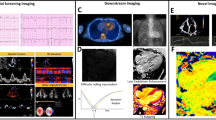
Abstract
Purpose of review
Imaging of cardiac amyloidosis has traditionally been hindered by nonspecific findings or diagnosis late in disease. Recent imaging techniques aim to address these gaps.
Recent findings
T1 mapping, extracellular volume (ECV) quantification, myocardial strain imaging, positron emission tomography/computed tomography (PET/CT), bone-seeking agents, and cardiac computed tomography angiography (CCTA) are a few of the imaging modalities and techniques used to image cardiac amyloidosis. Many offer the possibility of earlier cardiac amyloidosis detection. PET/CT and bone-seeking agents may allow for quantification of amyloid deposition as well as treatment response monitoring. Additionally, bone-seeking agents help diagnose transthyretin amyloidosis without the need for endomyocardial biopsy.
Summary
New imaging techniques have helped to expand the role of imaging in cardiac amyloidosis by offering the possibility of earlier disease detection, disease burden quantification, and cardiac amyloid subtype differentiation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amyloidosis is a systemic disease caused by the deposition of misfolded proteins (amyloid) into extracellular tissue. There are numerous precursor proteins implicated in the disease, although light-chain amyloidosis (AL) and transthyretin amyloidosis (ATTR) are the two most commonly associated with cardiac amyloidosis (CA) [1].
Historically, amyloidosis is associated with poor outcomes and survival. Patients with AL tend to have worse prognosis compared to ATTR [1]. Cardiac involvement in amyloidosis portends a worse prognosis compared to involvement of other organs, with a median survival of 6 months once symptomatic. Deposition of amyloid into the myocardium leads to mechanical and structural alterations resulting in myocardial thickening and impaired relaxation. Decreased diastolic volumes, reduced cardiac output, and arrhythmias are common features [2]. Although incompletely understood, there is supporting evidence of the cytotoxic effects of amyloid deposition [3].
CA is a relatively rare disease affecting 8 to 12 individuals per one million, although the diagnostic prevalence and incidence are increasing [4]. Low prevalence and lack of effective treatment likely contributed to under diagnosis. Nonetheless, relatively newly approved treatment options and promising treatments under investigation show great promise in potentially altering the natural history of this disease.
Treatment for AL and ATTR cardiac amyloidosis is different. As AL is a plasma cell dyscrasia, treatment is aimed at reducing light chain levels. For example, Daratumumab targets the CD38 receptor on plasma cells and has recently demonstrated efficacy in reducing serum light chain levels [5]. ATTR treatments target the TTR protein: tafamidis specifically binds to and stabilizes TTR, preventing it from dissociating and forming fibrils [6].
Newer treatments have focused on preventing deposition of amyloid fibrils into organs and tissue rather than removing preexisting amyloid deposits [2]. Thus, early detection of CA for treatment is crucial to prevent morbidity. Diagnosis of CA is challenging, as its clinical presentation is nonspecific and can mimic other diseases [7]. Imaging is used to support a diagnosis of CA and evaluate cardiac function.
Role of imaging of cardiac amyloidosis
Imaging findings of CA on echocardiography have been well-described. Left ventricular (LV) wall thickness greater than 1.2 cm, valve leaflet thickening, biatrial enlargement, and right ventricular free wall thickening are some of the characteristic morphologic changes. Functional abnormalities, such as diastolic dysfunction, reduced LV wall contractility, and decreased cardiac output, have also been described with CA [8]. Although useful in aiding diagnosis and monitoring cardiac function, echocardiographic findings are not specific to CA and may be seen with other disease; for example, LV hypertrophy can also be seen in the setting of chronic hypertension or hypertrophic cardiomyopathy. Thus, echocardiography alone is insufficient to establish a diagnosis of CA.
CA imaging has made considerable advancements over the last decade and expanded to include several additional modalities. Along with echocardiography, CMR, positron emission tomography/computed tomography (PET/CT), bone-seeking radiotracers, and cardiac computed tomography angiography (CCTA) are all used to evaluate CA. This review article will focus on the aforementioned relatively newer imaging options, which allow for earlier detection, subtype differentiation, treatment monitoring, and obviate the need for endomyocardial biopsy.
