Abstract
Purpose of Review
Rheumatic heart disease (RHD) is the predominant cause of mitral stenosis (MS) worldwide. This review provides an overview of MS diagnosis, assessing disease severity, and the hemodynamic impact of valve obstruction. Additionally, it examines different echocardiographic parameters and scoring systems employed to evaluate mitral valve morphology and determining suitability for percutaneous mitral commissurotomy (PMC).
Recent Findings
Echocardiography remains the cornerstone for diagnosing and assessing MS severity, while also evaluating valve morphology for potential interventions. Three-dimensional echocardiography planimetry is increasingly used in clinical practice as an as accurate method to measure the true mitral orifice area. Net atrioventricular compliance assessment can be useful for risk stratification, particularly in the presence of a discrepancy between anatomic severity and functional status. Speckle tracking echocardiography emerges as an innovative tool for early detection of left atrial dysfunction, predicting the onset of atrial fibrillation, and adverse outcomes in MS patients. Cardiac magnetic resonance imaging, with its multiparametric analysis, stands as one of the beneficial imaging methods in selected cases.
Summary
Individualized approaches based on symptoms, MS severity, mitral valve morphology are essential for precise management strategies that can improve patient outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Opinion Statement
Rheumatic MS remains a serious health concern, particularly affecting children and young adults in low-to-middle income countries. It is important to suspect the diagnosis in symptomatic patients, particularly those with dyspnea and cardiac murmurs in endemic areas. Transthoracic echocardiography stands as the primary imaging method for establishing the diagnosis and determining disease management. While a minority of patients necessitate further imaging tests like stress echocardiography, cardiac computed tomography, or cardiac magnetic resonance, echocardiography itself is cost-effective and non-invasive. However, its accessibility remains restricted for the most affected population, potentially leading to delays in diagnosis, which may impact survival rates and quality of life.
The treatment relies mainly on percutaneous mitral valve intervention, leading to a significant increase of the valve orifice and improvement in clinical outcomes. Hemodynamic benefits and the risk of procedural-related complications are primarily predicted by the anatomical features of the mitral valve. Patients with good post-procedural results show promising long-term outcomes, including excellent survival rates, no functional impairment, and reduced need for further surgeries or interventions. Nevertheless, persistent processes can lead to disease progression, resulting in valvular restenosis at varying intervals after the intervention, often necessitating valve replacement at that stage.
Accurate risk stratification tools incorporating clinical, imaging, and hemodynamic parameters assist in determining the optimal timing and approach for interventions in patients with MS, considering that percutaneous mitral commissurotomy has evolved as an effective alternative to surgery. Procedural success relies not only on mitral valve morphology but also on various other factors, including clinical characteristics, anatomic features of rheumatic MS, interventional management strategies, and operator expertise.
Introduction
Rheumatic heart disease (RHD) is the most important sequel of acute rheumatic fever (ARF), a disease caused by an abnormal immune response to Streptococcus pyogenes infection [1, 2]. RHD has significantly declined in developed countries. However, it remains a considerable challenge in low-to-middle income nations, where it stands as a primary cause of cardiovascular mortality among young individuals [3, 4, 5••].
The Global Burden of Disease Study in 2019 estimated a worldwide prevalence of RHD at 40.5 million people (95% UI: 32.1 to 50.1 million). This number has been on the rise since 1990 due to the disease's chronic nature, increased global awareness, wider availability of echocardiography for diagnosis, and improved survival rates. Nonetheless, in 2019, it accounted for 306,000 deaths and 10.7 million disability-adjusted life years (DALYs), primarily affecting regions such as Oceania, South Asia, the Caribbean, and sub-Saharan Africa. The most vulnerable and poorest populations bear the greatest impact [6••].
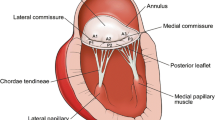
RHD predominantly affects the mitral valve, being the primary cause of mitral stenosis (MS) [7, 8••]. Rheumatic fever triggers distinct changes in the mitral valve, presenting diagnostic features including thickening along the leaflet edges, commissural fusion, and shortening and fusion of the chordae tendineae. These anatomic alterations generate a characteristic functional appearance of rheumatic MS [8••]. In its initial stages, the leaflets, relatively flexible, curve open during diastole due to restricted motion at their tips, predominantly noticeable in the anterior leaflet (Fig. 1). As the leaflets progressively undergo fibrotic and calcific changes, this diastolic doming diminishes. Commissure fusion results in a small oval-shaped central orifice (Fig. 2). In advanced stages, the thickened leaflets may adhere and stiffen, limiting their ability to fully open or close, occasionally causing both mitral stenosis and regurgitation.
