Opinion statement
The range of available treatment options for patients with multiple sclerosis (MS) has expanded tremendously in recent years, adding further complexity to the therapeutic decision-making process. The first-generation therapies interferon beta and glatiramer acetate have been safely used for more than 20 years, but are only partially effective. Many of the newly approved MS therapies such as oral agents and monoclonal antibodies are selective immunosuppressants that appear to have improved efficacy and/or are more convenient, albeit in the absence of a long-term safety record. Although some are known to be associated with serious adverse effects, these treatments provide evidence-based therapeutic options for patients with suboptimal response or breakthrough disease. In this new scenario, non-selective immunosuppressive drugs and autologous hematopoietic stem cell transplantation are still present but likely play a more limited role than before. In this review, we briefly summarize the current, recent, and most imminent immunosuppressive therapies, and present an overall summary along with a discussion of their role in the current MS treatment scenario.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is an autoimmune chronic inflammatory demyelinating disease of the central nervous system that typically strikes young adults, especially women. Despite considerable progress in our understanding of the MS pathogenesis, the exact mechanisms involved are still not completely understood. While classic disease-modifying therapies (DMTs) such as interferon beta (IFN-beta) and glatiramer acetate (GA) have been in use for more than 20 years, the repertoire of available therapeutic options for MS has been steadily increasing more recently. Most of the current treatments target the immune system by killing, attenuating, or disabling overactive autoreactive lymphocytes. These therapies are more effective in patients who are experiencing active inflammatory episodes, predominantly patients who are in the relapsing-remitting phase of the disease. Patients with relapsing-remitting MS (RRMS) often receive IFN-beta or GA as first-line DMT due to the immunomodulatory activity and relative safety of these treatment modalities, but their effectiveness is modest. Until a few years ago, immunosuppressants, natalizumab, and fingolimod were the only options for patients with suboptimal response or who had breakthrough disease. There are now 10 DMTs approved in Europe and 9 in the United States, offering several alternatives with various mechanisms of action and modes of administration, providing the potential to improve long-term outcomes for MS patients. However, the selection of an appropriate DMT is becoming increasingly more complex. In this review, we briefly summarize the existing and more recently approved immunosuppressive therapies, as well as emerging therapies under investigation, in order to present a scenario in which selective and non-selective immunosuppressive therapies will be considered for patients who have not responded to the earlier treatments.
Current approved immunosuppressive therapies for relapsing-remitting MS
Natalizumab
Natalizumab (Tysabri®) (NTZ) has been approved in Europe by the European Medicines Agency (EMA) as second-line therapy for patients with high disease activity despite treatment with IFN or GA, or as first-line treatment in rapidly evolving severe RRMS. In the U.S., NTZ is recommended for patients who have an aggressive course of the disease or those with an inadequate response or intolerance to other therapies. There is currently an active phase III clinical trial comparing NTZ with placebo in secondary progressive MS (SPMS).
Mechanism of action
NTZ is a humanized monoclonal antibody, administered intravenously, that targets the alpha-4 integrin on the surface of lymphocytes and monocytes and prevents lymphocytic migration across the blood–brain barrier.
Efficacy
Patients receiving NTZ monotherapy showed a 68 % reduction in annualized relapse rate (ARR), a 42 % decrease in disability progression, and a substantial reduction in MRI activity at two years compared with placebo [1]. Observational studies investigating the use of NTZ as monotherapy in clinical practice have shown that switching treatment to NTZ in patients with suboptimal response to first-line drugs is a good option, as a large percentage of these patients were free of relapse or disability progression during NTZ therapy as compared with a previous period [2, 3]. However, the quality of evidence is limited by the lack of a control group and the retrospective nature of studies.
Common side effects
There is a slightly increased rate of common infections (cold, urinary tract infections), and there have been cases of hepatotoxicity. Although NTZ is generally well tolerated, hypersensitivity reactions may occur in a small proportion of patients (5 %), usually associated with the presence of neutralizing antibodies [4].
Safety issues
The risk of progressive multifocal leukoencephalopathy (PML) is the primary serious concern. Risks factors for PML include exposure to the JC (John Cunningham) polyomavirus (JCV), duration of NTZ therapy, and prior use of immunosuppressants (Fig. 1). Risk stratification data for PML may be used to counsel individual patients; assessment of antibody titers may help to improve stratification, although this technique has not yet been validated [5, 6]. Retesting of JCV antibodies every six months is recommended in seronegative patients due to the risk of seroconversion, which has been estimated from 2 % to 14.5 % per year in recent series [7, 8]. Clinical or MRI evidence suggestive of PML should prompt NTZ discontinuation, and cerebrospinal fluid (CSF) analysis by polymerase chain reaction (PCR) should be performed to ascertain the presence of JCV DNA, with consideration of plasma exchange (PE) for the rapid removal of circulating NTZ. After discontinuing NTZ (with or without PE), there is a risk of immune reconstitution inflammatory syndrome (IRIS), characterized by severe clinical and radiological worsening within days to weeks after NTZ removal. Reports have also described a return of MS disease activity from three to six months after cessation of NTZ, and in some cases, a “rebound of activity” (increase of disease activity beyond pre-NTZ levels) has been noted [9]. An earlier transition to fingolimod (maximum two-month treatment gap) seems to reduce the risk of resumption of activity [10•].
Fingolimod
Fingolimod (Gilenya®) is approved by the EMA as second-line therapy, with the same indications as NTZ, and by the FDA as a first- or second-line agent for individuals without cardiovascular risk factors who desire once-daily oral therapy.
Mechanism of action
Fingolimod is a sphingosine-1-phosphate (S1P) modulator, binding to four of the five S1P receptor subtypes. Binding to the S1P1 receptor on lymphocytes prevents their exit from lymphoid tissues, thereby reducing infiltration into the CNS. Fingolimod also enters the CNS, potentially affecting the survival of oligodendrocyte precursor cells, and may promote protective effect on astrocytes.
