Abstract
The aim of this study is to compare alternative treatments on solvent-free extraction of high added value components from fermented grape pomace. Ultrasounds (US), pulsed electric fields (PEF) and high voltage electric discharges (HVED), which are physical treatments able to induce cell damages, were applied on aqueous suspensions of grape pomace. The efficiency of these technologies for phenolic compounds extraction, and particularly for anthocyanins recovery, was evaluated throughout the treatments at equivalent cell disintegration indexes (Z). HVED proved to be the most interesting technique to achieve higher phenolic compounds recovery with lower energy requirement than PEF and US at the same values of Z. However, HVED was less selective than PEF and US regarding the amount of anthocyanins recovered. At equivalent cell disintegration of Z = 0.8, PEF remarkably increased the extraction yield of total anthocyanins up to 22 and 55 % in comparison with US and HVED-assisted extractions. At this Z value, the ratio of total anthocyanins to TPC extracted reaches the respective values of 41.7, 34.9 and 14.1 % for PEF, US and HVED, thus demonstrating interesting differences of selectivity of the treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Grape pomace is known to contain appreciable quantities of antioxidant compounds, such as polyphenols. Over the last years, polyphenols have attracted a growing interest for their potential health benefits in preventing heart diseases and cancers (Craft et al. 2012; Quideau et al. 2011; Stintzing et al. 2002; Kähkönen and Heinonen 2003), thus being used for different food (e.g., colorants and antioxidants) and pharmaceutical applications (e.g., nutraceuticals) (Joana Gil‐Chávez et al. 2013).
These compounds exist in plants enclosed in particular structures such as the vacuoles of plant cells and lipoproteins bilayers (Agati et al. 2012). This fact complicates their recovery. Thus, the need for increasing the extraction yields has led to study deeper new non-conventional processes, including supercritical fluid extraction (Pereira and Meireles 2010), high hydrostatic pressure (Corrales et al. 2008, 2009), or microwaves (Routray and Orsat 2012; Krishnaswamy et al. 2013). Particularly, ultrasounds (USs), pulsed electric fields (PEFs), and high-voltage electric discharges (HVEDs) belong to the environmentally friendly and energy-efficient technologies being able to enhance mass transfer processes. These three technologies can physically affect the permeability of cell by different mechanisms (Boussetta and Vorobiev 2014; Chemat and Khan 2011; Toepfl et al. 2006; Vorobiev and Lebovka 2009).
Applications of ultrasounds have been widely developed in the industry and still are an active research field for enhancement of heat and mass transfer phenomena. Ultrasound waves after interaction with subjected plant material alter its physical and chemical properties, and their cavitational effect facilitates the release of extractable compounds and enhances the mass transport by disrupting the plant cell walls (Chemat and Khan 2011). The effect of ultrasounds on extraction yields is attributed to the microstreaming and heightened mass transfer produced by cavitation and bubble collapse. The temperature and the pressure at the moment of collapse have been estimated to be up to 5000 K and 2000 atm in an ultrasonic bath at room temperature. This creates hotspots that are able to accelerate dramatically the chemical reactivity and the turbulence into the medium, resulting in cell damages of the plant material in suspension and facilitating the release of bio-components (Chemat and Khan 2011). Its feasibility for the extraction of secondary metabolites of grapes has been highlighted in many research works (Novak et al. 2008; Vilkhu et al. 2008).
Pulsed electric field (PEF) treatment also increases the mass transfer (Vorobiev and Lebovka 2008, 2009) due to the permeabilization of cell membranes induced by the electroporation phenomenon (Kotnik et al. 2012). When subjected to an external electric field, the charge accumulation on the membrane surfaces induces the increase of transmembrane potential of both sides of the cell membrane (Barbosa-Cánovas et al. 1999). After exceeding a critical value of transmembrane potential, the expansion of pores present in weak areas of the membrane induces drastic increase of permeability (Zimmermann 1986; Knorr et al. 2001), thus facilitating the release of intracellular compounds (Delsart et al. 2014; Donsì et al. 2010).
The generation of high pulsed voltage using a point-to-plane electrode system induces electrical breakdowns in water (Boussetta et al. 2013a). This electrical discharge injects energy directly into the aqueous suspension and leads to the generation of hot, localized plasmas that strongly emit high-intensity UV light, produce shock waves of high pressure causing particle cell structure damages, and generate hydroxyl radicals during water photodissociation (Boussetta and Vorobiev 2014). The pressure shock wave is followed by a rarefaction wave that produces gaseous cavitation bubbles. The collapsing cavitations create strong secondary shocks with very short duration (≈60 ns that sometimes result in sonolumeniscence (excitation of light spikes)), and these shocks can interact with the weaken cellular structures and tissue disruption (Locke et al. 2006; Boussetta and Vorobiev 2014). These phenomena also cause particle fragmentation and creation of liquid turbulence that accelerate the release of biomolecules from the cytoplasm of the cells (Boussetta and Vorobiev 2014).