The narrowing of the valve orifice hinders blood flow from the left atrium (LA) to the left ventricle (LV), elevating LA pressure and the gradient across the mitral valve, which triggers LA dilation and impairs its function. This condition initiates an upstream surge in pulmonary venous pressure, prompting constriction in pulmonary arterioles and subsequently causing pulmonary hypertension. Consequently, increased right ventricular (RV) afterload occurs, resulting in RV hypertrophy, dilation, and eventual failure. Moreover, LV dysfunction can also be detected in MS related to various factors including rheumatic myocardial fibrosis, scarring of the subvalvular apparatus, decreased LV compliance, abnormal right-left septal interactions, increased afterload, and reduced LV filling [7, 8••, 9•].
Accurate diagnosis and evaluation play a pivotal role in identifying the optimal timing and procedure for valve intervention, thereby enhancing survival rates and quality of life [10, 11••]. Echocardiography stands as the most accurate method for diagnosing and assessing MS severity 8••, 11••]. However, in specific cases, emerging imaging tools such as cardiac computed tomography (CCT) and cardiac magnetic resonance (CMR) can be helpful.
The purpose of the present study is to provide an overview of the current diagnosis and evaluation methods for rheumatic MS.
Diagnosis
Clinical Presentation
Patients with MS typically manifest dyspnea, initially triggered by factors elevating pulmonary venocapillary pressure, such as physical exertion, pregnancy, or atrial fibrillation, gradually worsening over time [8••, 13, 15]. Additional symptoms include orthopnea, cough, hemoptysis, and chest pain. Dysphagia and dysphonia can also occurs due to pressure effects of a dilated left atrium on adjacent structures. Patients may also be diagnosed after a complication like heart failure, atrial fibrillation, embolic events, or infective endocarditis [10, 11••, 12, 13].
Signs in physical examination indicating moderate-to-severe MS typically include a prolonged diastolic murmur (with presystolic accentuation in normal sinus rhythm), a shortened interval between the second heart sound (S2) and the mitral opening snap (OS), along with indications of pulmonary arterial hypertension or RV overload [8••].
Imaging methods
Echocardiography stands as the most accurate method for diagnosing and evaluating MS. Transthoracic echocardiography (TTE) is recommended for all patients suspected of having MS to establish a diagnosis, determine its etiology, quantity its severity, and assess its hemodynamic consequences [Class 1, Level B] [11••, 12,13,14]. The 2023 WHF guideline for the echocardiographic diagnosis of RHD presents updated recommendations concerning population-based echocardiography and risk assessment strategies [11••].
The rheumatic process often involves the mitral valve distinctly, displaying characteristic features, including commissural fusion, leaflet thickening, restricted leaflet motion, chordal thickening and shortening [16•].
Transesophageal echocardiography (TEE) is performed to evaluate the presence of thrombi before percutaneous procedure and to determine mitral valve morphology and the severity of mitral regurgitation when there are uncertainties in the parameters obtained through transthoracic echocardiography. [Class 1, Level C] [12, 17]. Overall, echocardiography is crucial in managing MS progression, aiming to define the optimal timing for surgical intervention, guides catheter-based or surgical treatments during procedures, and diagnoses complications after intervention.
Although echocardiography is the cornerstone image method for diagnosis and evaluation of MS, cardiac computed tomography (CCT) and cardiac magnetic resonance (CMR) imaging gain importance when echocardiography is suboptimal or discordant with symptoms [18,19,20].
CCT may be useful in assessing the extent and localization of calcification and in determining the feasibility of an intervention, especially to guide transcatheter mitral valve procedures [19, 21, 22]. On the contrary of echocardiography and CMR, CCT lacks velocimetry data, limiting analyses to mitral valve orifice planimetry. Few studies have investigated the role of CCT in diagnosing MS, and the results remain insufficient to infer the reliability of this method. Similar to CMR, CCT often overestimates mitral valve area by planimetry [19, 20, 23]. Furthermore, CCT serves as an additional modality for detecting left atrial thrombus [24, 25].
