Abstract
The antioxidant profile of liquid foods is complex and includes different lipid and water-soluble compounds. These should be considered when assessing total antioxidant capacity (TAC) of these beverages, since it may act synergistically rather than individually. This study describes and compares the use of spectrophotometric methods (Trolox equivalent antioxidant capacity, TEAC and 2,2-diphenyl-1-picrylhydrazyl, DPPH), fluorometric (oxygen radical antioxidant capacity, ORAC), and photochemiluminescence (PCL) for the measurement of the TAC of different liquid foods (fruit juice mixed with milk and vegetables beverage). An evaluation was also made for the influence of certain compounds (ascorbic acid, phenolic compounds, total carotenoids, and tocopherols) with antioxidant capacity that was present in the samples studied. The different methods studied allow the determination of the TAC of the analyzed foods in a precise and accurate way. The TAC values in the studied samples differ from the applied method. An overall antioxidant potency composite index was calculated by assigning each test's equal weight. When an index score was applied, ORAC method had the higher antioxidant capacity values in the analyzed liquid foods in comparison with the other methods. The correlations among the different methods used for the determination of the antioxidant capacity depend on food, that is, mainly due to compounds (lipid and water soluble) of the different food matrix. In addition, ascorbic acid was the main contributor to antioxidant capacity of fruit juice mixed with milk beverages measured with the different methods. However, in vegetables beverages, phenolic compounds were found to correlate more significantly with antioxidant capacity values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Numerous in vitro studies have been conducted to evaluate total antioxidant capacity (TAC) of food products. So far, however, there is no official standardized method, and therefore, it is recommended that each evaluation should be made with various oxidation conditions and different methods of measurement (Karadag et al. 2009; Zulueta et al. 2009). The methods for measuring antioxidant capacity are basically classified into two groups, depending on the reaction mechanism: methods based on hydrogen atom transfer (HAT) and methods based on electron transfer (ET). Oxygen radical antioxidant capacity (ORAC) and Trolox equivalent antioxidant capacity (TEAC) assays are the most popularly used HAT and ET methods, respectively (Berker et al. 2012; Huang et al. 2005). Nowadays, the most widely used methods for measuring antioxidant activity are those that involve the generation of radical species and the presence of antioxidants determining the scavenging of these radicals (Arnao et al. 2001; Szydłowska-Czerniak et al. 2011).
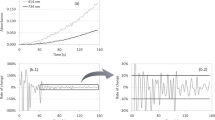
The TEAC assay or 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) assay is based on scavenging of the ABTS•+ radical cation by the antioxidants present in a sample. The ABTS•+ radical typically has a bluish-green color with maximum absorbance values at 645 nm, 734 nm, and 815 nm (Re et al. 1999). When there are antioxidant compounds in the reaction medium, they capture the free radical, which is translated into a loss of color and therefore, a reduction in absorbance, corresponding quantitatively to the concentration of antioxidants present. The DPPH assay is based on the reduction of the 2,2-diphenyl-1-picrylhydrazyl (DPPH•) radical reacting with antioxidant compounds present in a sample. DPPH is a stable free radical having a stable purple color. When free radical scavengers are added, DPPH is reduced and its color is changed to yellow, based on the efficacy of antioxidants. The radical color change is determined at 515 nm and quantification is done using standard solutions of ascorbic or Trolox® (Samaniego-Sánchez et al. 2007; Brand-Williams et al. 1995). In colorimetric methods, Trolox® (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) is used as standard. Food matrixes provide a complex of natural substances (hydrophilic and lipophilic) with different contributions to the antioxidant capacity of the final food product.
The ORAC method, developed initially by Cao et al. (1993) consists of measuring the decrease in the fluorescence of a protein as a result of the loss of its conformation when it suffers oxidative damage caused by a source of peroxyl radicals (ROO•). The method measures the ability of the antioxidants in the sample to protect the protein from oxidative damage. The protein used in the original method was β-phycoerythrin (β-PE), but it had a series of disadvantages, such as inconsistency between batches, photosensitivity, and interaction with phenolic compounds owing to nonspecific protein binding. To solve this problem, Ou et al. (2001) proposed fluorescein (3′,6′-dihydroxyspiro[isobenzofuran-1[3 H], 9′[9 H]-xanthen]-3-one) as the target compound.
