Abstract
Cereal β-glucan concentrates can be used in gluten-free breads to improve dough handling properties and quality of final products as well as to enhance their nutritional value; however, the presence of endogenous β-glucanases in rice flour, in combination with prolonged mixing, fermentation, and proofing time, can cause a substantial reduction in β-glucan molecular weight, affecting detrimentally their efficacy for bioactivity. In this study, microwave (MIWA) heating was applied to the rice flours before breadmaking at different flour water contents (13–25%) and treatment times (0-4 min) to reduce β-glucanase activity. Gluten-free breads made from the MIWA-treated rice flours were fortified with oat β-glucan concentrate to enhance their nutritional profile. The molecular weight of added β-glucan in the final products increased with increasing both flour water content and time of MIWA treatment, reflecting the magnitude of residual β-glucanase activity in the flour. Pretreatment with MIWA radiation for 4 min of the rice flour tempered at 25% moisture resulted in negligible residual β-glucanase activity and preserved to a great extent the molecular weight of β-glucans in the enriched breads. End-product quality was not affected by flour MIWA pretreatment, and even a slightly higher loaf specific volume was noted for breads made from the MIWA-treated flours (4 min MIWA at 25% moisture content) compared to that of untreated flour. These findings can contribute to the improvement of nutritional value of rice-based gluten-free breads for celiac consumers as well as of any β-glucan-containing yeast-leavened bakery product without altering its sensorial attributes. Additional studies are still required for further evaluation of the effect of more intense microwave treatment on rice flour and its application on breadmaking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Celiac disease (CD) is a genetically linked autoimmune disorder affecting the gastrointestinal system and characterized by life-long intolerance to the ingestion of gluten. CD has a diverse clinical picture ranging from tangible symptoms such as nutrient malabsorption, diarrhea, weight loss, and abdominal discomfort to vaguer symptoms such as iron and folic acid deficiency, arthralgia, fatigue, and osteoporosis (Sollid and Lundin 2009). The demand of gluten-free (GF) products is increasing as a result of the increased number of CD-diagnosed patients. Market trends have encouraged extensive research for the development of gluten-free products, especially breads and other bakery items (Houben et al. 2012; Foschia et al. 2016). Generally, dough and bread development without gluten involves the use of diverse ingredients and additives with the aim of imitating the viscoelastic properties of gluten and thereby obtaining high-quality products (Demirkesen et al. 2014; Hager and Arendt 2013; Sciarini et al. 2010). Hydrocolloids are one group of polysaccharides which can fulfill this need. They are used in gluten-free breads to improve the dough handling properties and enhance the quality attributes (volume, texture, moisture retention, etc.) and shelf-life of bread (Ahmad Mir et al. 2016). Moreover, some hydrocolloids, belonging to the dietary fiber group, are used in dough formulations to enhance the nutritional value of gluten-free breads. Nowadays, a wide range of non-starch polysaccharides, including cereal β-glucan (BG), are thought to exert several nutritive and physiological effects in the human digestive track.
The US Food and Drug Administration (USFDA) (2005) and, recently, the European Food Safety Authority (EFSA) have approved health claims according to which barley and oat β-glucan ingestion, with a daily consumption of 3 g of β-glucan-soluble fiber, leads to the reduction of blood plasma cholesterol levels, which is a major risk factor for the development of coronary heart disease (EFSA 2011a). Other health claims for oat and barley β-glucans were also approved by EFSA concerning the reduction in post-prandial glycemic responses, at recommended doses of about 4 g of β-glucans per 30 g of available carbohydrates in bread and pasta products (EFSA 2011b), and the increase of fecal bulk (EFSA 2011c); the latter claim can be used for foods containing barley or oat grain fiber of at least 6 g/100 g product or 3 g/100 kcal.
Our previous works have demonstrated the potential of baking rice-based gluten-free (GF) breads enriched with commercial BG concentrates to fulfill the EFSA health claim requirements as well as to provide products with acceptable quality (Perez-Quirce et al. 2014; Ronda et al. 2015). However, a reduction of the BG molecular weight in rice-based GF breads compared with that of the initial concentrates used as ingredients in the formulation mixture has been noted probably implying the presence of endogenous β-glucanase activity in rice flour (Hager et al. 2011; Ronda et al. 2015). It seems to be necessary to minimize BG depolymerization during food processing in order to retain the full physiological impact of β-glucans in formulated products, since it is related to the viscosity of β-glucan aqueous dispersions; the latter is a function of molecular weight and concentration of the polysaccharide (Tosh et al. 2008; Wood 2007; Wolever et al. 2010).