Efficacy
In a pivotal placebo-controlled phase III trial, fingolimod reduced the ARR by 54 %, disability progression by 30 %, and MRI activity by 74–82 % [11]. In comparison to intramuscular interferon beta-1a, fingolimod 0.5 mg reduced the ARR by 52 %, but it was not superior on disability progression. MRI activity was significantly lower for fingolimod, and brain volume loss was reduced by 31 % [12]. Post hoc analysis demonstrated the efficacy of fingolimod in patients previously treated with other DMTs (IFN, GA, NTZ), and observational studies support its real-life effectiveness [10•].
Common side effects
The most common adverse reactions are hypertension, headache, influenza, diarrhea, back pain, liver enzyme elevation, and asymptomatic reduction in pulmonary forced vital capacity or cough. Although few opportunistic infections have been recorded, there is a risk of herpes zoster infection, and documentation of an adequate serological response or immunization is required before the initiation of therapy. There is a 0.5 % risk of macular oedema, usually reversible and occurring within the three first months, which requires ophthalmological evaluation prior to beginning fingolimod treatment and again after three months of therapy. However, the risk is higher in patients with diabetes mellitus or prior uveitis [13].
Safety issues
Nodal trapping of lymphocytes causes mild lymphopenia. A six-hour observation period is necessary after the first dose because bradycardia and, less frequently, atrioventricular conduction block can occur. Fingolimod is contraindicated in patients who have experienced myocardial infarction, unstable angina, stroke, or heart failure within the past six months, as well as patients with a history of second-degree or third-degree atrioventricular block, baseline QT interval ≥500 ms, or treatment with Class Ia or Class III antiarrhythmic drugs.
Teriflunomide
Teriflunomide (Aubagio®) is a once-daily oral DMT approved by the FDA in September 2012 and by the EMA in August 2013 as first-line therapy for RRMS. It is also approved in Australia, Argentina, Chile, South Korea, and Mexico.
Mechanism of action
Teriflunomide, the active metabolite of the rheumatoid arthritis drug leflunomide, is an inhibitor of dihydroorotate dehydrogenase, the enzyme required for de novo pyrimidine synthesis, which reduces the proliferation of activated B and T lymphocytes. Additionally, it acts in the suppression of proinflammatory factors, prevention of T-cell interaction with antigen-presenting cells, and suppression of nuclear factor (NF)-κB activation.
Efficacy
In two phase III trials, teriflunomide in 7-mg and 14-mg doses were associated with a reduction in ARR of 31 %-37 % compared with placebo, but sustained reduction in disease progression was observed only in the 14-mg group. Patients treated with teriflunomide had significantly fewer gadolinium-enhancing lesions and unique active lesions. Teriflunomide 7 mg was inferior and 14 mg equal in time to treatment failure in a comparative study with subcutaneous interferon beta-1a [14]. The magnitude of these benefits was comparable to that of the current first-line injectable therapies.
Common side effects
Teriflunomide is generally well tolerated at the approved dose (14 mg). Common adverse effects include gastrointestinal symptoms (abdominal pain, diarrhea, and nausea), elevated liver enzymes, hypertension, alopecia, and skin rashes. Neutropenia and hepatotoxicity are the most serious adverse events. It has been described a 1-2 % incidence of peripheral neuropathy and 1 % of acute renal failure [15]. Monitoring of liver function is necessary, monthly for six months and then every two months.
Safety issues
No serious opportunistic infections have been reported, but two cases of PML were described in rheumatoid arthritis patients treated with leflunomide [16]. Both sexes must use effective contraception due to the risk of teratogenicity (pregnancy category X). Breast-feeding is also not recommended. Teriflunomide has a significant enterohepatic cycle and prolonged half-life, so it may take from several months up to two years after discontinuation for the drug to be fully eliminated from the body. Administration of cholestyramine or activated charcoal is recommended to accelerate elimination.
Dimethyl fumarate/BG-12
Dimethyl fumarate (Tecfidera®) (DMF) is an oral DMT approved by the FDA in March of 2013, with approvals in Canada and Australia as well. In February of 2014, it was approved by the EMA.
Mechanism of action
While the exact mechanism has not been completely elucidated, DMF appears to activate the nuclear factor-E2-related factor-2 transcriptional pathway, and to modulate NF-κB, reducing the production and release of inflammatory cytokines and oxidative stress.
Efficacy
The two phase III trials comparing DMF with placebo demonstrated that a dose of 240 mg twice a day was associated with significant reduction in the ARR (53 % and 44 %, respectively) and disability progression (38 % and 21 %, respectively) at two years. A 71 % reduction in MRI gadolinium-enhancing lesions and 90 % reduction in new T2-weighted lesions were observed. Compared to GA, which was included as a reference comparator in one of the phase III studies, DMF showed better results in relapse rate and MRI activity reduction, but without significant change in disability progression [17, 18].
Common side effects
Almost 30 % of patients experience self-limiting symptoms of flushing (lasting about one week and mitigated by taking the drug with food or aspirin). Gastrointestinal symptoms such as nausea, abdominal pain, and diarrhea are seen in up to 20 % of patients (lasting 2-4 weeks). There is a tendency toward elevation of liver enzymes during the first six months, and sporadic reversible proteinuria has also been reported.
Safety issues
A mean reduction of 30-50 % in lymphocyte count has been routinely observed in 10 % of patients treated with DMF, and regular complete blood cell count monitoring is recommended. To date, PML has not been reported with DMF, but four cases were described in association with the use of Fumaderm® or compounds of fumaric acid esters for the treatment of psoriasis in patients with chronic lymphopenia [19, 20].
Alemtuzumab
Alemtuzumab (Lemtrada®) is approved in Europe, Canada, Australia, and Latin America for the treatment of active RRMS, and may be used as first-line therapy. The FDA recently declined to register this therapy for treatment of MS.
