Abstract
Autologous haematopoietic stem cell transplantation (AHSCT) is a promising treatment for multiple sclerosis (MS) patients who have not adequately responded to conventional therapies. We retrospectively evaluated the safety and long-term clinical outcome of AHSCT in MS patients in China. Twenty-five patients with various types of MS were treated with AHSCT. Peripheral blood stem cells were derived by leukapheresis after mobilized with granulocyte colony-stimulating factor. Then CD34+ cell selection of the graft was performed and anti-thymocyte globulin was given for T-cell depletion, with the conditioning regimen BEAM adopted and early and late toxicities recorded. Long-term responses were evaluated by the expanded disability status scale (EDSS), progression-free survival and gadolinium-enhanced magnetic resonance imaging scans. 10, 7 and 8 patients experienced neurological improvement, stabilization and progression, respectively. The median EDSS scores observed over 1-year follow-up after transplantation (5.5–7.0) were consistently lower than the baseline (8.0). The progression-free survival rate was 74, 65 and 48% at 3, 6 and 9 years post-transplant. 58% cases (7/12) had active lesions at baseline and all turned to inactive status in the years of follow-up. 25% cases (3/12) experienced progression after transplantation but had no active lesions in MRI over the whole follow-up period. 17% cases (2/12) without active lesions at baseline progressed active lesions in MRI. The major early toxicity resulted in fever and late toxicity caused transplantation-related mortality due to severe pneumonia and varicella-zoster virus hepatitis, respectively. AHSCT is a feasible treatment for severe MS and its long-term efficacy is favorable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a T cell-mediated disorder in which aberrant immune responses lead to focal myelin destruction and secondary oligodendrocyte and axonal damage. The crude MS prevalence rate was 1.39 per 100,000 inhabitants (95% CI: 1.16–1.66 cases) in Shanghai and the female-to-male ratio was 1.8 [1]. Three treatment options including anti-inflammatory substances (corticosteroid hormone), immunomodulators (interferon-beta and immunoglobulin) and immunosuppressors (cyclophosphamide, azathioprine and mitoxantrone) are widely used in China. As MS is considered a heterogeneous disease with variable responses to these therapies, there are some cases continuing to develop despite the above treatments.
Autologous haematopoietic stem cell transplantation (AHSCT) has been reported as a promising treatment for MS patients who did not respond to the conventional therapies since 1997 [2]. Although the rationale for AHSCT treatment is still not clear, it is generally accepted that high-dose chemotherapy before AHSCT ablates the aberrant immune system and AHSCT regenerates a new and antigen-naive immune system [3, 4]. Moreover, laboratory studies observed that rodent and human bone-marrow cells can enter the brain to express gene products or proteins typical of neurons [5, 6].
Autologous haematopoietic stem cell transplantation has so far been performed in about 400 worldwide patients with different types of MS [7]. However, there are few data regarding its long-term clinical outcomes. We began to apply AHSCT to MS patients in 2000 and reported the efficacy and safety at three-year follow-up [8]. This report collected the update and new data on a larger group of 25 MS patients in our hospital and presented not only the safety but also the long-term treatment outcomes with a mean follow-up of 59.6 months.
Methods
Patients
From 2000 to 2007, 25 patients who were definitely diagnosed MS by the Poser criteria were enrolled in the study. Patient characteristics are shown in Table 1. 19 patients were defined as secondary-progressive MS (SP), one patient as primary progressive MS (PP), two patients as progressive relapsing MS (PR) and three patients as relapsing-remitting MS (RR). There were 19 females and 6 males with median age of 37.3 years (range 15–64). The median interval between diagnosis and transplantation was 48.0 months (range 7–147). Median score of expanded disability status scale (EDSS) before transplantation was 8.0 (range 3–9.5) as the baseline. 14 patients had active lesions appearing on MRI scans before transplantation. Mean follow-up was 59.6 months (range 4.5–111). All patients had previously failed in conventional therapies. The informed consent had been obtained from all patients before admission.