The activity of endogenous flour β-glucanases, in combination with the long contact time during mixing, fermentation, and proofing, can cause a substantial reduction in β-glucan molecular weight during production of β-glucan-containing yeast-leavened baked products (Aman et al. 2004; Andersson et al. 2004, 2009; Lazaridou et al. 2014; Ronda et al. 2015; Trogh et al. 2004). The inactivation of flour enzymes and the use of relatively short processing time have been proposed as effective means to minimize β-glucan degradation (Andersson et al. 2004; Lazaridou et al. 2014; Moriartey et al. 2010; Vatandoust et al. 2012). Some methods previously used for the β-glucanase inactivation were autoclaving, scalding, oven heating, microwave heating, and ethanol refluxing (Lazaridou et al. 2014; Rieder et al. 2015; Moriartey et al. 2010; Perez-Quirce et al. 2016). Among these options, the process with the greatest potential on the inactivation of endogenous β-glucanase rice flour seemed to be microwave heating since it has been previously found that a treatment for just 4 min after tempering of the flour to 25% moisture content resulted in enzyme inactivation (Perez-Quirce et al. 2016); moreover, the degree of crystallinity of the starch was unaffected and the side effects of such treatment on flour pasting and thermal properties were rather negligible. Lazaridou et al. (2014) and Perez-Quirce et al. (2016) have recently evaluated the β-glucanase inactivation including the flour tempering up to a certain moisture level before the thermal treatments as a critical parameter for the sufficient enzyme inactivation. However, maintaining the molecular weight of β-glucans in rice-based breads when they are fortified with these polysaccharides has not been yet verified. Furthermore, the effect of the microwave heating on quality attributes of the gluten-free breads made with the microwave-treated rice flours is still unknown.
Therefore, in the present study, gluten-free breads fortified with a high molecular weight β-glucan concentrate were made from rice flours pretreated by microwave (MIWA) radiation at different times and moisture levels aiming at the retention of β-glucan molecular size in breads and hence at maximizing their physiological functionality. Further to the amount and molecular weight of β-glucans found in the end-products, the effects of rice flour heat treatment on physical properties of flours and gluten-free breads were also explored.
Materials and Methods
Materials
Five samples of rice flour, varying in water content and time of microwave heating, were examined in this work. Rice flour from an Indica variety was supplied by Herba Ricemills SLU (Tarragona, Spain), having 13.12% moisture, 79.1% starch, 0.46% ash, 7.5% protein, and 0.49% fat. The particle size distribution of the flour was 6% > 150 μm, 150 μm > 63.2% > 100 μm, and 30.8% < 100 μm according to data provided by the manufacturer. Different combinations of flour water content and time of MIWA treatment that could lead to five almost equally spaced residual β-glucanase activities, corresponding to about 0, 25, 50, 75, and 100% β-glucanase inactivation, have been tested. These treatment conditions are shown in Table 1 and were adopted according to the findings from a previous work (Perez-Quirce et al. 2016).
A high molecular weight oat (1→3),(1→4)-β-d-glucan concentrate available on the market (PromOat™) was supplied by Biovelop AB (Kimstad, Sweden) and was further purified. The proximate composition of this commercial concentrate as provided by the supplier was 6% moisture, 54–56% carbohydrates (dextrins), <4.5% protein, 1–3% ash, 0.5–1% fat, and β-glucan content 33–36%. The purification protocol involved aqueous extraction of the polysaccharide (50 °C × 1 h) using an aqueous slurry of the PromOat™ flour (1:40 solid/liquid) followed by centrifugation (2400×g × 30 min). The supernatant was digested (37 °C × 3 h) by porcine pancreas α-amylase (100.000 U/g flour, Megazyme International Ltd., Bray, Ireland), concentrated (90 °C × 2 h to ½ of the volume), and β-glucan was precipitated with two volumes of ethanol (4 °C × 24 h). Finally, the polysaccharide was re-suspended in 2-propanol (4 °C × 24 h), filtered, and dried (50 °C × 18 h) to obtain a high molecular weight β-glucan preparation (HMW-BG). As a result of this purification scheme, the PromOat™ was concentrated from 33 to 72% β-glucan content (HMW-BG) as assessed by the mixed-linkage (1→3),(1→4)-β-d-glucan assay kit purchased from Megazyme.
The ingredients used in the breadmaking process, like salt, sugar, and sunflower oil, were obtained from the local market, whereas the hydroxypropyl methylcellulose (HPMC)-4KM preparation was a gift from Dow Chemical (Midland, EEUU).
Methods
Microwave Treatment of Rice Flour Samples
Rice flours were heated in a Panasonic Inverter NN-GD566M (Osaka, Japan) microwave oven following the method previously developed by Perez-Quirce et al. (2016), according to which samples of hydrated flours (50.0 g) were introduced and hermetically closed into polyamide and polypropylene bags and subsequently heat-treated with microwave power (900 W) applied in cycles of 20-s intervals combined with downtimes of 1 min. Several batches from each treatment were processed and then mixed in order to obtain the required amount of flour required for the breadmaking.
A particular attention was paid to achieve a uniform temperature and constant humidity during the MIWA process. The moisture contents of the flour before and after the microwave treatment were determined following the AACC 44-19 method (AACC 2000), and the water required to adjust it to a certain value was calculated as in a previous work (Perez-Quirce et al. 2016); the tempering procedure of flours before MIWA treatment was also described in the latter study.