These technologies have been previously studied for the improvement of phenolic compound recovery from grape wastes. US, PEF, and HVED pre-treatments of unfermented grape pomace (Pinot Meunier) in hot water (50 °C), at optimized parameters of treatment, increased by 3, 3.4, and 6.8 times, respectively, the content of polyphenols after 90 min of hydro-alcoholic diffusion at 50 °C compared to the extraction performed in the same conditions but without pre-treatment (Boussetta 2011). Similar results were obtained on vine shoots from Grenache blanc cultivar, with relative increases of 1.5, 2.1, and 3.1 of total phenolic compounds, at delivered energies of 3428, 762, and 254 kJ/kg for US, PEF, and HVED, respectively. Moreover, a previous work developed by Corrales et al. (2008) interestingly demonstrated that PEF pre-treatment (3 kV/cm, 10 kJ/kg) was efficient for anthocyanin recovery from Dornfelder grape skins during the subsequent liquid-to-solid diffusion, while US-assisted diffusion in a US bath did not induce any improvement of these compounds.
In spite of the literature dealing with the comparison of US, PEF, and HVED for extraction purposes, these three novel technologies had never been applied at equivalent processing conditions for the selective extraction of soluble phenolic bio-compounds, especially anthocyanins, from fermented grape pomace. Therefore, to better compare these alternative technologies and discuss the potential mechanisms that occur during the treatments, there is a need to use a common indicator of the process efficiency. Electrical conductivity index Z is often used as an indicator of cell damages, and it measures the effectiveness of the phenomena that could be observed for each of the above mentioned treatments. This is a simple innocuous tool for material characterization, inexpensive, and easy to assess, and it provides online information (Pliquett 2010; Vorobiev and Lebovka 2008; Lebovka et al. 2001, 2002). As US, PEF, and HVED are able to physically affect the cell structure, cell disintegration index Z was used to estimate the efficiency of the treatments for the release of solutes. However, Z index does not take into account numerous phenomena induced by some of studied treatments (e.g., bubbles cavitation, high local pressures, high-intensity UV light, and radical generation). The quality of the obtained extracts and the yields of extracted bio-compounds (proteins, polyphenols, and anthocyanins) are also compared. Anthocyanin profiles of the aqueous extracts are evaluated to assess the selectivity of studied alternative technologies.
Material and Methods
Winery By-product
Fermented grape pomace was obtained from Dunkelfelder variety cultivar (Vitisvinifera L.). This by-product was a residue of red wine processing and was composed of seeds (49 % of mass with particles of about 3 mm in diameter) and skins (51 % of mass with particles of about 6 × 6 × 3 mm). The pomace was collected immediately after pressing at 2 bar and was treated with 200 mg of SO2 per kilogram of raw material (RM). Samples were stored at 4 °C under vacuum until further processing. The dry matter was determined by the measurement of the mass of grape pomace before and after drying the samples at 105 °C overnight and was equal to 42.2 ± 0.7 % (w/w). The pH was 3.30.
Reference Extraction Procedure
A reference extraction of bio-compounds from grape pomace (40.0 ± 0.1 g) was carried out in water (liquid-to-solid ratio = 10) at 20 °C, during 2 h under mechanical stirring, in order to determine the maximal extractable polyphenols. Grape pomace was previously frozen (−18 °C) overnight and crushed for 90 s in a laboratory coffee grinder (SEB, Paris, France) (180 W, Ø < 2 mm).
Physical Treatments
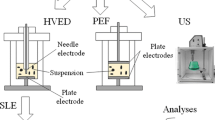
Fermented grape pomace (40 g ± 0.5 g) was suspended in water (400 mL) at 22 ± 1 °C, obtaining a liquid-to-solid ratio of 10 (v/w) which was selected based on preliminary studies. The setup of the experimental design is represented in Fig. 1.
Ultrasound Treatment
Ultrasound (US) treatment was carried out using an ultrasound processor UP 400S (Hielscher GmbH, Germany). The US processor operated at the maximal power of 400 W and frequency f of 24 kHz. The amplitude was fixed at 100 %, which corresponded to the nominal power of 400 W. The titanium USN probe (H14 Hielscher GmbH, Germany) has a diameter of 14 mm, and 90 % of the probe length was submerged in the liquid (total length, 100 mm). The probe was immersed in a narrow-necked glass flask of 1000 mL containing the grape pomace to be treated mixed with water (liquid-to-solid ratio, w/w, was 10). The potency density was 4 W/mL. The flask was immersed into a cooling bath, composed by an ice/water mixture, in order to avoid heating induced by US. The specific energy consumption W (kJ/kg) varied within 0–2727 kJ/kg and was calculated as
where t US is the total treatment duration (s) and m is the product mass (kg). The generator power was set to 400 W.