Transplantation
Peripheral blood stem cells (PBSCs) were mobilized with cyclophosphamide (CY) at 3,000–4,000 mg/m2, followed by daily subcutaneous injections of granulocyte colony-stimulating factor (G-CSF, Granocyte, Chugai Pharmaceutical Company, Japan) at 5–10 ug/kg body weight, starting from the fifth day after CY infusion. When leucocyte count was greater than 5.0 × 109/L and absolute CD34+ cell count were in excess of 0.01 × 109/L, a volume of 10,000–14,000 mL was leukapheresed by a continuous flow blood cell separator (Fenwal CS3000 plus; Baxter, Deerfield, IL, USA) so that the CD34+ cell count in leukapheresis reached over 2.0 × 106/kg body weight. Depletion of lymphocytes was performed by CD34+ enrichment using the Clini-Macs (AmCell GmbH, Bergisch Gladbach, Germany).
The CY/total body irradiation (TBI) as a conditioning regimen was used only in the first case as we described before [8]. The BEAM (BCNU, etoposide, arabinosylcytosine, melphalan) conditioning regimen was chosen in other 24 cases. In the BEAM regimen, BCNU 300 mg/m2 was given intravenously on day −6, etoposide 200 mg/m2 and arabinosylcytosine 200 mg/m2 was given intravenously on days −5 to −2, and melphalan 140 mg/m2 was taken orally on day −1. On day 0, the purged CD34+ cells were thawed and infused. The mean interval between mobilization and stem cell reinfusion was 36.0 days (range 16–92). On days +1 and +2, rabbit anti-thymocyte globulin (ATG) (Fresenius HemoCare Immune Therapy GmbH, Germany) at 10 mg/kg body weight/day was given with soluble methylprednisolone 1,000 mg/day. G-CSF was subcutaneously given at a dose of 5 ug/kg body weight/day starting from the day of stem cell reinfusion and continued until the absolute neutrophil was greater than 1.0 × 109/L for three consecutive days. Oral levofloxacin, fluconazole and acyclovir were given for prevention of infections.
Clinical evaluation
The clinical evaluation includes an EDSS evaluation and an MRI examination with gadolinium Gd64 (Gd–diethylenetriamine penta-acetic acid) enhancement. Evaluation of disease status was performed by skilled neurologists prior to haematopoietic cell mobilization (baseline) and subsequently at 6, 12 months, and then every year after AHSCT. MRI evaluations were obtained at baseline and every year after AHSCT. The EDSS was used to assess neurologic status. As MS patients tended to experience score fluctuations, any change in score (increase or decrease) had to be maintained for over 6 months in order to be confirmed. Confirmed disability progression was defined as one EDSS point or greater increase if EDSS score at entry was less than or equal to 5 or with an increase of 0.5 EDSS point or greater if EDSS score at entry was above 5. Confirmed PFS, the primary end-point of this study, was defined as the probability to be alive without confirmed disability progression, irrespective of the occurrence of a relapse, provided that the relapse did not cause a permanent increase in the EDSS score by 1 or 0.5 point compared with the baseline score, as mentioned above.
Adverse events
Adverse events were recorded during stem cell mobilization, conditioning, stem cell reinfusion and post-transplant period.
Statistical analysis
Kaplan–Meier estimator was used to assess the confirmed PFS, and GraphPad Prism 5.0 was used in the statistical analysis.
Results
PBSCs collection and engraftment
The mean number of 8.0 × 106/kg (range 2.0–36.0) CD34+ cells was collected by leukapheresis. The graft contained a mean number of 4.1 × 106 CD34+ cells/kg (range 0.88–18.76) after positive selection and cryopreservation. The mean intervals of the absolute neutrophil more than 0.5 × 109/L and platelet more than 50 × 109/L were 11.4 days (range 9–19) and 15.9 days (range 11–20), respectively, after transplantation. 24 patients needed transfusion support, 10 patients received an average of 2 units of red blood cells (range 2–4) and 24 patients received an average of 2.8 units of single-donor apheresis platelets (range 1–13).
Adverse events
12 patients had neutropenic fever without identification of pathogens or other clinical signs of infection. The remaining 13 cases developed confirmed bacterial infection. Transient elevation of liver enzymes occurred in six patients and transient elevation of creatinine occurred in one patient.
During the follow-up period, one patient died of pneumonia 4.5 months after transplantation. One patient suffered from varicella-zoster 10 months later and died of varicella-zoster virus hepatitis 15 months after transplantation. All adverse events were summarized in Table 2.