Cardiac magnetic resonance imaging
Late gadolinium enhancement
Cardiac magnetic resonance imaging (CMR) complements echocardiography by offering higher resolution for structural evaluation and more accurate quantification of cardiac function (Fig. 1a). Additionally, CMR allows for evaluation with late gadolinium enhancement (LGE) which can aid in the diagnosis of CA by revealing myocardial enhancement patterns characteristic of CA. Diffuse subendocardial and/or transmural delayed enhancement in a non-coronary distribution are the most common LGE patterns (Fig. 1b) [9]. These characteristic LGE patterns are highly specific for CA [10]. LGE is also an independent indicator of mortality for both AL and ATTR; transmural enhancement is associated with the poorest outcomes [11, 12].
Cardiac MRI. A 72-year-old male with biopsy proven systemic AL was referred for cardiac MRI. a 4-chamber view steady state free precession (SSFP) image demonstrates mild biatrial enlargement. Calculated left ventricular ejection fraction was 41%. The interventricular septum is thickened measuring 2 cm. b Phase sensitive inversion recovery (PSIR) late gadolinium enhancement (LGE) image shows diffuse transmural enhancement of the left and right ventricular myocardium. c TI scout shows myocardial nullification occurring prior to blood pool nullification. d T1 map of a different patient with a T1 of 953 ms (T1 values less than 1100 are considered normal at the author’s institution). Note the color bar on the left with T1 values ranging from 0 to 2000 ms.
Traditionally, LGE relied on magnitude inversion recovery (IR) imaging whereby an inversion time (TI) is selected by the operator as the time point for nullification of normal myocardium. TI selection can be challenging in the setting of diffusely abnormal myocardium. Blood pool nullification should occur before the myocardium nulls. Diffusely abnormal myocardium may null prior to blood pool (Fig. 1c). TI selection is crucial, as abnormal myocardium may be missed if the selected TI is incorrect. A relatively new technique, phase sensitive inversion recovery (PSIR), is less sensitive to a specific TI and can produce high quality images over a broad range of TIs. On PSIR, scar or infarcted tissue always has higher signal than normal myocardium regardless of the TI. PSIR has proven to be a more reliable sequence to detect LGE [12].
There are several shortcomings with LGE CMR sequences. Systemic amyloidosis can be associated with renal dysfunction, thereby restricting the use of intravenous gadolinium to only a subset of those suspected of having CA. LGE is also a late manifestation of CA, and therefore, this technique is insensitive to early disease, when additional treatment options might be considered. Furthermore, while a subendocardial enhancement pattern is more common in light chain amyloidosis (AL) and a transmural pattern is more common in transthyretin amyloidosis (ATTR), either pattern can be seen with both types of amyloidosis [12, 13], and therefore, LGE pattern alone cannot distinguish these two main types of amyloidosis.
T1 mapping
Another relatively new technique used to detect amyloid deposition is T1 mapping. In brief, T1 is the decay constant of tissue as it drifts towards equilibrium after the application of a radiofrequency pulse. The T1 in each voxel can be quantified and assigned a color from a predefined spectrum. An image with each voxel color coded allows for visual inspection for abnormality (Fig. 1d). A T1 value obtained without contrast is referred to as native T1, which will hereafter be referred to as T1. A thorough review of the principles and technique has been previously described [14, 15].
T1 values change based on tissue composition. Excess fluid within the myocardium, such as with myocardial edema, increases T1. T1 values also increase in CA [16]. T1 values decrease with fatty infiltration or iron deposition [17].
Recent studies highlight that, for patients with suspected CA, T1 mapping can be both sensitive and specific for detecting cardiac involvement of both AL and ATTR [18,19,20]. Furthermore, there is strong evidence to support the use of T1 for detecting early myocardial involvement, a limitation of LGE. In one study involving patients with confirmed systemic AL, T1 was elevated despite the absence of LGE [21], suggesting that T1 may be used to detect subtle amyloid deposition before it becomes apparent on LGE. Another benefit of T1 over LGE is that it can be performed without administering intravenous gadolinium.