The photochemiluminiscence (PCL) assay is based on the photo-induced autoxidation inhibition of luminol by antioxidants, mediated from the radical anion superoxide (O •−2 ) and is suitable to measure the radical scavenging properties of single antioxidants as well as more complex systems (Besco et al. 2007). The PCL method can be conducted by two different protocols, ACW and ACL, which consent to measure the antioxidant capacity of water- and lipid-soluble components, respectively. In the presence of substances, the intensity of chemiluminiscence is attenuated as a function of their concentration.
The antioxidant capacity can be expressed in different ways depending on the method, which makes it difficult to compare the results found in other studies. The expression of results of antioxidant capacity assays can be summarized in three categories: results based on measurements at a fixed end-point compared to a standard, results expressed considering lag-phase, and results based on kinetics parameters (Pérez-Jiménez et al. 2008).
The antioxidant profile of the liquid foods analyzed in this study is complex and includes ascorbic acid, as well as phenolic compounds in the water-soluble fraction and carotenoids, tocopherols, and other compounds in lipid fraction. These should be considered as a whole when assessing potential antioxidant activity, since antioxidants may act synergistically rather than individually (Besco et al. 2007; Lugasi and Hovari 2003). Other authors, when they studied the antioxidant capacity in different liquid foods measured with TEAC and ORAC assays observed changes in antioxidant capacity values depending on food matrix (Zulueta et al. 2009). Thus, this research involved the comparison of different methods for measuring antioxidant capacity, adapted to vegetable beverages and fruit juice mixed with milk.
Materials and Methods
Samples
Fruit Juice Mixed with Milk (FJM)
Oranges (Citrus sinensis L.) Navel cultivar and UHT skimmed milk (0.1 % fat) (Grupo Leche Pascual, SA, Burgos, Spain) were purchased from a local supermarket. Orange juice was extracted after appropriate washing and hygienization of the fruits. The orange juice–milk beverage, which was prepared in a laboratory (OJM) by mixing 50 % (v/v) of orange juice with the pulp removed, 20 % (v/v) of UHT skimmed milk, and 30 % (v/v) of water. Sugar (7.5 % w/v), citric acid (0.1 % w/v), and high methoxyl citrus pectin (0.3 % w/v) were added as a sweetener, preservative, and homogenizer of the samples, respectively. Solid ingredients were dissolved in water in the weight proportions indicated. The beverage was prepared just before use.
Commercial Fruit Juice Mixed with Milk Beverages (CFJM)
Three units from each of two batches of four different commercial juice–milk beverages marketed in Spain were purchased from a local supermarket (Valencia, Spain). All of them were kept at room temperature (20 ± 2 °C) because they had been sterilized. Tables 1 and 2 give details (as indicated on the label) of each of the samples analyzed. The measurements were taken in triplicate.
Vegetable Beverages (VB)
Vegetable beverage was prepared in a laboratory (VBL) by mixing the following ingredients purchased from a local supermarket in Valencia (Spain): tomato (Lycopersicon esculentum Mill., 33 %), green pepper (Capsicum annuum L., Italian pepper, 17 %), green celery (Apium graveolens L., 8.5 %), cucumber (Cucumis sativus L., 4 %), onion (Allium cepa L., 4 %), carrot (Daucus carota L., 4 %), lemon (Citrus limon L, 1.7 %), salt (1.7 %), virgin olive oil (Madrid, Spain, 0.8 %), and water to 100 %.
Commercial Vegetable Beverages (CVB)
Three units from each of two batches of four different commercial vegetable beverages marketed in Spain were purchased from a local supermarket (Valencia, Spain). All of them were kept at room temperature (20 ± 2 °C) because they had been sterilized. Tables 1 and 2 give details (as indicated on the label) of each of the samples analyzed. The measurements were taken in triplicate.