Clinical neurologic outcomes
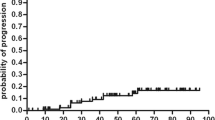
The median EDSS scores at baseline and follow-up years are shown in Fig 1. The median baseline EDSS score was 8.0 (range 3.0–9.5), while the median EDSS scores at every follow-up year after transplantation were consistently lower than that at baseline. The median EDSS score was the lowest (5.5 point) at the first year and was the highest (7.0 point) at 6, 7 and 8 follow-up years. 10 patients (40%) experienced neurological improvement, and their median EDSS scores decreased from 8.1 point (6.5–9.5) to 5.0 point (1.0–8.5) at the last follow-up year. 7 patients (28%) had disease stabilization. 8 patients (32%) experienced progression with the average of 40 month (range 12–72) after AHSCT, of whom 4 cases progressed with their EDSS scores surpassing baselines after an initial improvement. The post-transplant PFSR was 74, 65 and 48% at 3, 6 and 9 years, respectively, according to Kaplan and Meier survival curve (Fig 2).
Baseline MRI scans were available for all patients. 14 patients (56.0%) had Gd-enhancing active lesions at baseline. Post-transplant MRI scans were available for only 12 patients. Particularly, 58% cases (7/12) had active lesions at baseline and all turned to inactive status in the follow-up years. It was found that 25% cases (3/12) experienced progression after transplantation but had no active lesions in MRI in the whole follow-up period. 17% cases (2/12) without active lesions at baseline progressed active lesions in MRI.
Discussion
Autologous haematopoietic stem cell transplantation has been considered as a possible new treatment option for severe or refractory autoimmune diseases, including MS [9]. According to the published reports, 36–85% cases achieved stabilization or improvement at a median of 3 years post-transplant [10]. As MS is a chronic and highly heterogeneous disorder, it is worthwhile to observe more patients and take longer follow-up period to determine the efficacy of AHSCT on MS. This study was mainly aimed to demonstrate the long-term clinical outcomes of AHSCT on severe MS with the average follow-up of 59.6 months. The PFS decreased from 74% at 3 years to 65% at 6 years and became 48% at 9 years post-transplant. These figures are similar to those in other such trials [11, 12]. The median EDSS score of 25 patients during the whole follow-up period was consistently lower (range 5.5–7.0) than that at baseline (8.0) though it showed an increasing tendency from the first year post-transplant. The improvement and stabilization proportion of the neurological condition was 40 and 28%, respectively. According to the above data, AHSCT is still a feasible therapeutic approach for severe MS patients in the long run.
The data indicated a higher rate of adverse effect and TRM compared with the outcomes of European Group for Blood and Marrow Transplantation (EBMT) [13]. Neutropenic fever was the most common in early toxicity, 12 patients (48%) had fever of unknown origin and the rest 13 (52%) developed confirmed bacterial infection, whereas early adverse syndrome occurred only in 56% of the patients in EBMT study [13]. Late toxicity with the rate of 12% is also related to infection. Moreover, transplant-related mortality (TRM) was 8% which is higher than that obtained in EBMT analysis of 5.3% [13]. Two patients died of severe infection. The high toxicity and TRM can probably be explained by intensive immuno-suppression with double T-cell depletion. In the study, conditioning regime, BEAM with ATG and purged CD34+ stem cells which is known as in vivo and ex vivo T-cell depletion, respectively, was adopted. In theory, purging the graft of lymphocytes could eliminate all anti-myelin reactive lymphocytes among the autologous graft and decrease the relapse rate after transplantation. The outcome of early animal studies also suggested that autoreactive T cells survived the conditioning regimen and autoreactive T lymphocytes reinfused within the graft increased the relapse risk. However, ex vivo T-cell depletion seemed not to be associated with a more favorable clinical response. PFS of the study at 3- and 6-year follow-up was similar to that without ex vivo T-cell depletion [14]. EBMT analysis also indicated that double purging was associated with TRM even though they were not significant [13]. Non-myeloablative AHSCT had been applied in 21 elapsing-remitting MS patients and showed no TRM [15]. Controlled studies are needed to evaluate these conditioning regimes. We should emphasize that although BEAM is an intermediary intensity conditioning regime, toxic effects and TRM should be taken into account when both ex vivo and in vivo T-cell depletions are added on it.