Mechanism of action
Alemtuzumab is a monoclonal antibody against CD52, a cell surface marker on monocytes and lymphocytes, causing rapid and almost complete depletion by both complement-mediated and antibody-mediated cell lysis. The drug has been used for years as part of bone marrow transplantation conditioning protocols. Monocytes and B cells return to pre-alemtuzumab levels in approximately three to six months, but the reconstitution of memory B cells may take one year. T cells repopulate more slowly, with CD8+ T cells reaching baseline levels only after 30 months and CD4+ T cells after a median of 61 months. Thus, during the first six months, T-cell population is based on memory T cells with a regulatory phenotype and reduced cytokine expression.
Efficacy
The phase II study (CAMMS223) and the two phases III studies (CARE-MS1 and CARE-MS2) compared alemtuzumab with subcutaneous interferon beta-1a. In the phase II trial, alemtuzumab reduced the ARR by 74 % and sustained accumulation of disability by 71 %. In the CARE-MS1 study (treatment-naïve patients), ARR was reduced 55 %, but there was no significant decrease in sustained accumulation of disability. In the CARE-MS2 trial (patients who had relapsed on DMT), the reduction in ARR was 49 % and in disability progression was 42 %. Alemtuzumab also significantly increased the proportion of relapse-free patients in all three studies. Secondary MRI objectives provided additional evidence that alemtuzumab was more effective than interferon beta-1a, with a lower percentage of patients with active MRI lesions. A significant 40 % reduction of brain volume loss was observed in CARE-MS2 [21, 22].
Common side effects
Infusion-related reactions (rash, headache, fever, pruritus, and fatigue) occurred in 90-99 % of patients, but were graded severe in only 1-3 %. Globally, infections were reported more frequently in alemtuzumab-treated patients, but were predominantly mild or moderate. With the use of prophylactic acyclovir, the percentage of patients who had herpes infection (oral herpes or varicella zoster reactivation) in the month after alemtuzumab decreased considerably. Opportunistic infections such as listeria meningitis, esophageal candidiasis, and Pasteurella infection were observed, but no PML was reported [23].
Safety issues
Nearly 30 % of patients receiving alemtuzumab developed thyroid autoimmunity (typically hyperthyroidism and Graves’ disease). Immune thrombocytopenia occurred in 3 % of patients, and one of them died from an intracranial hemorrhage. Rare cases of immune-mediated hemolytic anemia and neutropenia also occurred, resulting in death in one patient. Renal failure resulting from Goodpasture syndrome was reported in four patients, and two required renal transplantation. There have been four malignancies related to alemtuzumab (three thyroid cancers and one fatal Burkitt lymphoma). A risk minimization and management program has been implemented [21, 22].
Emerging therapies under investigation
Daclizumab
Daclizumab is a humanized monoclonal antibody that targets CD25 (interleukin-2 receptor α), which is expressed on immune cells. This drug has been used for more than 10 years in the prevention of kidney transplant rejection. There is currently an active phase III clinical trial comparing daclizumab with interferon beta-1a (Avonex®) in RRMS.
Mechanism of action
Daclizumab induces the expansion of regulatory CD56bright natural killer cells, and it also modulates the function of dendritic cells, resulting in decreased T-cell activation.
Efficacy
The CHOICE phase II placebo-controlled trial showed that daclizumab added to IFN reduced the number of gadolinium-enhancing lesions and new or enlarging T2-weighted lesions at six months compared with placebo [24]. The SELECT trial, a phase IIb study, showed that subcutaneous daclizumab administered every four weeks reduced the ARR (50-54 %) and increased the percentage of relapse-free patients at one year compared with placebo. MRI endpoints were also significant for daclizumab-treated patients [25].
Common side effects and safety issues
Severe events were more frequent in patients treated with daclizumab than in those receiving placebo, including serious cutaneous events (1 %) and a greater than fivefold increase of liver enzyme levels (4 %). One patient died from a psoas abscess after recovering from a rash, but no opportunistic infections were seen [26].
Rituximab
Rituximab (RTX), a human/murine monoclonal antibody, was the first B-cell depletion therapy used for treatment of MS, and the agent has been utilized in the treatment of B-cell leukemia for more than 15 years. Four studies assessed the efficacy of RTX—one for primary progressive MS (PPMS), while the other three focused on RRMS—with a total of 599 patients included. However, the development program in MS has been suspended, and no phase III trial is ongoing.
Mechanism of action
RTX binds to the CD20 antigen, leading to B-cell depletion through a combination of cell-mediated and complement-dependent cytotoxicity. Near-complete depletion is observed by week 2, and is sustained for 6-8 months.
Efficacy
A phase II 48-week placebo-controlled trial in RRMS showed that patients treated with RTX had fewer gadolinium-enhancing lesions at weeks 12, 16, 20, and 24, and the results were sustained for 48 weeks (p < 0.001). The proportion of patients with relapses was also significantly lower [27]. With RTX used as add-on therapy with standard DMT, up to 74 % of post-treatment MRI scans were free of gadolinium-enhancing lesions, compared with 26 % at baseline [28]. In PPMS, RTX tended to delay the time to progression compared to placebo, but the difference was not statistically significant [29].
Common side effects
RTX has an attractive safety profile, with no significant differences in the short-term incidence of serious adverse events or infections compared with placebo. The most common side effects were infusion-associated events, including fever, chills, flushing, itching, and general flu-like symptoms (headache, fatigue, muscle weakness). Most were classified as mild or moderate, and tended to occur during the first administration, decreasing in both frequency and intensity during subsequent infusions.
Safety issues
No opportunistic infections, including LMP, have been reported in patients with MS. Two cases of PML resulting in death have been reported in patients receiving RTX for treatment of systemic lupus erythematosus, but they had multiple courses of immunosuppressant therapy prior to receiving RTX. Cases of PML have been previously reported in patients with lymphoid malignancies taking RTX in combination with other immunosuppressants.