Materials and Reagents
Acetonitrile, diethyl ether (HPLC grade), ethanol, hexane, methanol, phenolphthalein 1 % (w/v), and sodium chloride (special grade) were purchased from J.T. Baker (Deventer, The Netherlands). Potassium hydroxide and sodium sulphate (Scharlab, Barcelona, Spain), ascorbic acid, and chloroform (Merck, Darmstadt, Germany), butylhydroxytoluene (BHT), cholecalciferol, α-tocopherol, δ-tocopherol, and γ-tocopherol, Trolox® (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), ABTS (2,2'-azinobis(3-ethylbenzothiazoline 6-sulfonic acid)), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Folin–Ciocalteu reagent, and fluorescein sodium salt were purchased from Sigma-Aldrich (Steinheim, Germany) and ergocalciferol (Fluka, Buchs, Switzerland). Gallic acid was purchased from UCB (Brussels, Belgium). Sodium and disodium phosphates, L(+)-ascorbic acid, magnesium hydroxide carbonate (40–45 %), and 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH) were purchased from Panreac (Barcelona, Spain).
Instrumentation
A Metrohm 746 VA Trace Analyzer (Herisau, Switzerland) equipped with a Metrohm 747 VA stand was used for polarographic determination. The working electrode was a Metrohm multimode electrode operated in the dropping mercury mode. A platinum wire counter electrode and a saturated calomel reference electrode were used. A Perkin-Elmer UV/Vis Lambda 2 spectrophotometer (Perkin-Elmer, Jügesheim, Germany) was also used. The LC system consisted of two isocratic pumps (Prostar 210, Varian Inc., California, USA) with degasser (Degassit, MetaChem, USA), column thermostat (Prostar 510, Varian) and a UV–vis detector (Varian Inc, California, USA). A Wallac 1420 VICTOR2 multilabel counter (Perkin-Elmer, USA) with fluorescence filters was used, with an excitation wavelength of 485 nm and an emission wavelength of 535 nm. A Photochem instrument with ACW and ACL kit (Analytik Jena AG, Germany) was also used. The whole LC system was operated by a Varian Star Chromatography Workstation Ver. 6.0 (Varian Inc., California, USA).
Methods
Ascorbic Acid
The method used was in accordance with Zulueta et al. (2010). Beverage (5 mL) was diluted to 25 mL of the extraction solution (oxalic acid 1 % w/v, trichloroacetic acid 2 % w/v, sodium sulfate 1 % w/v). After vigorous shaking, the solution was filtered through a folded filter (Whatman no. 1). Oxalic acid solution (9.5 mL) 1 % (w/v) and 2 mL of acetic acid/sodium acetate 2 M buffer (pH = 4.8) were added to an aliquot of 0.5 mL of filtrate solution and the solution was transferred to the polarographic cell. Determinations were carried out using the peak heights and standard additions method. The amount of ascorbic acid present in the sample solution was determined by standard addition calibration. Standard ascorbic acid (Merck, Darmstadt, Germany) solution (100 μg/mL) in 1 % (w/v) oxalic acid solution was freshly prepared (Aparicio et al., 1992). To check the reliability and usefulness of the method, the analytical parameters were determined. Six levels of working calibration solutions with a mixture, each by duplicate, were used for linearity. A linear response was obtained for ascorbic acid contents in a polarographic cell (cuvette) in the range of 0.3–30 μg (n = 16) with a slope of 3.34 nA/μg, an intercept of −2.07 nA, and a correlation coefficient of 0.999. The limit of detection was calculated by preparing six reagent standard solutions and applying the quotient between three times the standard deviation and the slope of the calibration curve (LOD = 3 · S n − 1 / m). The detection limit was 0.76 μg/ mL (0.076 μg in cuvette) (S n − 1 = 0.085, m = 3.34 nA/μg). The limit of quantitation corresponds to the minimum quantity with which it was possible to quantify without uncertainty (LOQ = 10 · S n − 1 / m), and the result obtained was 2.5 μg/mL (0.250 μg in cuvette). Accuracy was estimated by means of recovery assays. A sample (61.65 μg/mL), to which known amounts of standard ascorbic acid (200 μg/mL) had been added, was subjected to the entire extraction and determination process. The recovery percentage obtained was 96 %. Instrumental precision was checked from six consecutive analysis of a sample extract and was expressed as relative standard deviations (RSD %). The method precision was determined by preparing six aliquots of the sample and was expressed as RSD (%). The precision values, expressed as the RSD, were 0.85 % (x = 57.35 nA, S n − 1 = 0.49, n = 6) and 2.9 (x = 156.67 μg/mL, S n − 1 = 4.62, n = 6) instrumental and method, respectively.