Current literature supports that CMR is feasible to diagnose MS as a diagnostic alternative when echocardiography proves inconclusive [18, 19, 26,27,28]. Initially confined to morphological evaluations, CMR now incorporates velocity and gradient assessments through phase-contrast image analysis. This expansion allows for the inclusion of mean transmitral gradient or PHT indicators in CMR, facilitating a multiparametric analysis. Comparisons of mitral valve area (MVA) measurements by planimetry or PHT exhibit strong correlations between echocardiography and CMR, albeit with CMR often overestimates valve area [18, 20, 27,28,29,30,31]. Conversely, CMR frequently underestimates peak velocities (E and A) and mitral valve gradients [18, 19, 26,27,28]. Hence, recognizing these anticipated discrepancies becomes crucial in decision-making processes when diagnosing MS using CMR.
In conclusion, the accessibility of CMR and CCT is limited, especially in low-income countries where the majority of MS cases occur. Additionally, the cost of these examinations is also frequently a limitation for these patients. Therefore, widespread diagnosis relying on these methods is unrealistic. While CMR remains more reliable than CCT, its use should be considered when echocardiography is insufficient for diagnosing MS. Current guidelines, therefore, do not advocate for the routine use of these methods in diagnosing and evaluating rheumatic MS [12, 14].
Evaluation
Assessment the Severity and Hemodynamic Impact of MS
Echocardiography is pivotal in assessing the severity of MS. Various parameters are utilized to evaluate the severity of MS, including mitral valve area (MVA), pressure half-time (PHT), transmitral mean pressure gradient, and systolic pulmonary artery pressure (SPAP) [13, 14].
MVA is the most important echocardiography parameter to define MS severity. Severe MS is defined when MVA ≤ 1.5 cm2 and very severe MS is indicated by MVA ≤ 1.0 cm2 [12, 13].
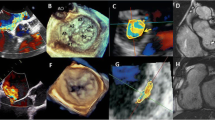
Planimetry is the preferred method to measure MVA [13, 32]. The imaging plane should be positioned at the tip of the valve to prevent overestimation, and this method remains unaffected by flow changes [13]. Three-dimensional (3D) echocardiography planimetry offers greater precision and consistency compared to two-dimensional measures (Fig. 3). It eliminates the need for assumptions and allows easy manipulation to accurately identify the true mitral orifice, particularly beneficial in cases of an eccentrically oriented or irregularly shaped mitral valve orifice [32,33,34,35]. 3D transesophageal echocardiography with multiplanar reconstruction can improve image alignment at the mitral tips [36].
The mitral valve area calculated through direct three-dimensional planimetry by transesophageal echocardiography and QLAB software version 15. This process involves deriving two orthogonal views of the mitral valve from a 3D zoom-mode acquisition, followed by meticulous alignment and tracing of the mitral valve orifice for accurate measurements. LV left ventricle, LA left atrium.
The pressure half-time (PHT), indicating the time for the instantaneous pressure gradient to decline by half from its peak during early mitral inflow, correlates inversely with MVA. PHT estimates functional valve area from the pressure decay between the LA and LV. A value of ≥ 150 ms indicates severe MS. This measurement is affected by the LA and LV compliance. Factors that can alter this compliance, such as the presence of LV hypertrophy, associated aortic regurgitation, and immediate post-percutaneous procedures may impact the results obtained by PHT. Significant MR reduces the reliability of PHT-derived MVA, potentially leading to an underestimation of the valve area [13].
Alternative methods to calculate MVA, such as the proximal isovelocity surface area (PISA) method and continuity equation, are not routinely utilized due to their complexity and susceptibility to multiple sources of error [13].
The transmitral mean gradient correlates with the severity of mitral stenosis: mild MS is indicated by < 5 mmHg, moderate falls between 5 to 9 mmHg, and severe by ≥ 10 mmHg, typically assessed at a heart rate of 60–80 bpm [13]. These gradients are influenced by flow dynamics and heart rate and should be complemented by other parameters for a comprehensive MS assessment.
Systolic pulmonary artery pressure (SPAP) reflects the hemodynamic impact of MS, calculated through the tricuspid regurgitation jet velocity and estimated atrial pressure by the inferior vena cava during respiration. SPAP levels ≥ 50 mmHg denote significant MS, 30–49 mmHg suggest moderate severity, and < 30 mmHg indicate mild MS [13, 14].