β-Glucanase Activity Determination
β-Glucanase activity in control (untreated) and heat-treated rice flours was assessed by measuring the rate of decrease in specific viscosity of a dilute solution of a pure β-glucan preparation, following addition of flour extracts, according to a method described in our previous works (Lazaridou et al. 2014; Perez-Quirce et al. 2016); flour extracts (1:10 w/w rice flour/water) were obtained by aqueous extraction (25 °C × 25 min), and they were mixed with an aqueous solution (0.1% w/v) of a high molecular weight (2 × 106) β-glucan preparation (purity 95%). The mixture was then transferred into an Ubbelohde glass capillary viscometer (UBBEL04NC, K 0.01, range 2–10 cSt, brand Paragon Scientific Ltd, Wirral, UK), and the specific viscosity (ηsp = (η − η0) / η0, where η and η0 is the viscosity of above mixture and water, respectively) was measured over a 1-h period at 20 ± 0.1 °C every 5-min intervals. The ηsp data versus time were fitted to a linear regression model, and the β-glucanase activity in rice flours was calculated from the slope of the fitted line and expressed as the decrease in specific viscosity per hour of the pure β-glucan solution upon addition of the flour extracts. Residual β-glucanase activity of each treated flour sample as well as enzyme activity of the untreated flour were analyzed at least in triplicate.
Pasting Properties of Rice Flour Aqueous Dispersions
Pasting properties were studied in the microwave-treated and control flours by the Rapid Visco Analyzer (RVA-4, Newport Scientific Pvt. Ltd., Australia) using the ICC Standard method 162. The pasting temperature, peak viscosity, holding strength, or trough viscosity, as well as breakdown, final, and setback viscosity, were calculated from the pasting curve using the Thermocline v.2.2 software. Viscoamylography of aqueous flour dispersions (3 g of 14% moisture basis flour in 28 g of total weight) was carried out in triplicate.
Breadmaking Process
A straight dough process was performed using the following formula on a 100 g rice flour basis: 92% water, 6% oil, 5% sucrose, 2% HPMC, 1.8% salt, and 3% dried yeast. GF dough-making was achieved by blending the solid ingredients first in a kitchen-aid professional mixer (Model 5KPM50, KitchenAid, St. Joseph, MI, USA) for 2 min at speed 2. Then, the liquid ingredients (oil and water at 20 ± 2 °C) were added and mixed for 8 min at speed 4. The dough (200 g) was placed into an aluminum pan and proofed at 30 °C and 90% relative humidity for 50 min. Subsequently, baking was carried out in a Sveba Dahlen oven (Fristad, Sweden) at 170 °C for 20 min with steam injection for 7 s at the beginning of the process. After baking, breads were removed from the pan and stored for 1 h at room temperature before further analysis. Breads from MIWA-treated rice flours enriched with the HMW-BG preparation were also made at 3.9% of pure β-glucan fortification level following the same breadmaking process.
Content and Molecular Weight Determination of β-Glucan in Breads
The content and molecular weights of the added BG in enriched breads were determined to evaluate any change during the breadmaking process due to endogenous β-glucanase activity of rice flour. The concentration of BG in bread was determined using the mixed-linkage (1→3),(1→4)-β-d-glucan assay kit of Megazyme. For molecular weight evaluation, the β-glucans were firstly extracted from the fortified breads according to an isolation protocol described in details elsewhere (Lazaridou et al. 2014); this includes an aqueous digestion with Termamyl 120 L (1% v/v, Novozymes), followed by a suspension with Fuller’s earth for protein removal, hydrolysis with xylanase (100 U/100 g bread, Megazyme), exhaustive dialysis, concentration, and repeated precipitations with ethanol. The contents of β-glucan concentrates derived from the bread crumbs were 55–80% as assessed by the respective assay kit of Megazyme.
The apparent peak molecular weight (Mp) of the extracted BG from breads was estimated following the method described in detail by Lazaridou et al. (2004, 2014) using a high-performance size exclusion chromatography system (HPSEC) system, which consisted of a single pump (Marathon IV, Rigas Labs, Thessaloniki, Greece), a guard TSKPWH column and two SEC columns in series, 7.5 × 300 mm TSK G6000 PW and 7.5 × 600 mm TSK G5000 PW (Tosoh Bioscience GmbH, Stuttgart, Germany), and a refractive index (RI) detector (ERC-7515A, ERC Inc., Nishiaoki, Kawaguchi City, Japan).
Evaluation of Bread Quality
For loaf-specific volume determination, breads were weighed after removal from the pan and cooling down and the loaf volume was measured in four replicates using a VolScan Profiler 300 analyzer (Stable Micro Systems, Surrey, UK). Texture parameters of the bread crumb were evaluated in quadruplicate samples with a TA.XT2 Texture Analyzer (Stable Micro Systems, Surrey, UK) using the software “Texture Expert”; for this analysis, an aluminum 20-mm-diameter cylindrical probe was employed to submit crumb specimens to a 2-cycle compression test (Texture Profile Analysis, TPA) at 1 mm/s speed test, with a 30-s delay between first and second compression, and at a deformation level up to 50%. This test was carried out at 20 ± 3 °C on bread slices, with 20 mm thickness, taken from the center of each loaf. Hardness (N), chewiness (N), cohesiveness, springiness, and resilience of the bread crumb were calculated from the TPA curves.