Total Phenolic Compounds
The total phenol contents of the samples were determined using the Folin–Ciocalteu method (Singleton and Rossi 1965). Sodium carbonate solution (3 mL) 2 % (w/v) and 100 μL of Folin–Ciocalteau reagent were added to an aliquot of 100 μL of sample. Absorbance was measured at 750 nm. The Folin–Ciocalteau method was linear over the working range between 0.1 and 0.5 mg/mL of gallic acid concentrations with a slope of 1.473 mg/mL, an intercept of 0.198, and a correlation coefficient of 0.999. The detection and quantification limits were 0.53 and 1.77 μg/mL (S n − 1 = 0.26, m = 1.47 mg/mL). The recovery percentage obtained was 92 %. The precision values, expressed as RSD, were 1.90 % and 5.94 %, instrumental and method, respectively.
Total Carotenoids
Extraction of total carotenoid was carried out in accordance with the method of Lee and Castle (2001). An aliquot of sample (2.5 mL) was homogenized with 5 mL of extracting solvent (hexane/acetone/ethanol, 50:25:25, v/v) and centrifuged for 5 min at 6,500 rpm at 5 °C. The top layer of hexane containing the color was recovered and transferred to glass tubes protected from light and homogenized with a Pasteur pipet. After that, 1 mL of this supernatant was transferred to a 25-mL volumetric flask, and the volume was completed with hexane. Total carotenoid determination was carried out on an aliquot of the hexane extract by measuring the absorbance at 450 nm. Total carotenoids were calculated according to Ritter and Purcell (1981) using an extinction coefficient of β-carotene, E 1% = 2505. Pure solvent (hexane)-based calibration and sample calibration curves of β-carotene were generated by plotting absorbance values versus the concentration of analyte. The method was linear over the working range between 0.015 and 4 μg/mL with a slope of 2.361 μg/mL, an intercept of 0.007, and a correlation coefficient of 0.999. The detection and quantification limits were 0.40 and 1.35 μg/mL (S n − 1 = 0.32, m = 2.36 mg/mL). The recovery percentage obtained was 96 %. The precision values, expressed as RSD, were 3.4 % and 6.5 %, instrumental and method, respectively.
Total Antioxidant Capacity
ABTS•+Assay
The method used was described by Re et al. (1999), based on the capacity of a sample to inhibit the ABTS radical (ABTS•+). The radical was generated using 440 μL of potassium persulfate (140 mM). The solution was diluted with ethanol until an absorbance of 0.70 was reached at 734 nm. Once the radical was formed, 2 mL of ABTS•+ was mixed with 100 μL of appropriately diluted beverage (1:25, v/v), and the absorbance was measured at 734 nm for 20 min in accordance with Zulueta et al. (2009).
DPPH Assay
The method used was as described by Brand-Williams et al. (1995). The reaction was begun by adding a suitable dilution (1:25, v/v) of the methanol beverage extract to the DPPH colored radical. Absorbance was measured at 515 nm every 15 min for 1 h until equilibrium was reached (Samaniego-Sánchez et al. 2007).
Oxygen Radical Absorbance Capacity (ORAC) Assay
The method used was in accordance to Zulueta et al.'s (2009). Samples had to be diluted 1:250 with a phosphate-buffered saline (PBS) (75 mM, pH = 7.0). For the measurements, 96 white flat-bottom wells (Sero-Wel, Bibby Sterilin, Ltd., Stone, UK) were used. In each well, 50 μL of fluorescein (78 mM) (Sigma–Aldrich, Steinheim, Germany) and 50 μL of sample, blank (PBS), or standard (Trolox, 20 μM) were placed and then 25 μL of 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH) (221 mM) (Sigma-Aldrich, Steinheim, Germany) were added. The measurement of fluorescence was carried out immediately after the addition, and measurements were then taken every 5 min until the relative at 37 °C on a Wallac 1420 VICTOR2 multilabel counter (Perkin-Elmer, Valencia, Spain) with fluorescence filters for an excitation wavelength of 485 nm and an emission wavelength of 535 nm. The measurements were taken in triplicate. The ORAC values, expressed as micromolar Trolox equivalents (μM TE) were calculated in accord to Zulueta et al. (2009).