Net atrioventricular compliance (Cn) assessment can be useful for risk stratification, particularly in the presence of a discrepancy between symptoms and severity of MS [37•]. Calculated non-invasively using echocardiography based on the formula: Cn (mL/mm Hg) = 1270 × [planimetric MVA (cm2) / E-wave downslope (cm/s2)]. A low Cn (≤ 4 mL/mm Hg) significantly predicts adverse outcomes in MS patients [37•, 38, 39•, 40], correlating with increased SPAP during exercise [37•, 41, 42].
In the echocardiographic evaluation of MS, another crucial aspect is assessment the size and function of the left atrium (LA). Enlargement of the LA indicates the hemodynamic burden imposed by MS and carries prognostic implications. It is crucial not only to assess enlargement but also the shape of the LA, as it contributes to the risk of thrombus formation and stroke [15]. Furthermore, impaired LA function may indicate atrial myopathy from rheumatic carditis, exacerbated by the chamber pressure overload. The integration of two-dimensional echocardiography with speckle tracking (2D-STE) to measure strain offers a non-invasive means to detect early LA dysfunction, facilitating risk stratification in these patients [43, 44]. Although LA contributes to ventricular filling through its three functions, including reservoir, conduit and contractile, the reservoir function represents the most crucial component of LA function in MS. Consequently, LA reservoir strain demonstrates prognostic value in MS, as evidenced by previous studies establishing its correlation with determining NYHA functional class and its association with heart failure symptoms [43, 44]. Additionally, LA reservoir strain is also a predict of new-onset AF in patients with rheumatic MS [43].
Right ventricular (RV) function significantly influences clinical symptoms and prognosis. RV dilation and reduced contractility often indicate pulmonary hypertension in MS [8••, 9•]. However, debate exists among researchers regarding the etiology of RV dysfunction, with some suggesting direct rheumatic involvement leading to myocyte necrosis, fibrosis, and calcification.
Stress Echocardiography
Stress echocardiography is indicated in MS when there are discrepancies between symptoms (NYHA functional class) and parameters of severity evaluated in echocardiography at rest [Class 1, Level C] [12, 14, 44]. It can determine the hemodynamic impact of MS, helping in clinical decision making [45].
Exercise echocardiography is the preferred choice due to its physiological nature, capacity to demonstrate symptoms and NYHA functional class, and the absence of drug-related side effects. The test should be maximum, limited by symptoms. Exercise can be conducted using either a bicycle or treadmill, depending on availability. The advantage of exercise in the bicycle is the possibility of assessing SPAP and mitral gradients throughout the examination. However, with treadmill exercise, capturing images immediately after exertion can pose practical limitations.
The most important parameters evaluated during the physical effort are SPAP and transmitral mean gradient, considered hemodynamic significant in rheumatic MS when > 60 mmHg and > 15 mmHg, respectively [8••, 13, 45]. SPAP at peak exercise predicts clinical outcomes and offers additional prognostic value beyond standard resting measurements, including valve area [37•]. Not only SPAP at peak exercise is important, but the rapid increase in SPAP and the decrease in Cn at lower loads can be associated with symptoms in MS patients [46, 47].
Dobutamine echocardiography can be an alternative for patients unable to undergo exercise testing. A transmitral mean gradient exceeding 18 mmHg predicts adverse events, especially in patients with moderate MS, permitting identify patients who may benefit from interventional procedures [48]. Notably, the assessment of SPAP is not recommended during dobutamine echocardiography [45].
Mitral Valve Morphology Evaluation for Percutaneous Mitral Commissurotomy
A comprehensive assessment of valve morphology, including leaflet thickness and mobility, degree and specific location of calcifications, and the extent of subvalvular involvement, is critical to ascertain suitability for the percutaneous mitral commissurotomy (PMC). Various echocardiographic parameters and scoring systems have been proposed to enhance patient selection and predict outcomes more effectively. Table 1 summarizes the main scores used in predicting outcomes after PMC [49,50,51,52,53,54,55,56,57,58,59,60]. The most widely method used was proposed by Wilkins et al. [49], which involves evaluating leaflet mobility, thickening, calcification, and subvalvular thickening. Despite its widespread use, this method presents several limitations. These include uneven distribution of pathological abnormalities, equal weighting given to all components without differentiation in their contributions, a semiquantitative evaluation leading to interobserver variations, and the lack of assessment of commissural involvement, impacting its accuracy in predicting MR.