Despite the promising benefits of T1 mapping, there are several limitations to the technique. Firstly, despite its correlation with LGE and extracellular volume (ECV), T1 itself has not yet been proven as an independent predictor of mortality as LGE and ECV have [21]. Secondly, there remains debate surrounding optimal T1 cutoff values to use. Baggiano et al., to generate high diagnostic accuracy, used a T1 < 1036 milliseconds (ms) to exclude CA and a TI > 1164 ms as positive for CA. However, this created an intermediate probability “gray zone” of TI ≥ 1036 ms and ≤ 1164 ms, in which 58% of their patients fell; for this subset of patients, the authors recommended further evaluation with ECV quantification, a technique that requires the administration of gadolinium and additional scanning time [18]. One could use a variety of cutoff values, including definition cutoffs without a gray zone, but each choice has a sensitivity/specificity trade-off. Lastly, while T1 mapping is an important new technique, standardized T1 CMR protocols have not been established. T1 values can vary based on CMR scanner systems, magnet strength (1.5 T vs 3 T), and even between views [22]; thus, these values cannot be applied universally.
Extracellular volume
The myocardium, at a cellular level, can be understood as having 3 main compartments: intravascular, intracellular, and interstitial. The ECV consists of the interstitium and intravascular compartments. Using measurements obtained from pre- and post-contrast T1 in combination with blood hematocrit information, an estimation of ECV becomes possible. A study by Duca et al. showed good correlation between the ECV estimated via CMR and histologic ECV calculated from endomyocardial biopsy (EMB) specimens [23]. Greater detail and review of this technique are beyond the scope of this review paper but have been described elsewhere [14].
ECV, like T1, can be elevated in patients with CA who have subclinical disease not yet demonstratable on LGE. This may be useful in diagnosing CA earlier, when more treatment options are available [24••]. ECV is also quantifiable and correlates with disease severity for both AL and ATTR amyloidosis [25]. Moreover, ECV can be monitored over time with serial CMR exams, potentially indicating CA disease trajectory. The ability to track amyloid burden would allow for treatment monitoring and assessment of disease progression [26]. Increased ECV is an independent prognostic marker for mortality in CA [21].
ECV varies based on disease states and reflects underlying tissue composition [27]. ECV increases with CA [16], but can also increase with other diseases, such as acute myocardial infarction and myocarditis. Underlying fibrosis and edema also have elevated ECV. Thus, as with T1, the specificity of ECV is limited. Evaluating patients with a high pre-test probability of CA and low pre-test probability of alternate diagnoses would improve test characteristics. As mentioned previously, another limitation to ECV assessment is the requirement of intravenous gadolinium administration.
Myocardial strain imaging
Myocardial strain refers to deformation of the myocardium after the application of force, i.e., during LV contraction. Strain can be measured as the percent change in myocardial length between end-systole and end-diastole in 3 directions: longitudinal strain (LS), circumferential strain (CS), and radial strain (RS). A detailed review of imaging principles and techniques has been previously summarized in detail [28].
The concept of altered myocardial strain in CA was initially applied to echocardiography, where early CA can show impaired LS but preserved RS [8]. LS in basal cardiac segments is more severely affected than in apical segments, which helps distinguish CA from other causes of LV hypertrophy [29].
With newer imaging techniques and postprocessing software, evaluating myocardial strain on CMR is feasible. Myocardial strain may provide diagnostic, as well as prognostic information. A recent study by Wan et al. showed that reduced global circumferential strain (GCS) may be seen in systemic amyloidosis patients without clinically diagnosed cardiac involvement, suggesting myocardial strain might detect early CA. Furthermore, a reduced GCS was independently associated with worse outcomes in all patients with AL [30]. The ability to detect abnormalities before the presence of LGE allows strain quantification to potentially serve as an early indicator of cardiac involvement, with specificity being highest in the setting of high pretest probability for CA.
Despite promising research, a number of limitations reduce the utility of strain quantification. Foremost, there are no standardized strain values due to variability in equipment, software, and technique. Therefore, cutoff values are likely institution specific and not generalizable. Secondly, validation studies on the use of CMR myocardial strain in CA are lacking [28].
Positron emission tomography/computed tomography
The use of PET/CT for amyloid imaging was originally developed to aid in the diagnosis of Alzheimer disease by binding to beta-amyloid (Aβ) deposits in the brain. Several agents including 11C-PiB, 18F-florbetapir, 18F-florbetapen, and 18F-flutemetamol have been studied for use in CA with promising results [31,32,33].