Ocrelizumab
Ocrelizumab is a humanized monoclonal antibody designed to selectively target CD20 B cells. Currently, two phases III studies in RRMS patients and one phase III study in PPMS patients are ongoing.
Mechanism of action
Ocrelizumab also binds to CD20 (at a distinct but overlapping epitope from RTX), and it is associated with increased antibody-dependent cell-mediated cytotoxicity and reduced complement-dependent cytotoxic effects in vitro. As a humanized molecule, it is expected to be less immunogenic with repeated infusions and to have a more favorable benefit–risk profile.
Efficacy
A phase II trial comparing ocrelizumab with intramuscular interferon beta-1a or placebo resulted in a significant reduction of gadolinium-enhancing lesions (89 % for the 600-mg dose and 96 % for the 2,000-mg dose). The ARR over 24 weeks was 80 % lower in the low-dose (600-mg) and 73 % lower in the high-dose (2,000-mg) groups compared to placebo. At week 144 of the open-label extension, MRI evidence of active disease was minimal, and the rate of relapse remained low [30].
Common side effects
Most infusion-related events occurred during the first infusion and were mild to moderate. The incidence of serious adverse events occurred at similar rates in all group of patients.
Safety issues
A rheumatoid arthritis program with ocrelizumab was discontinued due to the high rate of serious and opportunistic infections, some of which resulted in death. At this time, no opportunistic infections have been reported in MS patients, but the long-term safety profile of ocrelizumab in MS has yet to be established. Only one death was observed in an MS phase II trial, in which a contributory effect from ocrelizumab could not be excluded [30].
Ofatumumab
Ofatumumab is a fully human monoclonal antibody, directed against CD20.
Mechanism of action
Ofatumumab binds to a different epitope than RTX and ocrelizumab, resulting in different pharmacological properties. It acts through complement-mediated rather than antibody-mediated cytotoxicity.
Efficacy
In a phase II trial including 38 RRMS patients, all three intravenous doses evaluated (100 mg, 300 mg, and 700 mg) reduced the number of new gadolinium-enhancing lesions by >99 % at six months, with a statistically significant reduction compared to placebo [31].
Common side effects and safety issues
Ofatumumab given two weeks apart was not associated with any unexpected safety concerns and was well tolerated.
Non-selective immunosuppressive therapies
In recent decades, several non-selective immunosuppressive drugs have been administered in MS patients. The rationale for using these agents in MS is based on the experience of treating immune-mediated disorders that are refractory to conventional therapies. It is important to note that mitoxantrone is the only approved chemotherapy for MS, whereas the other immunosuppressants that will be reviewed are used off-label, and the level of evidence for their use is low. Today, non-selective immunosuppressants drugs are being used as monotherapy or in combination with injectable classical DMT only in selected cases due to their potential risk of further adverse events and their possible limitation for the future use of other, more selective immunosuppressants approved for MS.
Azathioprine
Azathioprine (AZA) is a purine analogue that competes with DNA nucleotides, approved in some countries for relapsing MS.
Efficacy
A recent meta-analysis concluded that AZA may be effective for RRMS patients in reducing the odds of clinical relapses and disability progression over 24-36 months compared to placebo [32•]. In one study, AZA (50 mg/day) added to interferon beta-1a was not shown to be beneficial in clinical or MRI outcomes [33]. The comparison of AZA (3 mg/kg/day) with IFN-beta showed a higher proportion of relapse-free patients in the AZA group than in the IFN-beta group (77 % vs. 57 %, p < 0.05) [34].
Common side effects and safety issues
Leukopenia, macrocytic anemia, and liver function abnormalities have been seen during treatment, but leukopenia tends to decrease over time. A possible long-term risk of cancer may be related to treatment duration and cumulative dosage.
Cyclophosphamide
Cyclophosphamide (CY) is an alkylating drug related to nitrogen mustards that bind to DNA and interfere with mitosis and cell replication. Several regimens have been described, but the most common is monthly intravenous infusions, initially at a dose of 1 g/m2, with a two-week post-infusion white blood cell count nadir of 2,000-2,500/mm3.
Mechanism of action
CY depletes lymphocytes, both B and T cells, but with preferential depletion of CD4 T cells. It decreases the secretion of interferon gamma and IL-12 by monocytes and increases the secretion of IL-4 and Il-10 from peripheral mononuclear cells, inducing a shift from Th1- to Th2-type cytokine profile.
Efficacy
Several open-label studies have suggested that patients with rapidly worsening treatment-refractory RRMS might benefit from intravenous administration of CY [35–39]. In patients with progressive MS, a single-blind placebo-controlled study did not demonstrate a beneficial effect [40, 41]. In general, it seems that CY may be of benefit for patients who are younger and have shorter disease duration, but still have a history of relapses or recent MRI activity. A few studies of CY in combination with IFN showed a reduction in clinical and MRI activity [42, 43]. A comparison study with mitoxantrone showed no differences in time to relapse, disease progression, or MRI variables [44].
Common side effects and safety issues
Infections, but no cases of PML, have been reported in MS patients treated with CY. Alopecia is seen in 40-60 % of patients, hemorrhagic cystitis in 7-15 %, and amenorrhea in 33 % of young women; permanent infertility is less common and is associated with older age. Bladder cancer has been occasionally reported.
Mitoxantrone
Mitoxantrone (Novantrone®) (MTX) is an anthracenedione drug approved in some countries for rapidly worsening RRMS or secondary progressive MS [45••].
Mechanism of action
MTX inhibits type II topoisomerase activity and disrupts DNA synthesis; it demonstrates effects on the proliferation of T and B cells and promotes maturation of natural killer cells.