Photochemiluminiscence Assay
The evaluation of TAC in the studied samples was based on the procedure described by Popov and Lewin (2000; 2001) and was carried out according to instructions of Photochem instrument assay kits. The standard and sample solutions were prepared by mixing 1.5 mL portion of Reagent 1 (sample solvent), 1 mL of Reagent 2 (reaction buffer), 25 μL of Reagent 3 (photosensitizer and detection reagent), and 0–30 μL of Reagent 4 (calibration standard for quantification of water-soluble antioxidants in ascorbic acid equivalents or lipophilic antioxidants in Trolox equivalents) or 10 μL of sample (beverage diluted with Reagent 1) and then measured by Photochem. The detector measures the current proportion to the generated luminescence as a function of measurement time. The detector signal, monitored for 1–3 min, includes a lag phase in which no luminescence can be detected. When the antioxidants are exhausted, the amount of radicals in the sample increases until the detected signal reaches the maximum. The length of the lag phase increases in function of the amount of the antioxidants in the sample, and it is calculated by determining the first derivative and the maximum point of the detected curve. The intersection point of the slope of the straight line with the x-axis defines the lag time. For ACW studies, the luminal reagent and Trolox work solution were prepared on the day during which they were needed according to the ACW protocol. The presence of Trolox (or any other antioxidants from the samples) retarded luminescence for a period; hence, a lag time was noted before a signal was measured. The concentration of the added sample was such that the generated luminescence fell within the limits of the standard curve. Therefore, the lag time (seconds) for the ACW assay was used as the radical scavenging activity and the antioxidant capacity calculated by comparison with a Trolox standard curve and then expressed as millimolar Trolox equivalents (mM TE). In ACL studies, the kinetic light emission curve, which exhibits no lag phase, was monitored for 3 min and expressed as millimolar Trolox equivalents. The areas under the curves were calculated using the PCLsoft control and analysis software. As greater concentrations of Trolox working solutions were added to the assay medium, a marked reduction in the magnitude of the PCL signal, and hence, the area calculated from the integral, was observed. This inhibition was used as a parameter for quantification and related to the decrease in the integral of PCL intensities caused by varying concentrations of Trolox. The observed inhibition of the signal was plotted against the concentration of Trolox added to the assay medium. The concentration of the added sample was such that the generated luminescence during the 3-min sampling interval fell within the limits of the standard curve.
Vitamin E (α-, γ- and δ-Tocopherol)
The HPLC system consisted of two isocratic pumps (Prostar 210, Varian Inc., California, USA) with degasser (Degassit, MetaChem, USA), column thermostat (Prostar 510, Varian), and UV–vis detector (Varian Inc., California, USA). The whole liquid chromatographic (LC) system was operated by a Varian Star Chromatography Workstation Ver. 6.0 (Varian Inc., California, USA). A 150 × 4.6 mm Kromasil reverse-phase C18 column with a particle size of 5 μm and a Kromasil precolumn (guard column) (30 × 4.6 mm i.d. cartridge with 5-μm particles) (Scharlab, Barcelona, Spain) were used. The α-, γ-, and δ-tocopherol were identified and quantified by HPLC with an ultraviolet/visible detector. The mobile phase used was acetonitrile–methanol (90:10, v/v) at a flow rate of 1 mL/min. Saponification was performed with 15 mL of KOH in ethanol (50 %, w/v), and BHT was added as antioxidant. The subsequent extraction was carried out with hexane. UV detection was used for the detection of all compounds at the same λ (265 nm). Method validation and validation parameters for the determination of vitamin E (α-, γ-, and δ-tocopherol) in the samples studied in the present work were detailed in a previous article published by Barba et al. (2011). Vitamin E activity was calculated using the factors for conversion of tocopherols to RRR-α-tocopherol equivalents (Eitenmiller and Landen 1999):
Statistical Analysis
A multiple regression analysis was performed to study the influence of bioactive compounds to antioxidant capacity (the results are shown in the significant cases, p < 0.05). A study was conducted with the aim of determining whether there were correlations between a pair of variables (Pearson's test). All statistical analyses were performed using SPSS® (Statistical Package for the Social Sciences) v.13.0 for Windows (SPSS Inc., Chicago, USA).