Another commonly used scoring system focuses on subvalvular disease and mitral calcification using fluoroscopy, categorizing patients into three groups based on the severity of subvalvular disease and valve calcification [50]. The Nunes score includes dichotomous variables with different weight, making evaluation more quantitative and precise to predict MR [51]. It assesses multiple parameters, including valve area, subvalvular disease extent, anterior mitral leaflet displacement, and the commissural area ratio. This scoring system proves valuable in predicting outcomes for patients falling within intermediate Wilkins score values [51]. Incorporating the extent of commissural calcification [52], an integrated evaluation has been proposed to accurately predict procedural outcomes. Among these approaches, the combination of the Wilkins, Sutaria, and Nunes scores demonstrated the most effective prediction of outcomes following PMC [53].
However, there have been no direct comparisons made between the existing scoring systems. Additionally, procedural success relies not only on mitral valve morphology but also on various other factors, including clinical characteristics, anatomic features of rheumatic MS, interventional management strategies, and operator expertise [60].
Conclusions
Rheumatic MS is an important cause of heart valve disease worldwide. Standard transthoracic echocardiography remains the cornerstone of imaging modality for diagnosing this condition. 3D echocardiography is increasingly utilized in clinical practice due to its ability to provide a more consistent and precise assessment of the valve area. The 2023 WHF guideline for the echocardiographic diagnosis of RHD presents updated recommendations concerning population-based echocardiography and risk assessment strategies. MS treatment relies mainly on valvular interventions, which results in a significant increase of valve orifice with improvement in clinical outcomes. Assessing the morphological characteristics of the mitral valve plays a pivotal role in determining intervention strategies.
References and Recommended Reading
Saxena A. Echocardiographic Diagnosis of Chronic Rheumatic Valvular Lesions. Glob Heart. 2013;8:203–12.
Abdelaziz HM, Tawfik AM, Abd-Elsamad AA, Sakr SA, Algamal AM. Cardiac magnetic resonance imaging for assessment of mitral stenosis before and after percutaneous balloon valvuloplasty in comparison to two- and three-dimensional echocardiography. Acta radiologica (Stockholm, Sweden: 1987). 2020;61(9):1176–85.
Al-Sabeq B, Chamsi-Pasha MA. Imaging in mitral stenosis. Curr Opin Cardiol. 2020;35(5):445–53.
Kim SS, Ko SM, Song MG, Chee HK, Kim JS, Hwang HK, et al. Quantification of stenotic mitral valve area and diagnostic accuracy of mitral stenosis by dual-source computed tomography in patients with atrial fibrillation: comparison with cardiovascular magnetic resonance and transthoracic echocardiography. Int J Cardiovasc Imaging. 2015;31(Suppl 1):103–14.
Tumenas A, Tamkeviciute L, Arzanauskiene R, Arzanauskaite M. Multimodality imaging of the mitral valve: morphology, function, and disease. Curr Probl Diagn Radiol. 2021;50(6):905–24.
Wunderlich NC, Dalvi B, Ho SY, Küx H, Siegel RJ. Rheumatic mitral valve stenosis: diagnosis and treatment options. Curr Cardiol Rep. 2019;21(3):14.
Unal Aksu H, Gorgulu S, Diker M, Celik O, Aksu H, Ozturk D, et al. Cardiac computed tomography versus echocardiography in the assessment of stenotic rheumatic mitral valve. Echocardiography (Mount Kisco, NY). 2016;33(3):346–52.
Choi BH, Ko SM, Hwang HK, Song MG, Shin JK, Kang WS, et al. Detection of left atrial thrombus in patients with mitral stenosis and atrial fibrillation: retrospective comparison of two-phase computed tomography, transoesophageal echocardiography and surgical findings. Eur Radiol. 2013;23(11):2944–53.
Yu S, Zhang H, Li H. Cardiac computed tomography versus transesophageal echocardiography for the detection of left atrial appendage thrombus: a systemic review and meta-analysis. J Am Heart Assoc. 2021;10(23): e022505.
Cawley PJ, Maki JH, Otto CM. Cardiovascular magnetic resonance imaging for valvular heart disease: technique and validation. Circulation. 2009;119(3):468–78.