These agents are highly sensitive in detecting CA. In a pilot study using 18F-florbetapen, cardiac radiotracer uptake was seen in all patients with biopsy proven CA, both AL and ATTR [31]. In this study, no uptake was detected in the control patients who had hypertension. Hypertension is a known cause of LV hypertrophy, and it can be challenging to differentiate from CA by other techniques. Similarly, in a separate study using 18F-florbetapir, all patients with endomyocardial or extracardiac biopsy proven CA (including AL and ATTR subtypes) demonstrated diffuse 18F-florbetapir uptake with none noted in control subjects [32]. These studies highlight the likely high diagnostic accuracy of PET/CT and its ability to differentiate CA from normal patients and those with other causes of LV hypertrophy.
Beyond being sensitive and specific, 18F-florbetapir was evaluated in a recent study by Cuddy et al. to correlate the degree of radiotracer uptake in active AL patients to varying degrees of cardiac involvement [34••]. The retention index (RI) was used to measure the amount of amyloid deposits. Patients with CA by consensus criteria had the highest RI, followed by patients with previous cardiac involvement who were in remission. All patients with active AL but no CA still demonstrated cardiac radiotracer uptake, albeit the lowest RI of the 3 subgroups. The ability to quantify amyloid burden is important not only for potential prognostic stratification but also for monitoring treatment response. The presence of uptake in patients with AL without known cardiac involvement suggests that PET/CT may play a role in detecting pre-clinical CA, although, notably, this study did not include control subjects free of amyloid disease.
Notwithstanding the promising research, the dearth of more confirmatory studies, short half-life of some of the agents, and high cost limit the usefulness of PET/CT in routine clinical care [35••].
Radionuclide bone-seeking agents
Nuclear medicine bone scintigraphy is an imaging technique originally developed to evaluate skeletal pathology. Incidental cardiac uptake was noted in patients with CA [36]. The exact mechanism for this uptake remains unclear, although binding to microcalcifications (which develops more frequently in ATTR) has been suggested [35, 37].
Agents used for CA imaging include 99mTc-labeled 3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD), 99mTc-labeled pyrophosphate (99mTc-PYP), and 99mTc-labeled hydroxymethylene diphosphonate (99mTc-HMDP). It is worth mentioning that 99mTc-labeled methylene diphosphonate (99mTc-MDP), which is commonly used in oncologic imaging to evaluate for bone metastases, has low sensitivity for CA and is not used for this purpose [38].
Distinguishing ATTR from AL is important, as their respective treatment options differ. Echocardiography, PET/CT, and CMR findings are often not sufficiently specific enough to distinguish ATTR from AL. However, the above radiotracers are both sensitive and specific for the detection of ATTR. In contrast, these same studies have demonstrated little to no uptake of bone-seeking agents in AL [39, 40].
In the setting of a negative urine protein electrophoresis (UPEP) and serum protein electrophoresis (SPEP), which are used to rule out the possibility of AL, the specificity of these radiotracers for the ATTR subtype approaches 100% [41]. In fact, guidelines for the diagnosis of ATTR have incorporated these specific agents into the diagnostic algorithm to help avoid endomyocardial biopsy when possible [35, 42]. If obtaining a 99mTc-PYP scan is not possible, endomyocardial biopsy may be necessary for diagnosis of ATTR. Although invasive, endomyocardial biopsies are performed preferentially over non-cardiac sites (such as abdominal fat pad), which are often low yield for ATTR [43].
Aside from distinguishing between ATTR and AL, bone-seeking radiotracers may also be helpful in detecting early cardiac involvement. Bone-seeking agent scintigraphy has been shown to be positive in asymptomatic patients who do not have signs of CA on echocardiography or CMR [44]. Given the new treatment options available, early detection of CA will become increasingly important to reduce morbidity and mortality.
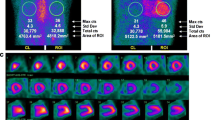
Like some of the other techniques described above, bone-seeking scintigraphy also offers the possibility of semi-quantitative assessment and prognosis. One study using 99mTc-HMDP calculated a heart to skull retention ratio (HR/SR) using regions of interests (ROI). Increased HR/SR correlated with increased CA severity based on LVEF and NT-proBNP. A HR/SR > 1.94 was associated with increased mortality [40]. In a different study using 99mTc-PYP, a heart to contralateral lung ratio ≥ 1.6 was associated with poorer outcomes [45]. Figure 2 demonstrates a 99mTc-PYP negative for ATTR and Fig. 3 shows a positive 99mTc-PYP scan.