Results and Discussion
Analytical Parameters of the Antioxidant Methods
To verify the quality and usefulness of the different antioxidant methods for the fruit juice mixed with milk and vegetable beverage, the analytical parameters, linearity, sensitivity, precision, and accuracy, were determined. Trolox was used as a calibration standard in all cases. A linear response was obtained for Trolox concentrations in the linearity ranges studied for the different antioxidant methods (see Table 3). The values obtained show that DPPH method is the most sensitive assay (LOD = 4.3 μM), although in all the cases, the value obtained for all the methods allow to detect the antioxidant capacity in the studied samples (5.0, 5.3, 8.1, and 13.5 μM for ACW, ORAC, TEAC, and ACL methods, respectively). The values obtained for LOQ were 13.1, 15.2, 16.0, 24.4, and 41.0 μM for DPPH, ACW, ORAC, TEAC, and ACL methods, respectively.
Instrumental and method precision values, expressed as the RSD are shown in Table 4. It should be noted that instrumental and method precision for the PCL (ACL and ACW) assay were higher in the FJM beverage compared with vegetables beverage. This may be due to the complexity of the formulation.
For verifying method accuracy, degree of recovery could be used, and known Trolox® amounts were added to the respective liquid foods, which were subsequently analyzed by using the experimental conditions described at the working procedure. Recovery percentages for fruit juice mixed with milk and vegetable beverage are shown in Table 4.
Ascorbic acid, phenolic, carotenoid, and tocopherol contents have been reported to be responsible for the TAC of liquid foods. TEAC, DPPH, ORAC, and PCL assays have been used to measure antioxidant capacity, and these results should correlate with those of bioactive compound and tocopherol contents. In order to verify the role of these compounds in determining the antioxidant capacity of the product, the correlations between antioxidant methods and ascorbic acid, phenolic, carotenoids, and tocopherols were calculated. Figure 1 shows the chromatograms obtained by applying the method established to determine vitamin E (α-, γ-, and δ-tocopherol) in samples of fruit juice–milk- and vegetable-based beverage. Tables 5 and 6 show the antioxidative potency (by TEAC, DPPH, ORAC, and PCL assays), bioactive compounds, and tocopherols contents of the different fruit juice–milk and vegetable beverages studied. As it can be observed, the magnitude of the antioxidant capacity in the different samples differs from the applied method. This fact makes difficult the comparison of the results obtained with the different antioxidant capacity assays. For this reason, an overall antioxidant potency composite index was determined by assigning all assays an equal weight, assigning an index value of 100 to the best score for each test, and then calculating an index score = [(sample score/best score) × 100]; the average of the values of all test for each beverage was then taken for the antioxidant potency composite index (Seeram et al. 2008). It should be noted that when the ORAC method was applied, the antioxidant capacity values were higher than those obtained by the other methods (Fig. 2). Orange juice mixed with milk prepared in laboratory had the most potent antioxidant capacity (7.28 ± 0.07 mM TE) followed by all vegetables beverages, prepared in a laboratory and commercial (6.59 ± 0.01–7.14 ± 0.08 mM TE) (see Fig. 2, Tables 5 and 6). This can be related with the nature and chemical structures of the different compounds included in food products that confers this antioxidant capacity, and also, it can be due to processing. The higher antioxidant index corresponded to commercial fruit juice mixed with milk samples 1A, 2B, and 3 C. This can be related with vitamins A (120 μg/100 mL), C (30 mg/100 mL), and E (1.5 mg/100 mL) added, as declared on the label (Table 2). However, the lowest value corresponded to sample 4D which also had a low vitamin C concentration (9 mg/100 mL) indicated in the label and a low juice percentage (7 %). In this sense, samples 2B and 3 C contained 25 % juice and sample 1A had 15 % juice, observing the lower antioxidant capacity for the last one. Vegetable beverages had lower antioxidant capacity index than those observed for fruit juice–milk beverages. Among all the vegetable beverages, the sample prepared in the laboratory had higher antioxidant activity in comparison with commercial beverages. This can be related with processing conditions. Likewise, commercial vegetable beverages did not have ascorbic acid (Table 6), and moreover, manufacturers did not declare the addition of any vitamin (Table 2).