Helvacioglu F, Yildirimturk O, Duran C, Yurdakul S, Tayyareci Y, Ulusoy OL, et al. The evaluation of mitral valve stenosis: comparison of transthoracic echocardiography and cardiac magnetic resonance. Eur Heart J Cardiovasc Imaging. 2014;15(2):164–9.
Lin SJ, Brown PA, Watkins MP, Williams TA, Lehr KA, Liu W, et al. Quantification of stenotic mitral valve area with magnetic resonance imaging and comparison with Doppler ultrasound. J Am Coll Cardiol. 2004;44(1):133–7.
Djavidani B, Debl K, Lenhart M, Seitz J, Paetzel C, Schmid FX, et al. Planimetry of mitral valve stenosis by magnetic resonance imaging. J Am Coll Cardiol. 2005;45(12):2048–53.
Lanjewar C, Ephrem B, Mishra N, Jhankariya B, Kerkar P. Planimetry of mitral valve stenosis in rheumatic heart disease by magnetic resonance imaging. J Heart Valve Dis. 2010;19(3):357–63.
Uygur B, Celik O, Ustabasioglu FE, Akinci O, Erturk M. Three-dimensional transesophageal echocardiography vs cardiac magnetic resonance in the assessment of planimetric mitral valve area in rheumatic mitral stenosis. Echocardiography (Mount Kisco, NY). 2018;35(10):1621–5.
Bleakley C, Eskandari M, Aldalati O, Moschonas K, Huang M, Whittaker A, et al. Impact of 3D echocardiography on grading of mitral stenosis and prediction of clinical events. Echo Research and Practice. 2018;5:105–11.
Binder TM, Rosenhek R, Porenta G, Maurer G, Baumgartner H. Improved assessment of mitral valve stenosis by volumetric real-time three-dimensional echocardiography. J Am Coll Cardiol. 2000;36:1355–61.
Min SY, Song JM, Kim YJ, Park HK, Seo MO, Lee MS, et al. Discrepancy between mitral valve areas measured by two-dimensional planimetry and three-dimensional transoesophageal echocardiography in patients with mitral stenosis. Heart (British Cardiac Society). 2013;99(4):253–8.
Ruiz JMM, Gomez JLZ. The Role of 2D and 3D Echo in Mitral Stenosis. J Cardiovasc Dev Dis. 2021;8(12):171.
Zhong X, Chen W, Shi Z, Huan Z, Ma L, Liu W. Three-dimensional transesophageal echocardiography measurement of mitral valve area in patients with rheumatic mitral stenosis: multiplanar reconstruction or 3D direct planimetry? Int J Cardiovasc Imaging. 2021;37:99–107.
• de Castro Faria SC, Costa HS, Hung J, de Miranda Chaves AG, de Oliveira FAP, da Silva JLP, et al. Pulmonary artery systolic pressure response to exercise in patients with rheumatic mitral stenosis: determinants and prognostic value. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2020;33(5):550–8 This study established an optimal cutoff value for systolic pulmonary artery pressure at peak exercise to predict outcomes.
Mahfouz RA, Elawady W, Hossein E, Yosri A. Impact of atrioventricular compliance on clinical outcome of patients undergoing successful percutaneous balloon mitral valvuloplasty. Echocardiography (Mount Kisco, NY). 2013;30(10):1187–93.
• Nunes MC, Hung J, Barbosa MM, Esteves WA, Carvalho VT, Lodi-Junqueira L, et al. Impact of net atrioventricular compliance on clinical outcome in mitral stenosis. Circ Cardiovasc Imaging. 2013;6(6):1001–8 This study emphasized the impact of net atrioventricular compliance on pulmonary pressure and confirmed its prognostic value in mitral stenosis.
Nunes MCP, Tan TC, Elmariah S, Lodi-Junqueira L, Nascimento BR, do Lago R, et al. Net atrioventricular compliance is an independent predictor of cardiovascular death in mitral stenosis. Heart (British Cardiac Society). 2017;103(23):1891–8.
Brochet E, Détaint D, Fondard O, Tazi-Mezalek A, Messika-Zeitoun D, Iung B, et al. Early hemodynamic changes versus peak values: what is more useful to predict occurrence of dyspnea during stress echocardiography in patients with asymptomatic mitral stenosis? Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2011;24(4):392–8.