Bread crust color was measured with a Minolta spectrophotometer CN-508i (Minolta Co., Ltd., Japan); results were obtained in the CIE L*a*b* and CIE L*C*h coordinates using the D65 standard illuminant and the 2° standard observer (International Commission on Illumination, CIE). Color determinations were made 4 × 4 times; i.e., color parameters were measured in four different bread loaves at four different points of their crust.
Statistical Analysis
The Statgraphics Centurion v.6 (Bitstream, Cambridge, MN, USA) was used for ANOVA analysis, and significant differences (p < 0.05) between samples were identified by the least significant difference (LSD) test.
Results and Discussion
Effect of Microwave Treatment on β-Glucanase Activity of Rice Flours
The β-glucanase activity in rice flour estimated from the rate of decrease in ηsp of a β-glucan solution-flour extract mixture significantly (p < 0.05) decreased with increase of MIWA treatment time and flour moisture content before heating (Table 1). The enzyme activity seemed to be eliminated when 4 min heating by microwave radiation applied to rice flour tempered at 25% moisture content before the treatment; these findings are in agreement with our previous study (Perez-Quirce et al. 2016) The flour temperatures at the end of the MIWA treatment were 84 °C (treatment 2), 93 °C (treatment 3), and 96 °C (treatments 4 and 5). The five microwave treatments (including no treatment) led to rice flours with β-glucanase activities 100, ∼75, ∼50, ∼25, and 0% of the value of the native flour (Table 1). This range allowed us to study the effect of β-glucanase activity of rice flour on the molecular weight of the resultant BG in the enriched breads.
Pasting Properties of Rice Flours
The pasting properties of aqueous dispersions of the treated rice flours were studied to evaluate the impact of microwave heat treatment on starch functionality. Minor differences were noted between the pasting properties of the native rice flour and those of the microwave-treated samples (Table 2) in agreement with our previous study (Perez-Quirce et al. 2016). Significant, although small, differences (p < 0.05) were only obtained between the control and the most intense microwave-treated flours, i.e., the sample tempered at the highest moisture level (25%) and submitted to the longest treatment time (4 min). The slight increase in trough viscosity in combination with the decrease in breakdown viscosity of the latter flour implies that microwave-treated flours are more stable during continuous heating and agitation as has been reported by several researchers for other hydrothermally treated flours (Adebowale et al. 2005; Hormdok and Noomhorm 2007; Olayinka et al. 2008; Watcharatewinkul et al. 2009). Such changes in pasting properties of heat-treated starches have been attributed to associations between the polymeric chains in the amorphous regions of the starch granule as well as to changes in crystallinity caused by the hydrothermal treatment. The structural modifications were found more pronounced as the flour moisture content before the hydrothermal treatment increased (Olayinka et al. 2008). As the intra-granular chain interactions strengthen (annealing effects), the reorganized starch structures require more heat energy for structural disintegration and paste formation; i.e., a higher pasting temperature, as found in the current study (Table 2), indicates a more dense cross-linking within the starch granules. A decrease of the peak viscosity was observed by Pinkrová et al. (2003) by increasing temperature and power output upon microwave treatment applied on rice grain at a moisture level of 30%. Luo et al. (2006) have reported even more marked changes in the starch granule structure when maize starch tempered at 30% moisture content and treated for 20 min with microwaves at 1 W/g. The absence of such effects in our MIWA-treated flours could be due to the shorter treatment times and the lower MIWA power applied as well as the use of completely airtight bags, in which water loss from the sample was probably hindered by the vapor pressure raised within the bag headspace (Perez-Quirce et al. 2016). Overall, the proposed MIWA treatment of rice flour, besides the efficient elimination of β-glucanase activity that was the main goal of the hydrothermal process, only slightly affected starch functionality.
Effect of Microwave Treatments on Gluten-Free Bread Quality Attributes
To explore the effect of the MIWA treatment on breadmaking properties of flours, breads were prepared using the microwave-treated rice flour under different conditions that result in various flour β-glucanase activities. The quality characteristics of the resultant breads are summarized in Table 3. Breads made from the most intensively treated flour (MIWA treatment 5) reached the highest specific volume among all breads, ∼7% higher than that of the control flour (MIWA treatment 1). On the other hand, the intermediate-treated flours (MIWA treatments 2 and 3) led to lower specific volumes, with a maximum decrease of 14% compared with the bread loaves which had the highest specific volume. This could be attributed to the high temperature reached during these MIWA treatments (84 and 93 °C, respectively) that can denature other enzymes than β-glucanases, including the α-amylases necessary for bread development (Caballero et al. 2007; Gujral et al. 2003); the reduction of α-amylase activity would explain the lower loaf volume. However, when the MIWA treatment became more intense (treatments 4 and 5), possibly the effect derived from the reduction of α-amylase activity is masked by the differences in the pasting properties, e.g., higher pasting temperature, peak viscosity, and final viscosity, which led to higher bread volume during the baking step compared to the control product. The higher viscosity of the dough matrix might restrict the coalescence phenomenon and allow a better retention of the gas produced during fermentation. At the same time, the higher pasting temperature would allow a greater development of the dough during baking before the fixation of the crumb structure upon baking (Ronda et al. 2016). Nevertheless, there were no large differences either in volume and appearance of bread loaves or in crumb structure with the use of MIWA-pretreated rice flours (Table 3 and Fig. 1).