Correlation of the Studied Methods for the Different Liquid Foods
There were significant correlations (p < 0.05) for ACL with ACW, DPPH, and TEAC (r = 0.854, r = 0.887, and r = 0.918, respectively) with the possible correlation (Pearson's test) between the various methods used for determining antioxidant capacity (ABTS, DPPH, ORAC, ACL, and ACW) in FJM. Also, there were correlations found between ACW with DPPH and TEAC (r = 0.937 and r = 0.757, respectively) and between DPPH and TEAC (r = 0.873).
Regarding vegetables beverage, significant correlations (p < 0.05) for ACL with ACW and ORAC (r = 0.716 and r = 0.800, respectively) were found. Also, correlations between ACW with DPPH and TEAC (r = 0.807 and r = 0.875, respectively), DPPH with TEAC (r = 0.963), and ORAC with TEAC (r = 0.830) were found.
Numerous authors have obtained correlations between the results found after analyzing antioxidant capacity with the TEAC and ORAC assays. However, this seems to be for foods in which the main antioxidants are water soluble and, as seen, the action of these antioxidants takes place easily with both methods (Barba et al. 2010; Proteggente et al. 2002; Stintzing et al. 2005; Taipong et al. 2006). On the other hand, Pérez et al. (2000) compared the ORAC and TEAC methods in samples of red wine and white wine and did not find correlations between the two methods. In addition, Zulueta et al. (2009) did not obtain statistically significant correlations between the ORAC and TEAC methods for changes in the milk concentration. In our study, milk percentage indicated by the manufacturer in the label did not overcome 10 % in the FJM beverages analyzed. This can explain the correlations observed among the different methods. Likewise, vitamins A, C ,and E were added to beverages, being correlated with an increase in TAC.
Bioactive Compound Contribution to Total Antioxidant Capacity
In order to evaluate the compounds that affect TAC, it was educed a multivariant regression analysis. Table 7 shows the results obtained. The R 2 value explained the percentage in which the models can explain the experimental values. In fruit juice mixed with milk beverages, ascorbic acid mainly contributed to antioxidant capacity determined by the different methods. This is similar to the result obtained by Gardner et al. (2000) and Sánchez-Moreno et al. (2003), who found that this compound had the greatest antioxidant capacity in different orange juices. Similarly, Rice-Evans and Miller (1996) observed that ascorbic acid was the chief contributor to antioxidant capacity in apple juice. However, in commercial vegetable beverages, without ascorbic acid in its composition, the higher contribution to antioxidant capacity is mainly due to phenolic compounds and tocopherols.
Conclusion
The analytical parameters: linearity, detection limit, precision, and accuracy assay show that the different methods studied are useful for measuring the TAC in liquid foods. This fact is important in order to quantify the changes in the antioxidant capacity of foods during processing/preservation treatment and subsequent storage. In this study, the correlations among the different methods used for the determination of the antioxidant capacity depend on the bioactive compounds (ascorbic acid, phenolic compounds, and carotenoids), and also, tocopherol content in the lipid and water-soluble fraction of the analyzed liquid foods. Further research will help to identify the specific components in food matrixes that provide protection against free radical damage.