Schwammenthal E, Vered Z, Agranat O, Kaplinsky E, Rabinowitz B, Feinberg MS. Impact of atrioventricular compliance on pulmonary artery pressure in mitral stenosis: an exercise echocardiographic study. Circulation. 2000;102(19):2378–84.
Stassen J, Butcher SC, Namazi F, Marsan NA, Bax JJ, Delgado V. Left atrial deformation imaging and atrial fibrillation in patients with rheumatic mitral stenosis. J Am Soc Echocardiogr. 2022;5:486–94.
Chien CY, Chen CW, Lin TK, Lin Y, Lin JW, Li YD, et al. Atrial deformation correlated with functional capacity in mitral stenosis patients. Echocardiography (Mount Kisco, NY). 2018;35(2):190–5.
Lancellotti P, Pellikka PA, Budts W, Chaudhry FA, Donal E, Dulgheru R, et al. The Clinical Use of Stress Echocardiography in Non-Ischaemic Heart Disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography. 2017;30(2):101–38.
Jung MH, Jung HO, Lee JW, Youn HJ. Decreases in left atrial compliance during early-stage exercise are related to exercise intolerance in asymptomatic significant mitral stenosis. Echocardiography (Mount Kisco, NY). 2017;34(11):1633–9.
Laufer-Perl M, Gura Y, Shimiaie J, Sherez J, Pressman GS, Aviram G, et al. Mechanisms of effort intolerance in patients with rheumatic mitral stenosis: combined echocardiography and cardiopulmonary stress protocol. JACC Cardiovasc Imaging. 2017;10(6):622–33.
Reis G, Motta MS, Barbosa MM, Esteves WA, Souza SF, Bocchi EA. Dobutamine stress echocardiography for noninvasive assessment and risk stratification of patients with rheumatic mitral stenosis. J Am Coll Cardiol. 2004;43(3):393–401.
Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60(4):299–308.
Iung B, Cormier B, Ducimetière P, Porte JM, Nallet O, Michel PL, et al. Immediate results of percutaneous mitral commissurotomy. A predictive model on a series of 1514 patients. Circulation. 1996;94(9):2124–30.
Nunes MC, Tan TC, Elmariah S, do Lago R, Margey R, Cruz-Gonzalez I, et al. The echo score revisited: Impact of incorporating commissural morphology and leaflet displacement to the prediction of outcome for patients undergoing percutaneous mitral valvuloplasty. Circulation. 2014;129(8):886–95.
Sutaria N, Northridge DB, Shaw TR. Significance of commissural calcification on outcome of mitral balloon valvotomy. Heart. 2000;84:398–402.
Gajjala OR, Durgaprasad R, Velam V, Kayala SB, Kasala L. New integrated approach to percutaneous mitral valvuloplasty combining Wilkins score with commissural calcium score and commissural area ratio. Echocardiography (Mount Kisco, NY). 2017;34(9):1284–91.
Padial LR, Freitas N, Sagie A, et al. Echocardiography can predict which patients will develop severe mitral regurgitation after percutaneous mitral valvulotomy. J Am Coll Cardiol. 1996;27:1225–31.
Cannan CR, Nishimura RA, Reeder GS, et al. Echocardiographic assessment of commissural calcium: a simple predictor of outcome after percutaneous mitral balloon valvotomy. J Am Coll Cardiol. 1997;29:175–80.
Rifaie O, Esmat I, Abdel-Rahman M, et al. Can a novel echocardiographic score better predict outcome after percutaneous balloon mitral valvuloplasty? Echocardiography. 2009;26:119–27.
Anwar AM, Attia WM, Nosir YF, et al. Validation of a new score for the assessment of mitral stenosis using real-time three-dimensional echocardiography. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2010;23:13–22.
Bhalgat P, Karlekar S, Modani S, Agrawal A, Lanjewar C, Nabar A, et al. Subvalvular apparatus and adverse outcome of balloon valvotomy in rheumatic mitral stenosis. Indian Heart J. 2015;67:428–33.
Prota-Filho LEB, Meneguz-Moreno RA, Queiroz CCV, Wohnrath FC, Carboni FAC, Silva GRC, et al. Novel Prognostic Score for Immediate and Late Success After Percutaneous Mitral Balloon Commissurotomy in Patients With Mitral Stenosis. J Invasive Cardiol. 2020;32:211–7.