Pulsed Electric Field Treatment
A custom-made pulsed high voltage power supply 40 kV–10 kA, frequency 0.5 Hz (Tomsk Polytechnic University, Tomsk, Russia), was used for the electrical treatments. For PEF, the stainless electrodes of the 1-L treatment chamber were two parallel disks with surface areas of 95 cm2. The electrodes were separated by a distance of 3 cm corresponding to an electric field strength of 13.3 kV/cm. PEF treatment consisted in applying W = 0–564 kJ/kg of energy input that was calculated as shown in Eq. (2):
where W PEF is the pulse energy (kJ/pulse), n is the number of pulses, and m is the product mass (kg). W PEF was determined from Eq. (3) where U is the voltage (V) and I is the current strength (A):
High-Voltage Electric Discharge Treatment
The HVED experiments were performed in a 1-L treatment chamber (inner diameter = 10 cm, wall thickness = 2.5 cm) equipped with needle-plate geometry electrodes, which were connected to a pulsed high-voltage power supply (Tomsk Polytechnic University, Russia). The stainless steel needle electrode of 10 mm in diameter and the grounded stainless plate electrode of 35 mm in diameter were separated by a distance of 1.25 cm. A positive peak pulse voltage (U) of 40 kV was applied to the needle electrode and the electrical discharges with a repetition rate of 0.5 Hz, which was imposed by the generator. Damped oscillations were obtained over a total duration t i of 10 μs. HVED treatment consisted in applying W = 0–218 kJ/kg of energy input. The specific energy input W (kJ/kg) was obtained from Eqs. (2) and (3), in which W PEF was replaced by pulse energy W HVED (kJ/discharge).
Temperature and pH Measurements
The temperature was measured using a thermocouple (Thermocoax, Suresnes, France) during the different treatments. The increase of the temperature was comparable for the three treatments and was less than 8 °C (≈25–30 °C). Moreover, this increase of the temperature led to a relatively low increase of phenolic compound extraction in tap water (Boussetta et al. 2009).
The pH of the solution was determined by using a pH meter (CONSOR C931, Bioblock Scientific, France) at 20 °C. The initial pH of the solution was 3.34 ± 0.07 and was stable during the different treatments.
Determination of Cell Disintegration Index Z
The conductivity was measured in the liquid medium using a conductivity meter InoLab pH/cond Level 1 (WTW, Weilheim, Germany) equipped with an independent probe, at 25 °C. Before each measurement, the treatment was stopped and the conductivity was recorded after 5 min in order to avoid interference with other phenomena. The degree of cell disintegration (Z) can be estimated from the electrical conductivity index (Vorobiev and Lebovka 2008; Lebovka et al. 2001, 2002):
where σ (μS/cm) is the electrical conductivity of sample, and the subscripts “u” and “d” refer to the electrical conductivities of the untreated and completely damaged samples, respectively. The value of σ d was estimated from the measurements of electrical conductivity of frozen and grinded grape pomace in water (reference extraction). Application of Eq. (4) gives Z = 0 for an intact tissue and Z = 1 for a maximally damaged tissue (reference extraction from grinded grape pomace).
Analyses
Total Phenolic Compounds
The samples taken during the extraction were centrifuged at 4000 rpm for 10 min. The quantification of phenolic compound content in the supernatant was performed according to the colorimetric method of Folin Ciocalteu reagent as described previously (Boussetta et al. 2012b). Results were expressed as mg gallic acid equivalent/L of aqueous extract (mg GAE/L).
Antioxidant Capacity
The DPPH method has been proposed as an easy and accurate method for measuring the antioxidant activity of grape pomace (Llobera and Cañellas 2008; Parry et al. 2011). In addition, DPPH assay is not specific to any particular antioxidant component, thus applying to the overall antioxidant capacity of the sample. The determination of the free radical scavenging capacity was evaluated with the stable radical 1,1-diphenyl-2-picrylhydrazyl (DPPH), according to the method described by Barba et al. (2013). Results were expressed as mM trolox equivalent (mM TE).
Total and Individual Anthocyanins
Anthocyanin profile was determined by high-performance liquid chromatography (HPLC) (Brianceau et al. 2014). The extracts were diluted in acidified water (0.1 % formic acid) and then filtered through nylon filters (diameter Ø = 0.45 μm). The system used for anthocyanin analysis was an Ultimate 3000 (Dionex, Idstein, Germany), equipped with a diode array detector. The separation was carried out with a Prontosil C18AQ column (4.6 × 250 mm, 5 μm, Bischoff Chromatography, Germany) operated at 25 °C in reverse phase. UV/Vis spectra were recorded in the range of 200–600 nm. Two mobile phases, (A) water/acetonitrile/formic acid (87:3:10, v/v/v) and (B) water/acetonitrile/formic acid (40:50:10, v/v/v), were used for the separation of phenolic compounds. The elution gradient had the following profile: t 0 min B (6 %), t 15 min B (30 %), t 30 min B (50 %), t 35 min B (60 %), t 41 min B (6 %), t 45 min B (6 %). The injection volume was 20 μL and the flow rate was set at 1.0 mL/min. Anthocyanins were detected at 518 nm. Individual anthocyanins were quantified using a calibration curve of malvidin-3-O-glucoside purchased from Extrasynthèse (Genay, France). Results were expressed as grams of malvidin-3-O-glucoside equivalent per liter of aqueous extract.
Statistical Analysis
Each experiment was repeated at least two times, and the analyses were performed at least two times. Data obtained during treatments were subjected to multivariate analysis of variance using Statistica 8 Software (Version 8.0.360.0, StatSof Inc., Tulsa, USA). Tukey tests were also performed on data for all pairwise comparisons of the mean responses to the different treatment groups. This test allows the determination of treatments which are statistically different from the other at a probability level of P = 0.05.