Efficacy
MTX has shown a moderate effect in reducing disability progression and ARR in patients affected by worsening RRMS, PRMS, and SPMS during short-term follow-up (two years) [46, 47]. In a phase III trial comparing MTX to placebo in SPMS, MTX at 12 mg/m2 every three months over a two-year period demonstrated a significant benefit. MTX showed superiority on the combined primary endpoint, including change from baseline expanded disability status score (EDSS), change from baseline ambulation index, number of treated relapses, time to first treated relapse, and change from baseline standardized neurological status at 24 months. MTX decreased the relapse rate by 68 % and significantly prolonged the time to progression. The results of this trial led to regulatory approval [48].
Common side effects and safety issues
At standard doses, use of MTX is limited to two years due to the risk of cumulative-dose-related cardiomyopathy. After initial widespread use, it was shown that systolic dysfunction occurred in about 12 % of patients, congestive failure in 0.4 %, and leukemia in approximately 0.8 %. These rates of complications, higher than expected, have limited its current use in clinical practice [49].
Hematopoietic stem cell transplantation in MS (bone marrow transplantation)
Treatment options for MS patients have expanded tremendously in recent years, but there are patients that continue to experience accumulated disability or rapidly worsening or fulminant MS, with frequent relapses, despite spite these new therapies. In recent years, intense immunosuppression followed by autologous hematopoietic stem cell transplantation (AHSCT) has been an option for these patients and for patients affected by other severe autoimmune disorders as well. The target of this treatment is the eradication of autoreactive cells, followed by the infusion of autologous hematopoietic stem cells to restore the aberrant hematolymphopoietic system. In addition to its clear immunosuppressive properties, this method can also result in a resetting of the immune system that may become tolerant to self-antigens for a long period of time. More than 500 MS cases have been treated worldwide with this procedure in the past few years, most of them in small phase I–II trials [50, 51•, 52–72]. Although AHSCT appears to be very effective, especially in selected MS cases, at this time there is no data available from prospective comparative studies. In addition, the heterogeneity of the reported studies makes comparisons difficult in terms of benefit–risk ratio.
Procedure
AHSCT is not a single treatment and involves various steps (Fig. 2) that have not been executed uniformly among different centers. The importance of age, disease phase, and intensity of the conditioning therapy are well-known factors influencing potential toxicity and outcome. For this reason, in 2012, an interdisciplinary group of experts published a proposal for uniform patient inclusion criteria and AHSCT procedures, such as conditioning regimen, to be implemented in clinical trials [73••]. A high-intensity conditioning regimen is myeloablative, and results in greater immunosuppression but riskier side effects. It includes total-body radiation or busulfan-containing drugs. Low-intensity refers to regimens that include CY alone, melphalan alone, or fludarabine-based regimens. Intermediate intensity includes combined drugs, such as BEAM (carmustine, etoposide, cytarabine, and melphalan), and the combined use of anti-thymocyte globulin, alemtuzumab, or RTX with high-dose CY or other chemotherapy. While no single conditioning regimen has demonstrated superiority over the others, BEAM has been the most widely used protocol in Europe [74].
AHSCT step-by-step procedure and proposed inclusion criteria on behalf of the European Group for Blood and Marrow Transplantation (EBMT), the Center for International Blood and Marrow Transplant Research (CIBMTR), and the Haematopoietic Stem Cell Transplantation (HSCT) in MS International Study Group, published in 2012 [73••]. MRI magnetic resonance imaging, Gd+ Gadolinium-positive, GCSF granulocyte-colony stimulating factor.
Efficacy
Despite the absence of comparative studies among different procedures, the heterogeneity of the patients included in the studies, and the differences in follow-up periods (Table 1), all of these studies suggest that AHSCT may lead to prolonged periods of stable disease (sustained progression-free survival beyond five years). The prognosis is more favorable in patients treated in the relapsing-remitting phase and/or showing inflammatory MRI activity, who are younger than 40 years, and who have shorter disease duration (within five years). It is excellent in aggressive malignant forms of MS.
Common side effects and safety profile
Transplant-related mortality rates have decreased from 7.3 % for the period 1995-2000 to 1.3 % during the period 2001-2007 [75]. This reduction can be attributed to better patient selection and type of conditioning, combined with improvements in supportive care and the experience of treating MS patients with AHSCT at accredited transplant centers. The most frequent cause of death has been severe systemic infections, but early non-neurological toxicity (first 100 days after AHSCT) has been seen in 56 % of patients [74]. It has been reported that the reconstituted immune system is predisposed to other autoimmune diseases within the first two years after AHSCT in 10 % of patients; the most frequent are autoimmune thyroiditis and immune cytopenia [76••].
Future view
Evidence-based data suggest the feasibility of AHSCT in severe forms of MS, but whether the procedure is really effective in modifying the progressive course of the disease deserves further assessment in comparative phase III trials. AHSCT appears to be most beneficial in patients transplanted during the relapse phase of the disease, and could be an alternative for those highly active and refractory to other conventional MS therapies.
Management algorithm for relapsing-remitting MS
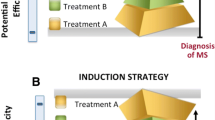
In current clinical practice, there are two general approaches that are considered at the time a therapeutic decision is made. The more common is the escalation strategy. The patient is initially treated with a first-line DMT, and if there is clinical and/or imaging evidence of disease activity or progression (suboptimal response or breakthrough disease), a second-line therapy with greater apparent efficacy is considered (Fig. 3). The choice of first-line DMT—first-generation injectable therapies with an impressive record of safety or new oral agents with a more convenient profile and maybe more efficacious—will depend on the characteristics of the patient (prior level of disease activity) and his/her concerns about unknown long-term adverse effects. Before escalation, patients may also switch to another first-line DMT with a different mechanism of action that may reduce the activity of the disease as shown by observational studies.
The key decision point is often JCV antibody status. If the patient is JCV-seronegative, switching to natalizumab is a clearly effective option, whereas fingolimod is more frequently considered for JCV-seropositive patients. For patients who do not achieve disease control with these therapies, or in the case of adverse events, alemtuzumab is an approved alternative in Europe that has, in fact, demonstrated superior efficacy to subcutaneous interferon beta-1a. Given the associated serious side effects, however, the risk-benefit ratio must be considered. Monoclonal antibodies such as ocrelizumab, ofatumumab, or daclizumab are promising as second-line therapies, but they are still in the experimental phase.