References
Aparicio P, Farré R, Frígola A (1992) An Bromatol 44:257–261
Arnao MB, Cano A, Acosta M (2001) Food Chem 73:239–244
Barba FJ, Esteve MJ, Frígola A (2010) J Agric Food Chem 58(18):10070–10075
Barba FJ, Esteve MJ, Frígola A (2011) Eur Food Res Technol 232:829–836
Berker KI, Demirata B, Apak R (2012) Food Anal Methods In press: doi 10.1007/s12161-011-9358-2
Besco E, Braccioli E, Vertuani S, Ziosi P, Brazzo F, Bruni R, Sacchetti G, Manfredini S (2007) Food Chem 102:1352–1356
Brand-Williams W, Cuvelier ME, Berset C (1995) LWT 28:25–30
Cao G, Alessio HM, Cutler RG (1993) Free Radic Biol Med 14:303–311
Eitenmiller RR, Landen WO (1999) Vitamin analysis for the health and food sciences. CRC Press LLC, Boca Ratón
Gardner PT, White TAC, McPhail DB, Duthie GG (2000) Food Chem 68:471–474
Huang D, Ou B, Prior RL (2005) J Agric Food Chem 53:1841–1856
Karadag A, Ozcelik B, Saner S (2009) Food Anal Methods 2:41–60
Lee HS, Castle WS (2001) J Agric Food Chem 49:877–882
Lugasi A, Hovari J (2003) Nahrung 47:79–86
Ou B, Hampsch-Woodill M, Prior RL (2001) J Agric Food Chem 49:4619–4626
Pérez D, Leighton F, Aspee A, Aliaga A, Lissi E (2000) Biol Res 33:71–77
Pérez-Jiménez J, Arranz S, Tabernero M, Díaz-Rubio ME, Serrano J, Goñi I, Saura-Calixto F (2008) Food Res Int 41(3):274–285
Popov IN, Lewin G (2000) Free Radic Med 17:267–271
Popov IN, Lewin G (2001) Photosensitized chemiluminescence. Its medical and industrial applications for antioxidizability tests. In: García-Campaña AM, Baeyens WRG (eds) Chemiluminescence in analytical chemistry. Marcel Dekker, New York-Basel, pp 497–527
Proteggente AR, Pannala A, Paganga G, Van Buren L, Wagner E, Wiseman S et al (2002) Free Radic Res 36:217–233
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Free Radic Biol Med 26:1231–1237
Rice-Evans CA, Miller NJ (1996) Biochem Soc T 24:790–800
Ritter ED, Purcell AE (1981) Carotenoid analytical methods. In: Bauernfeind (ed) Carotenoids as colorants and vitamin A precursors. Academic Press, New York, pp 815–923
Samaniego-Sánchez C, Troncoso-González AM, García-Parrilla MC, Quesada Granados JJ, López-García de la Serrana H, López-Martínez MC (2007) Anal Chim Acta 593:103–107
Sánchez-Moreno C, Plaza L, de Ancos B, Cano P (2003) J Sci Food Agric 83:430–439
Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M, Heber D (2008) J Agric Food Chem 56:1415–1422
Singleton VL, Rossi JA Jr (1965) Amer J Enol Vitic 16(3):144–158
Stintzing F, Herbach K, Mosshammer M, Carle R, Yi W, Sellappan S et al (2005) J Agric Food Chem 53:442–451
Szydłowska-Czerniak A, Tułodziecka A, Szłyk E (2011) Food Anal Methods In Press:doi 10.1007/s12161-011-9314-1
Taipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Hawkins D (2006) J Food Compos Anal 19:669–675
Zulueta A, Esteve MJ, Frígola A (2009) Food Chem 114(1):310–316
Zulueta A, Esteve MJ, Frígola A (2010) Innovat Food Sci Emerg Technol 11(1):84–90
Acknowledgments
This study was carried out with funds from the Spanish Ministry of Science and Technology and European Regional Development Funds (AGL2006-13320-C03-03 and AGL2010-22206-C02-01). F.J. Barba holds an award from the Generalitat Valenciana (Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barba, F.J., Esteve, M.J., Tedeschi, P. et al. A Comparative Study of the Analysis of Antioxidant Activities of Liquid Foods Employing Spectrophotometric, Fluorometric, and Chemiluminescent Methods. Food Anal. Methods 6, 317–327 (2013). https://doi.org/10.1007/s12161-012-9441-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-012-9441-3