Results
Effects of HVED, US, and PEF on Cell Disintegration Index Z
Figure 2 describes the variation of cell disintegration index Z, determined from Eq. (4), as a function of the energy input for different studied treatments. HVED, US, and PEF induce cell damages of fermented grape pomace and the release of intracellular components in the surrounding medium.
The degree value of Z increases significantly with the increase of energy input. This phenomenon reflects the extraction of ionic intracellular components from damaged cells modifying the electrical conductivity of the media (Zimmermann 1986; Lebovka et al. 2001, 2002). Extraction efficiency increases in the row of US < PEF < HVED. HVED is able to quickly induce fragmentation of the particles due to the propagation of shock waves of high pressure (Boussetta and Vorobiev 2014) and explosion/implosion of cavitation bubbles (Gros et al. 2003), thus facilitating the extraction of soluble biomolecules.
Although cell disintegration index Z is a useful indicator of cell damages induced by the process, it is necessary to better understand if it is adapted for evaluation of the extraction of particular targeted compounds. Indeed, the extraction of interesting biomolecules does not necessarily start with the first signs of cellular damage, since a minimal damage per cell or number of damaged cells is required to enhance the extraction (Vorobiev and Lebovka 2010).
Effects of US, PEF, and HVED on Solvent-Free Extraction of Bio-compounds Using Cell Disintegration Index Z as an Indicator of the Treatment Efficiency
To compare the influence of cell disruption induced by the three treatments, different cell damage levels were selected (Z = 0.2, 0.4, 0.6, and 0.8).
Effects of US, PEF, and HVED on Total Phenolic Compound Recovery and Antioxidant Capacity
Total Phenolic Compounds
US, PEF, and HVED treatments allowed recoveries of total phenolic compounds (TPCs) up to 44, 47, and 88 %, respectively, compared to the maximum TPCs extractable using reference extraction in water of grinded pomace (334.7 ± 22.5 mg/L, for completely damage cells by grinding (Z = 1)).
Figure 3a shows the effects of US, PEF, and HVED treatments on the content of TPCs of fermented grape pomace extracts at different Z indexes. TPC concentrations increased linearly for US (R 2 = 0.862), PEF (R 2 = 0.994), and HVED (R 2 = 0.981) treatments with Z. This index is consequently an interesting indicator of the process efficiencies as it is well correlated with TPC recovery.
US and PEF treatments were less effective than HVED for TPC recovery. At Z index above 0.4, HVED increased the polyphenol yield more than two times compared to US and PEF, with lower energy inputs. These results are in accordance with a previous study conducted by Rajha et al. (2014), who demonstrated that HVED better enhances TPC recovery from vine shoots than US and PEF, after a subsequent liquid-to-solid diffusion.
Antioxidant Capacity
Figure 3b shows the effects of the physical treatments on the antioxidant capacity of the aqueous extracts at equivalent cell disintegration index Z. The antioxidant capacity increased in the HVED- and US-treated extracts until Z = 0.6. It reached a maximal value of 3.29 ± 0.02 mM TE for HVED treatment at a value of 0.6 of Z index. Further decrease and stabilization of antioxidant property value were observed at Z = 0.8 for HVED and US, respectively. Antioxidant capacity did not follow the same behavior as TPCs regarding Z index for HVED and US. On the contrary, antioxidant capacity systematically increased with Z, thus following TPC behavior for PEF treatment.
Effects of US, PEF, and HVED on Anthocyanin Recovery
Dunkelfelder is a dark-skinned variety of grape used to obtain wines of deep and dark red color. This is due to its high content of anthocyanins that are the red pigments in grapes mainly located in the skin (Ribéreau-Gayon et al. 2012). The reference extraction of grinded pomace in water allowed the recovery of 12.09 ± 0.17 mg of anthocyanins per liter of extract. Anthocyanin extraction yields were up to 4.3, 5.3, and 3.4 times higher after US, PEF, and HVED treatments respectively (Z = 0.8) compared to reference extraction.
Similarly to TPCs, the concentrations of anthocyanins increased with the Z index for each treatment (Table 1). Consequently, Z index could be a useful indicator to evaluate the recovery of anthocyanins. However, the statistical analysis evidences that anthocyanin concentrations were significantly higher with PEF treatment at Z ≥ 0.6 compared to the those with other treatments. At equivalent Z index of 0.8, PEF increased the extraction yields of total anthocyanins up to 22 % in comparison with US, and up to 55 % compared to HVED-assisted extraction.
For each treatment, malvidin-3-O-glucoside concentration was higher than monoglucoside molecules of peonidin > petunidin > delphinidin. Only HVED extracts at index Z ≥ 0.6 showed different behaviors with equivalent concentrations of peonidin-3-O-glucoside and malvidin-3-O-glucoside. On the contrary, PEF remarkably enhanced the extraction of malvidin-3-O-glucoside compared to the amount of other individual anthocyanins. Malvidin-3-O-glucoside represents up to 57 % of total anthocyanins extracted at cellular damage index Z = 0.8 while these ratios are around 40 and 51 % for HVED and US treatments, respectively.