The crumb hardness values varied between 0.67 and 1.02 N (Table 3). Breads with the lowest specific volume showed the higher hardness, in agreement with the negative correlation between these parameters reported in earlier works (Perez-Quirce et al. 2014; Ronda et al. 2015); i.e., the lower the specific volume, the smaller the crumb air fraction and thus the more compact the structure. However, there was no apparent trend between the intensity of MIWA treatment and crumb hardness (Table 3). The treated flour breads showed lower resilience than the control bread, whereas breads made with untreated rice flours exhibited the highest crumb chewiness among all samples. Springiness of the control bread and those of the less treated flours were significantly lower than breads from flours heated for a longer time. Instead, crumb cohesiveness was not affected by rice flour pretreatment. However, the differences in texture parameters noted among the tested samples did not seem to be large. Furthermore, the flour tempered at the highest moisture level (25%) gave loaves with the highest water loss during the baking process, i.e., ∼7.4% higher weight loss than that of the control bread (Table 3). However, as can be seen in Table 4, the bread moisture content was not significantly different among all breads studied.
Regarding the crust color, breads made from flour with the greatest extent of enzyme inactivation (MIWA treatment 5) showed the highest L* value (Table 3). In addition, h and C* values increased as the MIWA treatment time of the flour increased. This means that bread made with treated flours were more yellowish, lighter, and with more vivid colors than the control bread. It is possible that some of the enzymes responsible for color development (α-amylases) have been partially inactivated by the heat treatment and thus the color is lighter since the Maillard reactions proceed at a lower rate and extent than in breads from the untreated flour (control), i.e., a lower concentration of reducing sugars in the fermented dough (Pyler and Gorton 2000).
Effect of β-Glucanase Inactivation of Flour on Molecular Weight of β-Glucan Isolated from Fortified Gluten-Free Breads
The β-glucan content in the final products fortified with the β-glucan isolate was similar to that expected from the amount of polysaccharide added to the dough formulation (Table 4); a slight decline in the BG content in breads was noted only in products made by flours with high β-glucanase activity (MIWA treatments 1 and 2) (Tables 1 and 4). The level (3.9% of pure β-glucan on a rice flour basis) of HMW-BG added to the gluten-free formulations can meet the health claim requirements of the US Food and Drug Administration (FDA 2005) and EFSA (EFSA 2011a) for the reduction of serum cholesterol and can be accomplished by a daily intake of ∼170–214 g product which, on average, corresponds to four servings of 50 g of the GF fortified with β-glucan breads.
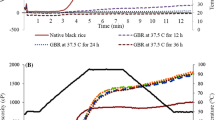
The molecular weight distributions of the HMW-BG preparation added to the gluten-free formulations as well as the β-glucan concentrates derived from breads made from rice flours submitted to different microwave pretreatments were analyzed by a HPLC-SEC-RI system to evaluate any changes in molecular weight of the polysaccharide during breadmaking (Fig. 2); the Mp values of these preparations were estimated from the peak fraction of the eluting peaks of the polysaccharides using a calibration curve made with β-glucan standards. A large portion of the eluting peak and the peak fraction of the HMW-BG sample were eluted in the void volume of the size exclusion columns, i.e., the β-glucan added to the gluten-free doughs had a Mp > 9 × 105 Da. However, for the β-glucans isolated from the control bread made from the untreated rice flour, there was a large reduction in Mp, as the main eluting peak was ∼0.23 × 105 Da, presumably due to polysaccharide degradation by endogenous β-glucanases of the rice flour during the breadmaking process (Table 1). Similarly, several researchers have previously noted a considerable reduction of β-glucan molecular weight during production of yeast-leavened bakery products from oat bran as well as rye and barley flour which was attributed to endogenous β-glucanase activity (Aman et al. 2004; Andersson et al. 2004, 2008, 2009; Lazaridou et al. 2014; Trogh et al. 2004); apparently, the molecular weight of β-glucans in the final products decreases with increasing mixing and dough fermentation time. Recently, an endogenous β-glucanase activity was also found in rice flour (Perez-Quirce et al. 2016) which can cause severe reduction of oat and barley β-glucans during breadmaking when these polysaccharides are added as concentrates to rice-based gluten-free bread formulations (Ronda et al. 2015).