Nunes MC, Nascimento BR, Lodi-Junqueira L, Tan TC, Athayde GR, Hung J. Update on percutaneous mitral commissurotomy. Heart. 2016;102(7):500–7.
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. Lancet (London, England). 2012;379(9819):953–64.
Meira ZM, Goulart EM, Colosimo EA, Mota CC. Long term follow up of rheumatic fever and predictors of severe rheumatic valvar disease in Brazilian children and adolescents. Heart (British Cardiac Society). 2005;91(8):1019–22.
Rwebembera J, Nascimento BR, Minja NW, de Loizaga S, Aliku T, Dos Santos LPA, et al. Recent advances in the rheumatic fever and rheumatic heart disease continuum. Pathogens. 2022;11(2):179.
Kingué S, Ba SA, Balde D, Diarra MB, Anzouan-Kacou JB, Anisubia B, et al. The VALVAFRIC study: a registry of rheumatic heart disease in Western and Central Africa. Arch Cardiovasc Dis. 2016;109(5):321–9.
•• Zühlke L, Engel ME, Karthikeyan G, Rangarajan S, Mackie P, Cupido B, et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J. 2015;36(18):1115–22. This study highlighted the significant burden of RHD specifically among young patients.
•• Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. This study presents the global estimated epidemiology of RHD and its impact in mortality and morbidity.
Esteves WAM, Lodi-Junqueira L, Soares JR, Athayde GRS, Goebel GA, Carvalho LA, et al. Impact of percutaneous mitral valvuloplasty on left ventricular function in patients with mitral stenosis assessed by 3D echocardiography. Int J Cardiol. 2017;248:280–28.
•• Silbiger JJ. Advances in rheumatic mitral stenosis: echocardiographic, pathophysiologic, and hemodynamic considerations. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2021;34(7):709-22.e1. This study presents a comprehensive review of all significant echocardiographic characteristics associated with rheumatic mitral stenosis.
• Vidula MK, Xu Z, Xu Y, Alturki A, Reddy BN, Kini P, et al. Cardiovascular magnetic resonance characterization of rheumatic mitral stenosis: findings from three worldwide endemic zones. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2022;24(1):24. This study described the cardiac structural and functional changes in a cohort of patients with rheumatic mitral stenosis.
Kumar RK, Antunes MJ, Beaton A, Mirabel M, Nkomo VT, Okello E, et al. Contemporary diagnosis and management of rheumatic heart disease: implications for closing the gap: a scientific statement from the american heart association. Circulation. 2020;142(20):e337–57.
•• Rwebembera J, Marangou J, Mwita JC, et al. 2023 World Heart Federation guidelines for the echocardiographic diagnosis of rheumatic heart disease . Nat Rev Cardiol. 2024;21(4):250–263. These World Heart Federation 2023 guidelines provide a concise and updated resource for clinical and research applications in RHD-endemic regions.
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: executive summary: a report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 2021;143(5):e35–71.
Pandian NG, Kim JK, Arias-Godinez JA, Marx GR, Michelena HI, Chander Mohan J, et al. Recommendations for the use of echocardiography in the evaluation of rheumatic heart disease: a report from the american society of echocardiography. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2023;36(1):3–28.
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632.
Nunes MC, Handschumacher MD, Levine RA, Barbosa MM, Carvalho VT, Esteves WA, et al. Role of LA shape in predicting embolic cerebrovascular events in mitral stenosis: mechanistic insights from 3D echocardiography. JACC Cardiovasc Imaging. 2014;7(5):453–61.
• Remenyi B, ElGuindy A, Smith SC Jr, Yacoub M, Holmes DR Jr, et al. Valvular aspects of rheumatic heart disease. Lancet. 2016;387:1335–46. This study provides an overview of RHD including clinical presentations and management strategies.
Funding
MCPN is National Council for Scientific and Technological Development (CNPq) scholarship recipient.
Ethics declarations
Conflict of Interest
No potential conflicts of interest relevant to this article were reported.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva, V.R., De Castro Faria, S.C., de Azevedo Figueiredo, F. et al. Rheumatic Mitral Stenosis: Update in Diagnosis and Evaluation. Curr Treat Options Cardio Med 26, 207–220 (2024). https://doi.org/10.1007/s11936-024-01042-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11936-024-01042-6