Ratio of Total Anthocyanins to TPCs Extracted
Figure 4 gives the ratio of total anthocyanins to TPCs extracted at different Z indexes for US-, PEF-, and HVED-treated grape pomace. US, PEF, and HVED systematically lead to a higher ratio of total anthocyanins to TPCs extracted compared to the reference extraction of grinded pomace in water (Z = 1). These processes allowed the specific recovery of anthocyanins and the production of extracts with specific biochemical composition that cannot be achieved by conventional extraction procedure, such as grinding combined to diffusion.
HVED treatment first induced a decrease of this ratio (from 31.5 to 14.1 % between Z values of 0.2 and 0.4) and then stabilization, meaning that the phenomena induced by the process, including cell damages, allowed the recovery of anthocyanins in equivalent proportions than TPCs.
On the contrary, US treatment systematically increased the aforementioned ratio with the increase of Z index. This ratio was increased from 19.6 to 34.9 % between 0.2 and 0.8 of Z index. These results suggest that US treatment may exhibit some selectivity with respect to the extraction of anthocyanins from fermented grape pomace.
Similarly to US, PEF treatment exhibits the selective recovery of anthocyanin compounds. At Z ≥ 0.6, the ratio of total anthocyanins to TPCs extracted reaches the highest value of 41.7 % with PEF treatment. However, the decrease of resulting ratio below energy input of 100.0 kJ/kg suggests that the energy threshold required by PEF to initiate the specific extraction of anthocyanins from grape pomace was not attained.
Discussion
This set of examinations underlined the differences of efficiency and selectivity for extraction purposes between the studied alternative treatments. Particularly, The specific group of polyphenols, their relevant location in plant tissue (Agati et al. 2012; Conn et al. 2003; Markham et al. 2000), and their particular bounding to the plant matrix (Le Bourvellec and Renard 2005; Pinelo et al. 2006) might be crucial in defining the physical treatment to be used to obtain extracts with specific biochemical profiles.
Potential Mechanisms Involved and Their Impact on Selective Extraction
HVED proved to be the most efficient tool to extract TPCs. This is mostly attributed to product fragmentation (Boussetta et al. 2013a). A previous study has shown that HVED allows reducing the grape seed size (Boussetta et al. 2012a). At the macroscopic level, the treated grape pomace was clearly fragmented after the application of electrical discharges. The increase of the exchange surface promoted the release of non-cell-wall phenolic components. Moreover, the observed HVED enhancement of TPC extraction could be ascribed to the release of cell-wall-linked phenolic compounds (Boussetta et al. 2013b), and particularly proanthocyanidins which are known to interact with polysaccharides (Le Bourvellec and Renard 2011). On the other side, the HVED enhancement of anthocyanins compared to the reference extraction procedure (grinding of grape pomace followed by a diffusion step) implies that the increase of mass transfer phenomena induced by HVED is not only related to the product fragmentation (increase of exchange surface). This might be due to the highly turbulent conditions that accelerate the convection of these components from particles to the surrounding medium (Boussetta et al. 2011a).
Contrarily to HVED treatment, US and PEF did not lead to the fragmentation of the grape pomace, which was visually intact after the treatments. Because of the different mechanisms involved by the processes, TPCs were extracted to a lower extent with US and PEF, than with HVED. However, US and PEF were more efficient than HVED for anthocyanin extraction. This could be partly ascribing to specific and localized disruptions of the grape skins tissue.
Grape skin is composed of two distinct tissues—epidermis and hypodermis—that appear constituted by several cell layers (González-Paramás et al. 2004) with abundant phenolic compounds and particularly anthocyanins. A previous study showed that PEF had an important effect on hystological structure and composition of cell wall grape berry, with a particular impact in the deepest layers of the skin, which corresponds to the cell layers of the hypodermis (Cholet et al. 2014). PEF treatment causes irreversible perforations in the cell wall of the outer hypodermis and distention of the fiber cell wall polysaccharides at the inner hypodermis (Cholet et al. 2014). This electroporation phenomenon may allow the specific recovery of anthocyanins that are located in the upper cell layers of the hypodermis by facilitating the solvent penetration to particular skin tissues of grape. These results are in agreement with previous studies (Corrales et al. 2008; Brianceau et al. 2014) in which an improved extraction of monoglucoside anthocyanins from Dornfelder and Dunkelfelder grape pomaces using PEF was described.
Selective anthocyanin recovery observed in US-treated samples seems to be rather due to localized microfractures of the cell walls. When the cavitation bubbles induced by US reach a critical size, they collapse onto the surface of the solid material. The high pressure (2000 atm) and temperature (5000 K) released generate hotspots that are able to accelerate dramatically the chemical reactivity into the medium. Some microjets directed toward the grape skin matrix are created and can destroy locally the cell walls (Li et al. 2004; Balachandran et al. 2006). Noticeably, previous observations (Fava et al. 2011) revealed that moderate US treatment (2–3 min, 600 W, 20 kHz) of grape berry generates structural changes of the skin berry, and particularly fractures in the inner layer of the epidermis, where vacuolar anthocyanins were located.