Negative 99mTc-PYP Scan. A 77-year-old female with severe global hypokinesis and reduced left ventricular ejection fraction of 20–25% on echocardiography. a Planar imaging of a 99mTc-PYP scan demonstrates no significant radiotracer uptake in the expected location of the heart. b Same patient with ROI drawn over the expected location of the heart and the contralateral lung. Heart to contralateral lung ratio (H/CL) was calculated to be 1.1. A ratio of 1.5 or greater is considered positive for ATTR. c SPECT/CT fusion image for the same patient in the axial plane demonstrates no radiotracer activity beyond background within the myocardium.
Positive 99mTc-PYP Scan. An 88-year-old male with concentric left ventricular hypertrophy and normal ejection fraction (65%) on echocardiography. a Planar imaging of a 99mTc-PYP scan demonstrates noticeable radiotracer uptake in the location of the heart. b Same patient with ROI drawn over the heart and contralateral lung. H/CL ratio was 2. A ratio greater than 1.5 is considered positive for ATTR. c SPECT/CT fusion image for the same patient in the axial plane demonstrates radiotracer uptake of the left ventricular myocardium.
One recently discovered limitation of these bone-seeking agents is the lack of avidity for certain mutations. In a study by Musumeci et al., 99mTc-DPD and 99mTc-HMDP tracers had low sensitivity (10.5%) for ATTR with the Phe64Leu mutation [46]. The broad implication of this new finding is unknown, as there are over 100 known TTR genetic mutations. In a systematic review, Waddington-Cruz et al. found that the Phe64Leu mutation accounted for 4.4% of the genotypes in patients with ATTR polyneuropathy [47].
Cardiac computed tomography angiography
Although cardiac computed tomography angiography (CCTA) has not been traditionally used to evaluate CA, advances in technology have made CCTA a promising modality in diagnosing CA. Dual-energy CCTA has been used to calculate ECV [48], with the potential to provide diagnostic information similar to that of CMR-derived ECV measurements. Recently, Scully et al. demonstrated the ability to calculate ECV without dual energy by instead obtaining a delayed phase [49•]. Detection of regional myocardial strain with 4DCT has also been demonstrated in the last couple years [50]. More research is needed to establish the true utility of CCTA in CA imaging.
Conclusion
Options for imaging CA have rapidly expanded over the last several years. New imaging techniques promise increased diagnostic accuracy, earlier detection, improved prognostication, subtype differentiation, and the possibility of treatment response monitoring. In combination with new therapies, the ultimate goal of decreased morbidity and improved outcomes is increasingly possible.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337(13):898–909. https://doi.org/10.1056/NEJM199709253371306.
Witteles RM, Liedtke M. AL amyloidosis for the cardiologist and oncologist. JACC: CardioOncology. 2019;1(1):117–30. https://doi.org/10.1016/j.jaccao.2019.08.002.
Imperlini E, Gnecchi M, Rognoni P, Sabido E, Ciuffreda MC, Palladini G, et al. Proteotoxicity in cardiac amyloidosis: amyloidogenic light chains affect the levels of intracellular proteins in human heart cells. Sci Rep. 2017;7(1):15661. https://doi.org/10.1038/s41598-017-15424-3.
Gilstrap LG, Dominici F, Wang Y, El-Sady MS, Singh A, Di Carli MF, et al. Epidemiology of cardiac amyloidosis-associated heart failure hospitalizations among fee-for-service Medicare beneficiaries in the United States. Circ Heart Fail. 2019;12(6):e005407. https://doi.org/10.1161/CIRCHEARTFAILURE.118.005407.
Kaufman GP, Schrier SL, Lafayette RA, Arai S, Witteles RM, Liedtke M. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood. 2017;130(7):900–2. https://doi.org/10.1182/blood-2017-01-763599.
Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–16. https://doi.org/10.1056/NEJMoa1805689.
Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135(14):1357–77. https://doi.org/10.1161/CIRCULATIONAHA.116.024438.
Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2—evidence base and standardized methods of imaging. J Nucl Cardiol. 2019;26(6):2065–123. https://doi.org/10.1007/s12350-019-01760-6.
Oda S, Kidoh M, Nagayama Y, Takashio S, Usuku H, Ueda M, et al. Trends in diagnostic imaging of cardiac amyloidosis: emerging knowledge and concepts. Radiographics. 2020;40(4):961–81. https://doi.org/10.1148/rg.2020190069.
Bhatti S, Watts E, Syed F, Vallurupalli S, Pandey T, Jambekar K, et al. Clinical and prognostic utility of cardiovascular magnetic resonance imaging in myeloma patients with suspected cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2016;17(9):970–7. https://doi.org/10.1093/ehjci/jew101.
Raina S, Lensing SY, Nairooz RS, Pothineni NVK, Hakeem A, Bhatti S, et al. Prognostic value of late gadolinium enhancement CMR in systemic amyloidosis. JACC Cardiovasc Imaging. 2016;9(11):1267–77. https://doi.org/10.1016/j.jcmg.2016.01.036.
Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132(16):1570–9. https://doi.org/10.1161/CIRCULATIONAHA.115.016567.
Dungu JN, Valencia O, Pinney JH, Gibbs SD, Rowczenio D, Gilbertson JA, et al. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7(2):133–42. https://doi.org/10.1016/j.jcmg.2013.08.015.
Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 mapping: basic techniques and clinical applications. JACC Cardiovasc Imaging. 2016;9(1):67–81. https://doi.org/10.1016/j.jcmg.2015.11.005.
Aherne E, Chow K, Carr J. Cardiac T1mapping: Techniques and applications. J Magn Reson Imaging. 2020;51(5):1336–56. https://doi.org/10.1002/jmri.26866.
Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, et al. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J. 2015;36(4):244–51. https://doi.org/10.1093/eurheartj/ehu444.
Germain P, El Ghannudi S, Jeung MY, Ohlmann P, Epailly E, Roy C, et al. Native T1 mapping of the heart - a pictorial review. Clin Med Insights Cardiol. 2014;8(Suppl 4):1–11. https://doi.org/10.4137/CMC.S19005.
Baggiano A, Boldrini M, Martinez-Naharro A, Kotecha T, Petrie A, Rezk T, et al. Noncontrast magnetic resonance for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2020;13(1 Pt 1):69–80. https://doi.org/10.1016/j.jcmg.2019.03.026.
Fontana M, Banypersad SM, Treibel TA, Maestrini V, Sado DM, White SK, et al. Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7(2):157–65. https://doi.org/10.1016/j.jcmg.2013.10.008.
Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6(4):488–97. https://doi.org/10.1016/j.jcmg.2012.11.013.
Lin L, Li X, Feng J, Shen KN, Tian Z, Sun J, et al. The prognostic value of T1 mapping and late gadolinium enhancement cardiovascular magnetic resonance imaging in patients with light chain amyloidosis. J Cardiovasc Magn Reson. 2018;20(1):2. https://doi.org/10.1186/s12968-017-0419-6.
Karamitsos TD, Papanastasiou CA, Wan K, Sun J, Yang D, Liu H, Wang J, Cheng W, Zhang Q, Zeng Z, Zhang T, Greiser A, Jolly MP, Han Y, Chen YA-O. Cardiac magnetic resonance T1 mapping for cardiac amyloidosis: the best way forward. Left Ventricular Myocardial Deformation on Cine MR Images: Relationship to Severity of Disease and Prognosis in Light-Chain Amyloidosis. (1876–7591 (Electronic)).
Duca F, Kammerlander AA, Panzenbock A, Binder C, Aschauer S, Loewe C, et al. Cardiac magnetic resonance T1 mapping in cardiac amyloidosis. JACC Cardiovasc Imaging. 2018;11(12):1924–6. https://doi.org/10.1016/j.jcmg.2018.06.010.
•• Pan JA, Kerwin MJ, Salerno M. Native T1 mapping, extracellular volume mapping, and late gadolinium enhancement in cardiac amyloidosis: a meta-analysis. JACC Cardiovasc Imaging. 2020;13(6):1299–310. https://doi.org/10.1016/j.jcmg.2020.03.010 Paper compares the diagnostic value of T1, ECV, and LGE in evaluating cardiac amyloidosis.