HPSEC elution profiles detected by RI and apparent peak molecular weight, Mp (slanted solid arrows) of the eluting peaks of the high molecular weight oat β-glucan concentrate (HMW-BG) added to the gluten-free formulations and of the β-glucan extracted from the crumbs of the fortified breads made by the microwave (MIWA)-treated flours; MIWA treatment conditions (moisture content and heating time) of the rice flours are also given on the respective elution curves. The slanted dotted arrows show the Mp of minor eluting peaks or the molecular weight of the fraction of shoulders. The vertical arrows indicate the elution time of the peak fraction of six (1 → 3),(1 → 4)-β-d-glucan standards (Mp: 15, 33, 83, 186, 340, and 466 × 103 Da) used for plotting of the standard curve (inset); (1 → 3),(1 → 4)-β-d-glucan standard with Mp 941 × 103 Da is eluted at the void volume of the columns. All Mp values showed on the figure is expressed in Daltons (Da)
Tempering of rice flour up to 16% moisture content followed by 1 min microwave heating resulted in bread with higher Mp (0.61 × 105 Da) compared to that made from the untreated flour (Fig. 2), presumably due to a partial decrease in β-glucanase activity as evidenced from the data in Table 1. A minor fraction of β-glucans in both breads from the control and this treated (MIWA treatment 1) flour preserved their initial molecular size, since small peaks representing 3 and 6% of the chromatograph area, respectively, had Mp > 9 × 105 Da (Fig. 2, slanted dotted arrow). With increasing time of MIWA treatment up to 4 min for the flour tempered at 16% moisture, there was a gradual increase in β-glucan molecular weight as shown by the shifting of polysaccharide eluting peaks to lower retention times and increase of Mp up to 4.37 × 105 Da (Fig. 2); this observation is consistent with the decline in β-glucanase activity of the respective treated flours (Table 1). The eluting profile of β-glucans in bread prepared from the flour heated with MIWA for 2 min showed two main peaks with Mp values of 1.41 and 0.23 × 105 Da which represent 61 and 39% of the total chromatograph area, respectively (Fig. 2). Similar bimodal molecular weight distributions of β-glucans isolated from oat, rye, and barley breads have been previously reported and attributed also to the endogenous β-glucanase action in the flours (Aman et al. 2004; Andersson et al. 2004, 2009).
With increase in flour moisture level to 25% before MIWA treatment, there was a further increase in the molecular weight of β-glucan in bread, as indicated by the higher Mp value, 5.01 × 105 Da, compared to that from flour with 16% moisture level treated with MIWA for the same time, 4 min (Fig. 2). In contrast, for MIWA treatment 5, it seemed that the β-glucans maintained to a considerable extent their initial molecular size, since a large portion of the eluted polysaccharides were of high molecular weight (>9 × 105 Da) and appeared as a shoulder of the main peak eluted in the void volume of the chromatograph (slanted dotted arrow). This fact is in accordance with the β-glucanase activity of the respective treated flour used for breadmaking which appeared to be negligible, i.e., apparently, non-detectable activity by the employed viscometric method (Table 1). As found previously, increased levels of flour moisture are crucial for adequate β-glucanase inactivation, most likely due to the drop of denaturation temperature of the enzyme with increasing water content (Lazaridou et al. 2014; Perez-Quirce et al. 2016). Perez-Quirce et al. (2016) have recently reported that residual activity of endogenous β-glucanase in rice flour decreased with increasing time of microwave heating and moisture level of the flour; for instance, an increase in initial water content up to 19 and 25% in rice flour followed by microwave treatment for 8 and 4 min, respectively, resulted in non-measurable β-glucanase activity using the same viscometric method. In the present study, the slight drop of β-glucan molecular weight during production of breads made by the MIWA treatment 5 flour (with no apparent β-glucanase activity) could be attributed either to minor residual enzyme activity, non-detectable by the viscometric method, or to oxidative degradation reactions involving β-glucans during breadmaking; apparently, there is evidence for hydroxyl radical mediated depolymerization of β-glucans that could occur in cereal baked products (Kivela et al. 2011).
Conclusions
Microwave pretreatment of hydrated rice flours used as a base material to produce gluten-free breads, fortified with β-glucans, do not cause reduction in the molecular weight of the bioactive polysaccharide upon baking, presumably due to inactivation of the endogenous rice flour β-glucanases. The β-glucan molecular weight in the final product increased with time of microwave heating and initial moisture content of the flour in accordance with the magnitude of the residual endogenous β-glucanase activity found in the treated flours. A slight increase in loaf volume was also observed for the breads made from the rice flour treated for the longest time, while no practically important changes in the pasting properties of the flour as well as in texture and color of the final products were noted as a result of flour microwave treatment. The findings of the present study could contribute to improving the quality and bioactivity of rice-based gluten-free baked products, containing cereal β-glucan concentrates, to broaden the food item choices for celiac consumers. Additional studies are still required to extensively evaluate the effect of more intense microwave treatments on rice flour functionality and its applicability on the breadmaking process.