The second approach is induction therapy, proposed for patients with aggressive or rapidly worsening MS, with the idea of stabilizing and inducing sustained remission with powerful, intensive treatment, and then resuming safer DMT therapy. Mitoxantrone, cyclophosphamide, and, more recently, rituximab have been used and have demonstrated their effectiveness in observational studies; alemtuzumab has also been proposed as an option to evaluate in this setting. Finally, intense immunosuppression, followed by AHSCT, should be reserved for patients with very active RRMS who have failed treatment with other approved therapies. In both therapeutic strategies, however, the selection of the more suitable drug must be carefully considered in light of the risk of severe adverse events such as secondary neoplasms or the occurrence of PML. The lack of significant efficacy in progressive MS precludes the use of immunosuppressive drugs in this subtype of patients.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Havrdova E, Galetta S, Hutchinson M, Stefoski D, Bates D, Polman CH, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009;8(3):254–60.
Castillo-Trivino T, Mowry EM, Gajofatto A, Chabas D, Crabtree-Hartman E, Cree BA, et al. Switching multiple sclerosis patients with breakthrough disease to second-line therapy. PLoS One. 2011;6(2):e16664.
Prosperini L, Gianni C, Leonardi L, De Giglio L, Borriello G, Galgani S, et al. Escalation to natalizumab or switching among immunomodulators in relapsing multiple sclerosis. Mult Scler. 2012;18(1):64–71.
O’Connor P, Goodman A, Kappos L, Lublin F, Polman C, Rudick RA, et al. Long-term safety and effectiveness of natalizumab redosing and treatment in the STRATA MS Study. Neurology. 2014;83(1):78–86.
Fernandez O, Garcia-Merino JA, Arroyo R, Alvarez-Cermeno JC, Izquierdo G, Saiz A, et al. Spanish consensus on the use of natalizumab (Tysabri)-2013. Neurologia. 2013 Dec 18.
Warnke C, Ramanujam R, Plavina T, Bergstrom T, Goelz S, Subramanyam M, et al. Changes to anti-JCV antibody levels in a Swedish national MS cohort. J Neurol Neurosurg Psychiatry. 2013;84(11):1199–205.
Gorelik L, Lerner M, Bixler S, Crossman M, Schlain B, Simon K, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol. 2010;68(3):295–303.
Outteryck O, Zephir H, Salleron J, Ongagna JC, Etxeberria A, Collongues N, et al. JC-virus seroconversion in multiple sclerosis patients receiving natalizumab. Mult Scler. 2013 Sep 26.
Salhofer-Polanyi S, Baumgartner A, Kraus J, Maida E, Schmied M, Leutmezer F. What to expect after natalizumab cessation in a real-life setting. Acta Neurol Scand. 2014;130(2):97–102.
Jokubaitis VG, Li V, Kalincik T, Izquierdo G, Hodgkinson S, Alroughani R, et al. Fingolimod after natalizumab and the risk of short-term relapse. Neurology. 2014;82(14):1204–11. This study evaluated the early risk of relapse after switch from other first-line therapies to fingolimod.
Aktas O, Kury P, Kieseier B, Hartung HP. Fingolimod is a potential novel therapy for multiple sclerosis. Nat Rev Neurol. 2010;6(7):373–82.
Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–15.
Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355(11):1124–40.
Vermersch P, Czlonkowska A, Grimaldi LM, Confavreux C, Comi G, Kappos L, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler. 2014;20(6):705–16.
O’Connor PW, Li D, Freedman MS, Bar-Or A, Rice GP, Confavreux C, et al. A Phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology. 2006;66(6):894–900.
Rahmlow M, Shuster EA, Dominik J, Deen Jr HG, Dickson DW, Aksamit Jr AJ, et al. Leflunomide-associated progressive multifocal leukoencephalopathy. Arch Neurol. 2008;65(11):1538–9.
Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098–107.
Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–97.
van Oosten BW, Killestein J, Barkhof F, Polman CH, Wattjes MP. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med. 2013;368(17):1658–9.
Ermis U, Weis J, Schulz JB. PML in a patient treated with fumaric acid. N Engl J Med. 2013;368(17):1657–8.
Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819–28.
Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–39.
Bourdette D. Alemtuzumab and multiple sclerosis: is it safe? Neurology. 2014;83(1):17–8.
Wynn D, Kaufman M, Montalban X, Vollmer T, Simon J, Elkins J, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurol. 2010;9(4):381–90.
Gold R, Giovannoni G, Selmaj K, Havrdova E, Montalban X, Radue EW, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet. 2013;381(9884):2167–75.
Giovannoni G, Gold R, Selmaj K, Havrdova E, Montalban X, Radue EW, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECTION): a multicentre, randomised, double-blind extension trial. Lancet Neurol. 2014;13(5):472–81.
Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–88.
Naismith RT, Piccio L, Lyons JA, Lauber J, Tutlam NT, Parks BJ, et al. Rituximab add-on therapy for breakthrough relapsing multiple sclerosis: a 52-week phase II trial. Neurology. 2010;74(23):1860–7.
Hawker K, O’Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460–71.
Kappos L, Li D, Calabresi PA, O’Connor P, Bar-Or A, Barkhof F, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378(9805):1779–87.
Sorensen PS, Lisby S, Grove R, Derosier F, Shackelford S, Havrdova E, et al. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology. 2014;82(7):573–81.
Filippini G, Del Giovane C, Vacchi L, D’Amico R, Di Pietrantonj C, Beecher D, et al. Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev. 2013;6, CD008933. This meta-analysis summarizes the efficacy of total approved MS disease-modifying therapies: immunomodulators, immunosuppressants and monoclonal antibodies.
