formulation and its main components determined by DSC and DMTA. Carbohydrate Polymers, 76(4), 528–534.
Sharma, N., & Niranjan, K. (2017). Foxtail millet: properties, processing, health benefits and uses. Food Reviews International. https://doi.org/10.1080/87559129.2017.1290103.
Sharma, S., Saxena, D. C., & Riar, C. S. (2015). Antioxidant activity, total phenolics, flavonoids and antinutritional characteristics of germinated foxtail millet (Setaria italica). Cogent Food & Agriculture, 1(1), 1081728.
Simha, H. V., Pushpadass, H. A., Franklin, M. E. E., Kumar, P. A., & Manimala, K. (2016). Soft computing modelling of moisture sorption isotherms of milk-foxtail millet powder and determination of thermodynamic properties. Journal of Food Science and Technology, 53(6), 2705–2714.
Sjollema, A. (1963). Some investigations on the free-flowing properties and porosity of milk powders. Netherlands Milk and Dairy Journal, 17(3), 245–259.
Sodipo, M. A., & Fashakin, J. B. (2011). Physicochemical properties of a complementary diet prepared from germinated maize, cowpea and pigeon pea. Journal of Food, Agriculture and Environment, 9(3–4), 23–25.
VICH Steering Committee (2002). Testing of residual formaldehyde. VICH International Cooperation on Harmonization of Technical Registration of Veterinary Medical Products, 4–6.
Wu, F., Chen, H., Yang, N., Wang, J., Duan, X., Jin, Z., & Xu, X. (2013). Effect of germination time on physicochemical properties of brown rice flour and starch from different rice cultivars. Journal of Cereal Science, 58(2), 263–271.
Yu, Y., Ge, L., Ramaswamy, H. S., Wang, C., Zhan, Y., & Zhu, S. (2016). Effect of high-pressure processing on moisture sorption properties of brown rice. Drying Technology, 34(7), 783–792.
Zhang, Q., Ge, L., Ramaswamy, H. S., Zhu, S., Yu, L., & Yu, Y. (2017a). Modeling equilibrium moisture content of Brown Rice as affected by high-pressure processing. Transactions of the ASABE, 60(2), 551–559.
Zhang, L., Li, J., Han, F., Ding, Z., & Fan, L. (2017b). Effects of different processing methods on the antioxidant activity of 6 cultivars of foxtail millet. Journal of Food Quality. https://doi.org/10.1155/2017/8372854.
Acknowledgements
Nitya Sharma gratefully acknowledges the support provided by the Commonwealth Scholarship Commission (INCN-2015-124).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, N., Goyal, S.K., Alam, T. et al. Effect of Germination on the Functional and Moisture Sorption Properties of High–Pressure-Processed Foxtail Millet Grain Flour. Food Bioprocess Technol 11, 209–222 (2018). https://doi.org/10.1007/s11947-017-2007-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-2007-z