Biochemical Modifications
Concomitant mechanical and chemical actions induced by the processes may also be responsible of some results observed in this work. Indeed, free radicals can be formed via the thermal dissociation of water during electrical discharge and ultrasound treatments. Under these extreme conditions, antioxidant capacity can be affected (Boussetta et al. 2011b).
Energy Costs of the Processes
Energy consumptions required to extract 1 mg of targeted bio-compounds were compared for the three physical treatments at different Z indexes. The results evidenced that HVED is the less energy consuming process, followed by PEF and US, for both recoveries of TPCs and total anthocyanins (Table 2).
The energy required to extract 1 mg of TPCs by HVED was statistically stable (P > 0.05) for the different values of Z. On the contrary, the energies required were linearly correlated and increased with cell disintegration index Z induced by the process for PEF (R 2 = 0.936) and US (R 2 = 0.981). The achievement of higher Z index is necessary to recover targeted compounds but requires increased costs for PEF and US.
Conclusion
The studied physical extraction treatments proved to be efficient for the recovery of soluble bio-compounds from fermented grape pomace, in aqueous medium. Z index is an interesting tool to discuss the possible mechanisms involved, to compare the efficiencies of the treatments and to predict the extraction yields.
HVED was the most interesting technique in terms of phenolic compound yield and energy requirement. However, HVED was less selective than PEF and US regarding the amount of anthocyanins recovered. Consequently, the location of targeted compounds with respect to tissue structures seems to be a key issue to optimize their extraction. Overall, further studies are needed to understand how and when phenomena that physically affect the cells can occur.
References
Agati, G., Azzarello, E., Pollastri, S., & Tattini, M. (2012). Flavonoids as antioxidants in plants: location and functional significance. Plant Science, 196, 67–76.
Balachandran, S., Kentish, S. E., Mawson, R., & Ashokkumar, M. (2006). Ultrasonic enhancement of the supercritical extraction from ginger. Ultrasonics Sonochemistry, 13(6), 471–479.
Barba, F. J., Esteve, M. J., Tedeschi, P., Brandolini, V., & Frígola, A. (2013). A comparative study of the analysis of antioxidant activities of liquid foods employing spectrophotometric, fluorometric, and chemiluminescent methods. Food Analytical Methods, 6(1), 317–327.
Barbosa-Cánovas, G. V., Pothakamury, U. R., Gongora-Nieto, M. M., & Swanson, B. G. (1999). Preservation of foods with pulsed electric fields. San Diego: Academic.
Boussetta, N. (2011). Intensification de l'extraction des polyphénols par électrotechnologies pour la valorisation des marcs de Champagne. Université de Technologie de Compiègne.
Boussetta, N., & Vorobiev, E. (2014). Extraction of valuable biocompounds assisted by high voltage electrical discharges: a review. Comptes Rendus Chimie.
Boussetta, N., Lebovka, N., Vorobiev, E., Adenier, H., Bedel-Cloutour, C., & Lanoiselle, J.-L. (2009). Electrically assisted extraction of soluble matter from Chardonnay grape skins for polyphenol recovery. Journal of Agricultural and Food Chemistry, 57(4), 1491–1497.
Boussetta, N., Reess, T., Vorobiev, E., & Lanoisellé, J.-L. (2011a). Pulsed electrical discharges: Principles and application to extraction of biocompounds.
Boussetta, N., Vorobiev, E., Deloison, V., Pochez, F., Falcimaigne-Cordin, A., & Lanoisellé, J. L. (2011b). Valorisation of grape pomace by the extraction of phenolic antioxidants: application of high voltage electrical discharges. Food Chemistry, 128(2), 364–370.
Boussetta, N., Vorobiev, E., Le, L. H., Cordin-Falcimaigne, A., & Lanoisellé, J. L. (2012a). Application of electrical treatments in alcoholic solvent for polyphenols extraction from grape seeds. LWT - Food Science and Technology, 46(1), 127–134.
Boussetta, N., Vorobiev, E., Reess, T., De Ferron, A., Pecastaing, L., Ruscassié, R., & Lanoisellé, J. L. (2012b). Scale-up of high voltage electrical discharges for polyphenols extraction from grape pomace: effect of the dynamic shock waves. Innovative Food Science & Emerging Technologies, 16, 129–136.
Boussetta, N., Lesaint, O., & Vorobiev, E. (2013a). A study of mechanisms involved during the extraction of polyphenols from grape seeds by pulsed electrical discharges. Innovative Food Science & Emerging Technologies, 19, 124–132.
Boussetta, N., Turk, M. F., De Taeye, C., Larondelle, Y., Lanoisellé, J., & Vorobiev, E. (2013b). Effect of high voltage electrical discharges, heating and ethanol concentration on the extraction of total polyphenols and lignans from flaxseed cake. Industrial Crops and Products, 49, 690–696.
Brianceau, S., Turk, M., Vitrac, X., & Vorobiev, E. (2014). Combined densification and pulsed electric field treatment for selective polyphenols recovery from fermented grape pomace. Innovative Food Science & Emerging Technologies.