References
Adebowale, K. O., Afolabi, T. A., & Olu-Owolabi, B. I. (2005). Hydrothermal treatments of finger millet (Eleusine coracana) starch. Food Hydrocolloids, 19, 974–983.
Ahmad Mir, S., Ahmad Shah, M., Rashid Naik, H., & Ahmad Zargar, I. (2016). Influence of hydrocolloids on dough handling and technological properties of gluten-free breads. Trends in Food Science & Technology, 51, 49–57.
Aman, P., Rimsten, L., & Andersson, R. (2004). Molecular weight distribution of β-glucan in oat-based foods. Cereal Chemistry, 81, 356–360.
American Association of Cereal Chemists (AACC). (2000). Method 44–19. In Anonymous approved methods of the AACC (10th ed.). St. Paul: The Association.
Andersson, A. A. M., Armo, E., Grangeon, E., Fredriksson, H., Andersson, R., & Aman, P. (2004). Molecular weight and structure units of (1-3, 1-4)-β-glucans in dough and bread made from hull-less barley milling fractions. Journal of Cereal Science, 40, 195–204.
Andersson, A. A. M., Ruegg, N., & Aman, P. (2008). Molecular weight distribution and content of water-extractable β-glucan in rye crisp bread. Journal of Cereal Science, 47, 399–406.
Andersson, R., Fransson, G., Tietjen, M., & Aman, P. (2009). Content and molecular-weight distribution of dietary fiber components in whole-grain rye flour and bread. Journal of Agricultural and Food Chemistry, 57, 2004–2008.
Caballero, P. A., Gomez, M., & Rosell, C. M. (2007). Bread quality and dough rheology of enzyme-supplemented wheat flour. European Food Research Technology, 224, 525–534.
Demirkesen, I., Kelkar, S., Campanella, O. H., Sumnu, G., Sahin, S., & Okos, M. (2014). Characterization of structure of gluten-free breads by using X-ray Microtomography. Food Hydrocolloids, 36, 37–44.
EFSA. (2011a). Scientific opinion on the substantiation of a health claim related to barley beta-glucans and lowering of blood cholesterol and reduced risk of (coronary) heart disease pursuant to article 14 of regulation (EC) no. 1924/2006. EFSA Journal, 9(2470), 14.
EFSA. (2011b). Scientific opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13 (1) of Regulation (EC) No. 1924/2006. EFSA Journal, 9(2207), 21.
EFSA. (2011c). Scientific opinion on the substantiation of health claims related to oat and barley grain fibre and increase in faecal bulk (ID 819, 822) pursuant to Article 13 (1) of Regulation (EC) No. 1924/2006. EFSA Journal, 9(2249), 13.
Foschia, M., Horstmann, S., Arendt, E. K., & Zannini, E. (2016). Nutritional therapy—facing the gap between coeliac disease and gluten-free food. International Journal of Food Microbiology, 239, 113–124.
Gujral, H. S., Haros, M., & Rosell, C. M. (2003). Starch hydrolyzing enzymes for retarding the staling of rice bread. Cereal Chemistry, 80(6), 750–754.
Hager, A. S., & Arendt, E. K. (2013). Influence of hydroxypropylmethylcellulose (HPMC), xanthan gum and their combination on loaf specific volume, crumb hardness and crumb grain characteristics of gluten-free breads based on rice, maize, teff and buckwheat. Food Hydrocolloids, 32, 195–203.
Hager, A. S., Ryan, L. A. M., Schwab, C., Ganzle, M. G., O’Doherty, J. V., & Arendt, E. K. (2011). Influence of the soluble fibres inulin and oat beta-glucan on quality of dough and bread. European Food Research and Technology, 232(3), 405–413.
Hormdok, R., & Noomhorm, A. (2007). Hydrothermal treatments of rice starch for improvement of rice noodle quality. LWT–Food Science and Technology, 40, 1723–1731.
Houben, A., Hoechstoetter, A., & Becker, T. (2012). Possibilities to increase the quality in gluten-free bread production: an overview. European Food Research and Technology, 235(2), 195–208.
Kivela, R., Sontag-Strohm, T., Loponen, J., Tuomainen, P., & Nystrom, L. (2011). Oxidative and radical mediated cleavage of β-glucan in thermal treatments. Carbohydrate Polymers, 85, 645–652.
Lazaridou, A., Biliaderis, C. G., Micha-Screttas, M., & Steele, B. R. (2004). Acomparative study on structure-function relations of mixed linkage (1→3), (1→4) linear β-D-glucans. Food Hydrocolloids, 18, 837–855.
Lazaridou, A., Marinopoulou, A., Matsoukas, N. P., & Biliaderis, C. G. (2014). Impact of flour particle size and autoclaving on β-glucan physicochemical properties and starch digestibility of barley rusks as assessed by in vitro assays. Bioactive Carbohydrates and Dietary Fibre, 4(1), 58–73.