Selection of patients is a key and controversial issue on AHSCT for MS though criteria were established for patient-selection in Milan Conference in 1998 [14]. In this study, patient no. 5, a 15-year-old boy with 9.5 EDSS score before transplantation recovered dramatically after AHSCT was adopted. His EDSS score decreased to 1 point at 6-month follow-up and sustained for the last follow-up (111 months). For patient no. 24, her EDSS score dropped from 8 point at baseline to 6 point at 6 months, increased slightly to 1.5 point at 2 years from 1 point at 12 months and increased to 2.0 at 3 years post-transplant (last follow-up). Although we cannot establish some criteria based on the outcome of only two patients, at least, it led us to explore which kind of patients can get the maximum benefits from AHSCT. Here we propose that young SP MS patient (age 15 and 33) with high EDSS score (9.5 and 8.0) and Gd-enhancing lesions on MRI before AHSCT might benefit more from AHSCT. Fagius et al. [16] selected 9 patients with “malignant” MS and achieved 3.5 points (1.0–7.0) improvement on median EDSS at 29 months post-transplant. A malignant form of MS is a term applied to severe cases of MS patients who have a rapidly evolving clinical course with progression to severe disability or even death in a short period of time, usually less than 5 years [17]. The two cases exactly conform to this definition in our study. Some other reports also found the favorable outcomes of malignant MS after AHSCT [16, 18–20]. In term of pre-transplant EDSS, both criteria established in Milan conference [14] and the ongoing multicentre, prospective randomized phase II study supported by the EBMT [15] required that EDSS scores before AHSCT should be lower than 6.5. However, Burt et al. [21] proposed that patients with higher EDSS scores could be considered if they have malignant MS manifest by rapid clinical deterioration and striking Gd enhancement on MRI. Some patients in this study also arrived at EDSS of 8 or 9 within a short period from onset like patient no. 5 and had beneficial outcome after AHSCT. Hence we suggested that EDSS was not an absolute exclusion criteria for patient admission. In terms of the type of MS, most studies were done in SP patients [22]. But recently Burt et al. [15] thought that demyelination is mediated by immune cells during the RR phase of MS rather than SP phase and applied AHSCT to RR MS patients. All 21 patients in this study were free from progression and 16 were free of relapse after the mean of 37-month follow-up. In this study, most cases (20/25) were SP MS and only 3 cases were RR MS who showed improvement, stabilization and progression, respectively, during the follow-up period. The 10-year observation conducted by single centre [23] showed that there was no significant difference in PFS outcome between patients with EDSS <6 and EDSS >6 and patients with relapsing multiple sclerosis course, disease duration <5 years and age <35 years had a more favorable outcome. Which type of patients could get better results will be evaluated in a randomized trial.
As to MRI data, no active lesions were registered in patients without disease progression. A few reports also illustrated that AHSCT can suppress Gd-enhancing MRI activity obviously and enduringly [24–26]. However, three patients experienced progression after transplantation had no active lesion in MRI. More data on MRI such as brain atrophy are needed to explain it. Whole-brain atrophy has a stronger, yet moderate, imaging association with physical disability, and it is a stronger predictor of future disability than T1-hypointense and T2-hyperintense lesion load [27].
In conclusion, based on the long-term follow-up study on 25 MS patients treated with AHSCT, it was proved that AHSCT is a feasible treatment with favorable long-term efficacy for severe MS. More random and controlled clinical trials would be required to fully assess the long-term efficacy of AHSCT for MS.