Chemat, F., & Khan, M. K. (2011). Applications of ultrasound in food technology: processing, preservation and extraction. Ultrasonics Sonochemistry, 18(4), 813–835.
Cholet, C. L., Delsart, C., Petrel, M., Gontier, E., Grimi, N., L’Hyvernay, A., Ghidossi, R., Vorobiev, E., Mietton-Peuchot, M., & Geny, L. (2014). Structural and biochemical changes induced by pulsed electric field treatments on Cabernet Sauvignon grape berry skins: impact on cell wall total tannins and polysaccharides. Journal of Agricultural and Food Chemistry, 62(13), 2925–2934.
Conn, S., Zhang, W., & Franco, C. (2003). Anthocyanic vacuolar inclusions (AVIs) selectively bind acylated anthocyanins in Vitis vinifera L. (grapevine) suspension culture. Biotechnology Letters, 25(11), 835–839.
Corrales, M., Toepfl, S., Butz, P., Knorr, D., & Tauscher, B. (2008). Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: a comparison. Innovative Food Science & Emerging Technologies, 9(1), 85–91.
Corrales, M., García, A. F., Butz, P., & Tauscher, B. (2009). Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. Journal of Food Engineering, 90(4), 415–421.
Craft, B. D., Kerrihard, A. L., Amarowicz, R., & Pegg, R. B. (2012). Phenol‐based antioxidants and the in vitro methods used for their assessment. Comprehensive Reviews in Food Science and Food Safety, 11(2), 148–173.
Delsart, C., Cholet, C., Ghidossi, R., Grimi, N., Gontier, E., Gény, L., Vorobiev, E., & Mietton-Peuchot, M. (2014). Effects of pulsed electric fields on Cabernet Sauvignon grape berries and on the characteristics of wines. Food and Bioprocess Technology, 7(2), 424–436.
Donsì, F., Ferrari, G., & Pataro, G. (2010). Applications of pulsed electric field treatments for the enhancement of mass transfer from vegetable tissue. Food Engineering Reviews, 2(2), 109–130.
Fava, J., Hodara, K., Nieto, A., Guerrero, S., Alzamora, S. M., & Castro, M. A. (2011). Structure (micro, ultra, nano), color and mechanical properties of Vitis labrusca L. (grape berry) fruits treated by hydrogen peroxide, UV–C irradiation and ultrasound. Food Research International, 44(9), 2938–2948.
González-Paramás, A. M., Esteban-Ruano, S., Santos-Buelga, C., de Pascual-Teresa, S., & Rivas-Gonzalo, J. C. (2004). Flavanol content and antioxidant activity in winery byproducts. Journal of Agricultural and Food Chemistry, 52(2), 234–238.
Gros, C., Lanoisellé, J., & Vorobiev, E. (2003). Towards an alternative extraction process for linseed oil. Chemical Engineering Research and Design, 81(9), 1059–1065.
Joana Gil‐Chávez, G., Villa, J. A., Fernando Ayala‐Zavala, J., Basilio Heredia, J., Sepulveda, D., Yahia, E. M., & González‐Aguilar, G. A. (2013). Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Comprehensive Reviews in Food Science and Food Safety, 12(1), 5–23.
Kähkönen, M. P., & Heinonen, M. (2003). Antioxidant activity of anthocyanins and their aglycons. Journal of Agricultural and Food Chemistry, 51(3), 628–633.
Knorr, D., Angersbach, A., Eshtiaghi, M. N., Heinz, V., & Lee, D.-U. (2001). Processing concepts based on high intensity electric field pulses. Trends in Food Science & Technology, 12(3), 129–135.
Kotnik, T., Kramar, P., Pucihar, G., Miklavcic, D., & Tarek, M. (2012). Cell membrane electroporation-part 1: the phenomenon. IEEE Electrical Insulation Magazine, 28(5), 14–23.
Krishnaswamy, K., Orsat, V., Gariépy, Y., & Thangavel, K. (2013). Optimization of microwave-assisted extraction of phenolic antioxidants from grape seeds (Vitis vinifera). Food and Bioprocess Technology, 6(2), 441–455.
Le Bourvellec, C., & Renard, C. (2005). Non-covalent interaction between procyanidins and apple cell wall material. Part II: quantification and impact of cell wall drying. Biochimica et Biophysica Acta (BBA) - General Subjects, 1725(1), 1–9.
Le Bourvellec, C., & Renard, C. M. G. C. (2011). Interactions between polyphenols and macromolecules: quantification methods and mechanisms. Critical Reviews in Food Science and Nutrition, 52(3), 213–248.
Lebovka, N. I., Bazhal, M. I., & Vorobiev, E. (2001). Pulsed electric field breakage of cellular tissues: visualisation of percolative properties. Innovative Food Science & Emerging Technologies, 2(2), 113–125.
Lebovka, N. I., Bazhal, M. I., & Vorobiev, E. (2002). Estimation of characteristic damage time of food materials in pulsed-electric fields. Journal of Food Engineering, 54(4), 337–346.
Li, H., Pordesimo, L., & Weiss, J. (2004). High intensity ultrasound-assisted extraction of oil from soybeans. Food Research International, 37(7), 731–738.