Luo, Z., He, X., Fu, X., Luo, F., & Gao, Q. (2006). Effect of microwave radiation on the physicochemical properties of normal corn, waxy corn and amylomaize V starches. Starch-Starke, 58, 468–474.
Moriartey, S., Temelli, F., & Vasanthan, T. (2010). Effect of formulation and processing treatments on viscosity and solubility of extractable barley beta-glucan in bread dough evaluated under in vitro conditions. Cereal Chemistry, 87(1), 65–72.
Olayinka, O. O., Adebowale, K. O., & Olu-Owolabi, B. I. (2008). Effect of heat-moisture treatment on physicochemical properties of white sorghum starch. Food Hydrocolloids, 22, 225–230.
Perez-Quirce, S., Collar, C., & Ronda, F. (2014). Significance of healthy viscous dietary fibres on the performance of gluten-free rice-based formulated breads. International Journal of Food Science and Technology, 49, 1375–1382.
Perez-Quirce, S., Ronda, F., Melendre, C., Lazaridou, A., & Biliaderis, C. G. (2016). Inactivation of endogenous rice flour β-glucanase by microwave radiation and impact on physico-chemical properties of the treated flour. Food and Bioprocess Technology, 9(9), 1562–1573.
Pinkrová, J., Hubácková, B., Kadlec, P., Příhoda, J., & Bubník, Z. (2003). Changes of starch during microwave treatment of rice. Czech Journal of Food Sciences, 21, 176–184.
Pyler, E. J., & Gorton, L. A. (2000). Fundamental bakery dough processes. In Baking science & technology, volume II: formulation and production. Kansas City: Sosland Publishing Company.
Rieder, A., Ballance, S., & Knutsen, S. H. (2015). Viscosity based quantification of endogenous β-glucanase activity in flour. Carbohydrate Polymers, 115, 104–111.
Ronda, F., Perez-Quirce, S., Lazaridou, A., & Biliaderis, C. G. (2015). Effect of barley and oat β-glucan concentrates on gluten-free rice-based doughs and bread characteristics. Food Hydrocolloids, 48, 197–207.
Ronda, F., Pérez-Quirce, S., & Villanueva, M. (2016). Rheological properties of gluten-free bread doughs. In J. Ahmed, P. Ptaszek, S. Basu, & W. P. Elsevier (Eds.), Cap 12 Relationship with bread quality, in Advances in food rheology and its applications (pp. 297–334).
Sciarini, L. S., Ribotta, P. D., Leon, A. E., & Perez, G. T. (2010). Effect of hydrocolloids on gluten-free batter properties and bread quality. International Journal of Food Science and Technology, 45, 2306–2313.
Sollid, L. M., & Lundin, K. E. A. (2009). Diagnosis and treatment of celiac disease. Mucosal Immunology, 2, 3–7.
Tosh, S. M., Brummer, Y., Wolever, T. M. S., & Wood, P. J. (2008). Glycemic response to oat bran muffins treated to vary molecular weight of β-glucan. Cereal Chemistry, 85, 211–217.
Trogh, I., Courtin, C. M., Andersson, A. A. M., Aman, P., Sorensen, J. F., & Delcour, J. A. (2004). The combined use of hull-less barley flour and xylanase as a strategy for wheat/hull-less barley breads with increased arabinoxylan and (1-3, 1-4) β- D-glucan levels. Journal of Cereal Science, 40, 257–267.
US Food and Drug Administration (USFDA). (2005). Food labeling: Soluble dietary fibre from certain foods and coronary heart disease. Federal Register, 70, 76150–76162.
Vatandoust, A., Ragaee, S. M., Wood, P. J., Tosh, S. M., & Seetharaman, K. (2012). Detection, localization, and variability of endogenous β-glucanase in wheat kernels. Cereal Chemistry, 89(1), 59–64.
Watcharatewinkul, Y., Puttanlek, C., Rungsardthong, V., & Uttapap, D. (2009). Pasting properties of a heat-moisture treated canna starch in relation to its structural characteristics. Carbohydrate Polymers, 75, 505–511.
Wolever, T. M. S., Tosh, S. M., Gibbs, A. L., Brand-Miller, J., Duncan, A. M., Hart, V., Lamarche, B., Thomson, B. A., Duss, R., & Wood, P. J. (2010). Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: a randomized clinical trial. The American Journal of Clinical Nutrition, 92, 723–732.
Wood, P. J. (2007). Cereal beta-glucans in diet and health. Journal of Cereal Science, 46, 230–238.
Acknowledgements
The authors gratefully acknowledge the financial support of the Spanish Institutions Ministerio de Economía y Competitividad and the European Regional Development Fund (FEDER) (Projects AGL2012-35088 and AGL2015-63849-C2-2-R).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez-Quirce, S., Ronda, F., Lazaridou, A. et al. Effect of Microwave Radiation Pretreatment of Rice Flour on Gluten-Free Breadmaking and Molecular Size of β-Glucans in the Fortified Breads. Food Bioprocess Technol 10, 1412–1421 (2017). https://doi.org/10.1007/s11947-017-1910-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1910-7