References
Cheng Q, Miao L, Zhang J et al (2007) A population-based survey of multiple sclerosis in Shanghai. China Neurol 68:1495–1500
Fassas A, Anagnostopoulos A, Kazis A et al (1997) Peripheral blood stem cell transplantation in the treatment of progressive multiple sclerosis: first results of a pilot study. Bone Marrow Transplant 20:631–638
Muraro PA, Abrahamsson SV (2010) Resetting autoimmunity in the nervous system: the role of hematopoietic stem cell transplantation. Curr Opin Investig Drugs 11:1265–1275
Van Wijmeersch B, Sprangers B, Dubois B et al (2008) Autologous and allogeneic hematopoietic stem cell transplantation for multiple sclerosis: perspective on mechanisms of action. J Neuroimmunol 197:89–98
Mezey E, Key S, Vogelsang G et al (2003) Transplanted bone marrow generates new neurons in human brains. Proc Natl Acad Sci 100:1364–1369
Brazelton TR, Rossi FM, Keshet GI et al (2000) From marrow to brain: expression of neuronal phenotypes in adult mice. Science 290:1775–1779
Mancardi G, Saccardi R (2008) Autologous haematopoietic stem-cell transplantation in multiple sclerosis. Lancet Neurol 7:626–636
Ni XS, Ouyang J, Zhu WH et al (2006) Autologous hematopoietic stem cell transplantation for progressive multiple sclerosis: report of efficacy and safety at three yr of follow up in 21 patients. Clin Transplant 20:485–489
van Bekkum DW (2004) Autologous stem cell transplantation in animal models of autoimmune diseases. In: Burt RK, Marmont AM (eds) Stem cell therapy for immune diseases. Landes Biosciences, Georgetown, pp 237–244
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372:1502–1517
Saiz A, Saccardi R, Mancardi GL et al (2008) Autologous HSCT for severe progressive multiple sclerosis in the Italian prospective, multicentre GITMO-Neuro trial: long term follow-up. Bone Marrow Transplant 41:S17
Saiz A, Blanco Y, Berenguer J et al (2008) Clinical outcome 6 years after autologous hematopoietic stem cell transplantation in multiple sclerosis. Neurologia 23:405–407
Saccardi R, Kozak T, Bocelli-Tyndall C et al (2006) Autologous stem cell transplantation for progressive multiple sclerosis: update of the European Group for blood and marrow transplantation autoimmune diseases working party database. Mult Scler 12:814–823
Comi G, Kappos L, Clanet M et al (2000) Guideline for autologous blood and marrow stem cell transplantation in multiple sclerosis: a consensus report written on behalf of the European Group for blood and marrow transplantation and the European Charcot Foundation. J Neurol 247:376–382
Burt RK, Loh Y, Cohen B et al (2009) Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol 8:244–253
Fagius J, Lundgren J, Oberg G (2009) Early highly aggressive MS successfully treated by hematopoietic stem cell transplantation. Mult Scler 15:229–237
Matthews WB (1991) Clinical aspects. Course and prognosis. In: Matthews WB (ed) McAlpine’s multiple sclerosis, second edition edn. Churchill Livingstone, Edinburgh, pp 139–163
Mancardi GL, Murialdo A, Rossi P et al (2005) Autologous stem cell transplantation as rescue therapy in malignant forms of multiple sclerosis. Mult Scler 11:367–371
Havrdova E (2005) Aggressive multiple sclerosis—is there a role for stem cell transplantation? J Neurol 252:III34–III37
Kimiskidis V, Sakellari I, Tsimourtou V et al (2008) Autologous stem-cell transplantation in malignant multiple sclerosis: a case with a favorable long-term outcome. Mult Scler 14:278–283
Burt RK, Cohen B, Rose J et al (2005) Hematopoietic stem cell transplantation for multiple sclerosis. Arch Neurol 62:860–864
ASTIMS “Autologous Stemcell Transplantation International Multiple Sclerosis trial”. http://www.astims.org
Krasulová E, Trneny M, Kozák T et al (2010) High-dose immunoablation with autologous haematopoietic stem cell transplantation in aggressive multiple sclerosis: a single centre 10-year experience. Mult Scler 16:685–693
Mancardi GL, Saccardi R, Filippi M et al (2001) Autologous hematopoietic stem cell transplantation suppresses Gd-enhanced MRI activity in MS. Neurology 57:62–68
Saiz A, Blanco Y, Carreras E et al (2004) Clinical and MRI outcome after autologous hemtopoietic stem cell transplantation in MS. Neurology 62:282–284
Fassas A, Kimiskidis VK, Sakellari I et al (2011) Long-term results of stem cell transplantation for MS: a single-center experience. Neurology 76:1066–1070
Zivadinov R, Bakshi R (2004) Central nervous system atrophy and clinical status in multiple sclerosis. J Neuroimaging 14:27–35
Acknowledgment
This work was supported by the Project of Jiangsu Province’s Laboratory of Clinical Immunology (NO.2003-19).
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Zhou and B. Chen contribute equally to this article and should be considered as co-first authors.
Rights and permissions
About this article
Cite this article
Chen, B., Zhou, M., Ouyang, J. et al. Long-term efficacy of autologous haematopoietic stem cell transplantation in multiple sclerosis at a single institution in China. Neurol Sci 33, 881–886 (2012). https://doi.org/10.1007/s10072-011-0859-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-011-0859-y