Llobera, A., & Cañellas, J. (2008). Antioxidant activity and dietary fibre of Prensal Blanc white grape (Vitis vinifera) by‐products. International Journal of Food Science & Technology., 43(11), 1953–1959.
Locke, B., Sato, M., Sunka, P., Hoffmann, M., & Chang, J.-S. (2006). Electrohydraulic discharge and nonthermal plasma for water treatment. Industrial & Engineering Chemistry Research, 45(3), 882–905.
Markham, K. R., Gould, K. S., Winefield, C. S., Mitchell, K. A., Bloor, S. J., & Boase, M. R. (2000). Anthocyanic vacuolar inclusions—their nature and significance in flower colouration. Phytochemistry, 55(4), 327–336.
Novak, I., Janeiro, P., Seruga, M., & Oliveira-Brett, A. M. (2008). Ultrasound extracted flavonoids from four varieties of Portuguese red grape skins determined by reverse-phase high-performance liquid chromatography with electrochemical detection. Analytica Chimica Acta, 630(2), 107–115.
Parry, J. W., Li, H., Liu, J.-R., Zhou, K., Zhang, L., & Ren, S. (2011). Antioxidant activity, antiproliferation of colon cancer cells, and chemical composition of grape pomace. Food and Nutrition Sciences, 2, 530–540.
Pereira, C. G., & Meireles, M. A. A. (2010). Supercritical fluid extraction of bioactive compounds: fundamentals, applications and economic perspectives. Food and Bioprocess Technology, 3(3), 340–372.
Pinelo, M., Arnous, A., & Meyer, A. S. (2006). Upgrading of grape skins: significance of plant cell-wall structural components and extraction techniques for phenol release. Trends in Food Science & Technology, 17(11), 579–590.
Pliquett, U. (2010). Bioimpedance: a review for food processing. Food Engineering Reviews, 2(2), 74–94.
Quideau, S., Deffieux, D., Douat‐Casassus, C., & Pouysegu, L. (2011). Plant polyphenols: chemical properties, biological activities, and synthesis. Angewandte Chemie International Edition, 50(3), 586–621.
Rajha, H. N., Boussetta, N., Louka, N., Maroun, R. G., & Vorobiev, E. (2014) A comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Research International.
Ribéreau-Gayon, P., Dubourdieu, D., & Donèche, B. (2012) Handbook of oenology. In The chemistry of wine. Stabilization and treatments (vol 2. 2nd edn. pp. 451). Wiley.
Routray, W., & Orsat, V. (2012). Microwave-assisted extraction of flavonoids: a review. Food and Bioprocess Technology, 5(2), 409–424.
Stintzing, F. C., Stintzing, A. S., Carle, R., Frei, B., & Wrolstad, R. E. (2002). Color and antioxidant properties of cyanidin-based anthocyanin pigments. Journal of Agricultural and Food Chemistry, 50(21), 6172–6181.
Toepfl, S., Mathys, A., Heinz, V., & Knorr, D. (2006). Review: potential of high hydrostatic pressure and pulsed electric fields for energy efficient and environmentally friendly food processing. Food Reviews International, 22(4), 405–423.
Vilkhu, K., Mawson, R., Simons, L., & Bates, D. (2008). Applications and opportunities for ultrasound assisted extraction in the food industry—a review. Innovative Food Science & Emerging Technologies, 9(2), 161–169.
Vorobiev, E., & Lebovka, N. (2008). Electrotechnologies for extraction from food plants and biomaterials. New York: Springer.
Vorobiev, E., & Lebovka, N. (2009) Pulsed-electric-fields-induced effects in plant tissues: Fundamental aspects and perspectives of applications. In Electrotechnologies for extraction from food plants and biomaterials (pp. 39–81).
Vorobiev, E., & Lebovka, N. (2010). Enhanced extraction from solid foods and biosuspensions by pulsed electrical energy. Food Engineering Reviews, 2(2), 95–108.
Zimmermann, U. (1986). Electrical breakdown, electropermeabilization and electrofusion. Reviews of Physiology Biochemistry and Pharmacology, 105, 175–256.
Acknowledgments
This study received financial support from the Agence National de Recherche under the first transnational call of ECO-INNOVERA (ERA-NET). The authors would like to thank the Ecoled’ingénieurs de Changins (EIC, Switzerland), and particularly Ms. Anna-Claire Silvestri for providing grape pomace. F.J. Barba wishes to thank the Valencian Autonomous Government (Conselleríad’Educació, Cultura I Esport. Generalitat Valenciana) for the postdoctoral fellowship of the VALi+d program “ProgramaVALi+d per a investigadors en fase postdoctoral 2013” (APOSTD/2013/092).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barba, F.J., Brianceau, S., Turk, M. et al. Effect of Alternative Physical Treatments (Ultrasounds, Pulsed Electric Fields, and High-Voltage Electrical Discharges) on Selective Recovery of Bio-compounds from Fermented Grape Pomace. Food Bioprocess Technol 8, 1139–1148 (2015). https://doi.org/10.1007/s11947-015-1482-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1482-3