Havrdova E, Zivadinov R, Krasensky J, Dwyer MG, Novakova I, Dolezal O, et al. Randomized study of interferon beta-1a, low-dose azathioprine, and low-dose corticosteroids in multiple sclerosis. Mult Scler. 2009;15(8):965–76.
Etemadifar M, Janghorbani M, Shaygannejad V. Comparison of interferon beta products and azathioprine in the treatment of relapsing-remitting multiple sclerosis. J Neurol. 2007;254(12):1723–8.
Harrison DM, Gladstone DE, Hammond E, Cheng J, Jones RJ, Brodsky RA, et al. Treatment of relapsing-remitting multiple sclerosis with high-dose cyclophosphamide induction followed by glatiramer acetate maintenance. Mult Scler. 2012;18(2):202–9.
Gladstone DE, Peyster R, Baron E, Friedman-Urevich S, Sibony P, Melville P, et al. High-dose cyclophosphamide for moderate to severe refractory multiple sclerosis: 2-year follow-up (investigational new drug No. 65863). Am J Ther. 2011;18(1):23–30.
Khan OA, Zvartau-Hind M, Caon C, Din MU, Cochran M, Lisak D, et al. Effect of monthly intravenous cyclophosphamide in rapidly deteriorating multiple sclerosis patients resistant to conventional therapy. Mult Scler. 2001;7(3):185–8.
de Bittencourt PR, Gomes-da-Silva MM. Multiple sclerosis: long-term remission after a high dose of cyclophosphamide. Acta Neurol Scand. 2005;111(3):195–8.
Gobbini MI, Smith ME, Richert ND, Frank JA, McFarland HF. Effect of open label pulse cyclophosphamide therapy on MRI measures of disease activity in five patients with refractory relapsing-remitting multiple sclerosis. J Neuroimmunol. 1999;99(1):142–9.
Zephir H, de Seze J, Duhamel A, Debouverie M, Hautecoeur P, Lebrun C, et al. Treatment of progressive forms of multiple sclerosis by cyclophosphamide: a cohort study of 490 patients. J Neurol Sci. 2004;218(1–2):73–7.
Perini P, Gallo P. Cyclophosphamide is effective in stabilizing rapidly deteriorating secondary progressive multiple sclerosis. J Neurol. 2003;250(7):834–8.
Patti F, Reggio E, Palermo F, Fiorilla T, Politi G, Nicoletti A, et al. Stabilization of rapidly worsening multiple sclerosis for 36 months in patients treated with interferon beta plus cyclophosphamide followed by interferon beta. J Neurol. 2004;251(12):1502–6.
Smith DR, Weinstock-Guttman B, Cohen JA, Wei X, Gutmann C, Bakshi R, et al. A randomized blinded trial of combination therapy with cyclophosphamide in patients-with active multiple sclerosis on interferon beta. Mult Scler. 2005;11(5):573–82.
Zipoli V, Portaccio E, Hakiki B, Siracusa G, Sorbi S, Amato MP. Intravenous mitoxantrone and cyclophosphamide as second-line therapy in multiple sclerosis: an open-label comparative study of efficacy and safety. J Neurol Sci. 2008;266(1–2):25–30.
Castro-Borrero W, Graves D, Frohman TC, Flores AB, Hardeman P, Logan D, et al. Current and emerging therapies in multiple sclerosis: a systematic review. Ther Adv Neurol Disord. 2012;5(4):205–20. This review provide general information regarding mechanism of action, indications, side effects and safety of approved therapies for MS, emerging therapies, and drugs that can be considered for off-label use in MS.
Edan G, Miller D, Clanet M, Confavreux C, Lyon-Caen O, Lubetzki C, et al. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry. 1997;62(2):112–8.
Millefiorini E, Gasperini C, Pozzilli C, D’Andrea F, Bastianello S, Trojano M, et al. Randomized placebo-controlled trial of mitoxantrone in relapsing-remitting multiple sclerosis: 24-month clinical and MRI outcome. J Neurol. 1997;244(3):153–9.
Hartung HP, Gonsette R, Konig N, Kwiecinski H, Guseo A, Morrissey SP, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018–25.
Edan G, Morrissey S, Le Page E. Rationale for the use of mitoxantrone in multiple sclerosis. J Neurol Sci. 2004;223(1):35–9.
Fassas A, Anagnostopoulos A, Kazis A, Kapinas K, Sakellari I, Kimiskidis V, et al. Autologous stem cell transplantation in progressive multiple sclerosis —an interim analysis of efficacy. J Clin Immunol. 2000;20(1):24–30.
Fassas A, Kimiskidis VK, Sakellari I, Kapinas K, Anagnostopoulos A, Tsimourtou V, et al. Long-term results of stem cell transplantation for MS: a single-center experience. Neurology. 2011;76(12):1066–70. This article showed the results of 11 years follow-up after bone marrow transplantation in one single center.
Fassas A, Passweg JR, Anagnostopoulos A, Kazis A, Kozak T, Havrdova E, et al. Hematopoietic stem cell transplantation for multiple sclerosis. A retrospective multicenter study. J Neurol. 2002;249(8):1088–97.
Mancardi GL, Saccardi R, Filippi M, Gualandi F, Murialdo A, Inglese M, et al. Autologous hematopoietic stem cell transplantation suppresses Gd-enhanced MRI activity in MS. Neurology. 2001;57(1):62–8.
Kozak T, Havrdova E, Pit’ha J, Gregora E, Pytlik R, Maaloufova J, et al. Immunoablative therapy with autologous stem cell transplantation in the treatment of poor risk multiple sclerosis. Transplant Proc. 2001;33(3):2179–81.
Kozak T, Havrdova E, Pit’ha J, Gregora E, Pytlik R, Maaloufova J, et al. High-dose immunosuppressive therapy with PBPC support in the treatment of poor risk multiple sclerosis. Bone Marrow Transplant. 2000;25(5):525–31.
Nash RA, Bowen JD, McSweeney PA, Pavletic SZ, Maravilla KR, Park MS, et al. High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood. 2003;102(7):2364–72.
Burt RK, Cohen BA, Russell E, Spero K, Joshi A, Oyama Y, et al. Hematopoietic stem cell transplantation for progressive multiple sclerosis: failure of a total body irradiation-based conditioning regimen to prevent disease progression in patients with high disability scores. Blood. 2003;102(7):2373–8.
Saiz A, Blanco Y, Carreras E, Berenguer J, Rovira M, Pujol T, et al. Clinical and MRI outcome after autologous hematopoietic stem cell transplantation in MS. Neurology. 2004;62(2):282–4.
Saccardi R, Mancardi GL, Solari A, Bosi A, Bruzzi P, Di Bartolomeo P, et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood. 2005;105(6):2601–7.
Capello E, Saccardi R, Murialdo A, Gualandi F, Pagliai F, Bacigalupo A, et al. Intense immunosuppression followed by autologous stem cell transplantation in severe multiple sclerosis. Neurol Sci. 2005;26 Suppl 4:S200–3.
Ni XS, Ouyang J, Zhu WH, Wang C, Chen B. Autologous hematopoietic stem cell transplantation for progressive multiple sclerosis: report of efficacy and safety at three yr of follow up in 21 patients. Clin Transplant. 2006;20(4):485–9.
Xu J, Ji BX, Su L, Dong HQ, Sun XJ, Liu CY. Clinical outcomes after autologous haematopoietic stem cell transplantation in patients with progressive multiple sclerosis. Chin Med J (Engl). 2006;119(22):1851–5.
Samijn JP, te Boekhorst PA, Mondria T, van Doorn PA, Flach HZ, van der Meche FG, et al. Intense T cell depletion followed by autologous bone marrow transplantation for severe multiple sclerosis. J Neurol Neurosurg Psychiatry. 2006;77(1):46–50.
Atkins H, Freedman M. Immune ablation followed by autologous hematopoietic stem cell transplantation for the treatment of poor prognosis multiple sclerosis. Methods Mol Biol. 2009;549:231–46.
Shevchenko YL, Novik AA, Kuznetsov AN, Afanasiev BV, Lisukov IA, Kozlov VA, et al. High-dose immunosuppressive therapy with autologous hematopoietic stem cell transplantation as a treatment option in multiple sclerosis. Exp Hematol. 2008;36(8):922–8.
Burt RK, Loh Y, Cohen B, Stefoski D, Balabanov R, Katsamakis G, et al. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol. 2009;8(3):244–53.
Krasulova E, Trneny M, Kozak T, Vackova B, Pohlreich D, Kemlink D, et al. High-dose immunoablation with autologous haematopoietic stem cell transplantation in aggressive multiple sclerosis: a single centre 10-year experience. Mult Scler. 2010;16(6):685–93.
Chen B, Zhou M, Ouyang J, Zhou R, Xu J, Zhang Q, et al. Long-term efficacy of autologous haematopoietic stem cell transplantation in multiple sclerosis at a single institution in China. Neurol Sci. 2012;33(4):881–6.
Hamerschlak N, Rodrigues M, Moraes DA, Oliveira MC, Stracieri AB, Pieroni F, et al. Brazilian experience with two conditioning regimens in patients with multiple sclerosis: BEAM/horse ATG and CY/rabbit ATG. Bone Marrow Transplant. 2010;45(2):239–48.
Mancardi GL, Sormani MP, Di Gioia M, Vuolo L, Gualandi F, Amato MP, et al. Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: the Italian multi-centre experience. Mult Scler. 2012;18(6):835–42.
Bowen JD, Kraft GH, Wundes A, Guan Q, Maravilla KR, Gooley TA, et al. Autologous hematopoietic cell transplantation following high-dose immunosuppressive therapy for advanced multiple sclerosis: long-term results. Bone Marrow Transplant. 2012;47(7):946–51.
Burman J, Iacobaeus E, Svenningsson A, Lycke J, Gunnarsson M, Nilsson P, et al. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. J Neurol Neurosurg Psychiatry. 2014 Feb 19.
Saccardi R, Freedman MS, Sormani MP, Atkins H, Farge D, Griffith LM, et al. A prospective, randomized, controlled trial of autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: a position paper. Mult Scler. 2012;18(6):825–34. This article suggested that all randomized control trials of haematopoietic stem cell transplantation should have similar objectives, patient selection and transplant technology and outcome assessment in order to compare results.
Saccardi R, Kozak T, Bocelli-Tyndall C, Fassas A, Kazis A, Havrdova E, et al. Autologous stem cell transplantation for progressive multiple sclerosis: update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Mult Scler. 2006;12(6):814–23.
Reston JT, Uhl S, Treadwell JR, Nash RA, Schoelles K. Autologous hematopoietic cell transplantation for multiple sclerosis: a systematic review. Mult Scler. 2011;17(2):204–13.
Atkins HL, Freedman MS. Hematopoietic stem cell therapy for multiple sclerosis: top 10 lessons learned. Neurotherapeutics. 2013;10(1):68–76. This review highlight the most relevant issues to consider of hematopoietic stem cell therapy.
Compliance with Ethics Guidelines
Conflict of Interest
N. Sola-Valls, M. Sepúlveda, Y. Blanco, and A. Saiz each declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Multiple Sclerosis and Related Disorders
Rights and permissions
About this article
Cite this article
Sola-Valls, N., Sepúlveda, M., Blanco, Y. et al. Current Role of Chemotherapy and Bone Marrow Transplantation in Multiple Sclerosis. Curr Treat Options Neurol 17, 324 (2015). https://doi.org/10.1007/s11940-014-0324-3
Published:
DOI: https://doi.org/10.1007/s11940-014-0324-3