Barison A, Aquaro GD, Pugliese NR, Cappelli F, Chiappino S, Vergaro G, et al. Measurement of myocardial amyloid deposition in systemic amyloidosis: insights from cardiovascular magnetic resonance imaging. J Intern Med. 2015;277(5):605–14. https://doi.org/10.1111/joim.12324.
Martinez-Naharro A, Abdel-Gadir A, Treibel TA, Zumbo G, Knight DS, Rosmini S, et al. CMR-verified regression of cardiac AL amyloid after chemotherapy. JACC Cardiovasc Imaging. 2018;11(1):152–4. https://doi.org/10.1016/j.jcmg.2017.02.012.
Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson. 2016;18(1):89. https://doi.org/10.1186/s12968-016-0308-4.
Amzulescu MS, De Craene M, Langet H, Pasquet A, Vancraeynest D, Pouleur AC, et al. Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. Eur Heart J Cardiovasc Imaging. 2019;20(6):605–19. https://doi.org/10.1093/ehjci/jez041.
Phelan D, Collier P, Thavendiranathan P, Popovic ZB, Hanna M, Plana JC, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98(19):1442–8. https://doi.org/10.1136/heartjnl-2012-302353.
Wan K, Sun J, Yang D, Liu H, Wang J, Cheng W, et al. Left ventricular myocardial deformation on cine MR images: relationship to severity of disease and prognosis in light-chain amyloidosis. Radiology. 2018;288(1):73–80. https://doi.org/10.1148/radiol.2018172435.
Law WP, Wang WY, Moore PT, Mollee PN, Ng AC. Cardiac amyloid imaging with 18F-Florbetaben PET: a pilot study. J Nucl Med. 2016;57(11):1733–9. https://doi.org/10.2967/jnumed.115.169870.
Dorbala S, Vangala D, Semer J, Strader C, Bruyere JR, Carli MFD, et al. Imaging cardiac amyloidosis: a pilot study using 18F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging. 2014;41(9):1652–62. https://doi.org/10.1007/s00259-014-2787-6.
Lee SP, Lee ES, Choi H, Im HJ, Koh Y, Lee MH, et al. 11C-Pittsburgh B PET imaging in cardiac amyloidosis. JACC Cardiovasc Imaging. 2015;8(1):50–9. https://doi.org/10.1016/j.jcmg.2014.09.018.
•• Cuddy SAM, Bravo PE, Falk RH, El-Sady S, Kijewski MF, Park MA, et al. Improved quantification of cardiac amyloid burden in systemic light chain amyloidosis: redefining early disease? JACC Cardiovasc Imaging. 2020;13(6):1325–36. https://doi.org/10.1016/j.jcmg.2020.02.025 Article demonstrates the ability for PET/CT to detect early cardiac amyloidosis as well as the possibility for quantifying disease burden.
•• Martinez-Naharro A, Baksi AJ, Hawkins PN, Fontana M. Diagnostic imaging of cardiac amyloidosis. Nat Rev Cardiol. 2020;17(7):413–26. https://doi.org/10.1038/s41569-020-0334-7 Excellent review of the various modalities and techniques used to image cardiac amyloidosis.
Wizenberg TA, Muz J, Sohn YH, Samlowski W, Weissler AM. Value of positive myocardial technetium-99m-pyrophosphate scintigraphy in the noninvasive diagnosis of cardiac amyloidosis. Am Heart J. 1982;103(4 Pt 1):468–73. https://doi.org/10.1016/0002-8703(82)90331-3.
Stats MA, Stone JR. Varying levels of small microcalcifications and macrophages in ATTR and AL cardiac amyloidosis: implications for utilizing nuclear medicine studies to subtype amyloidosis. Cardiovasc Pathol. 2016;25(5):413–7. https://doi.org/10.1016/j.carpath.2016.07.001.
Rapezzi C, Gagliardi C, Milandri A. Analogies and disparities among scintigraphic bone tracers in the diagnosis of cardiac and non-cardiac ATTR amyloidosis. J Nucl Cardiol. 2019;26(5):1638–41. https://doi.org/10.1007/s12350-018-1235-6.
Perugini E, Guidalotti PL, Salvi F, Cooke RM, Pettinato C, Riva L, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46(6):1076–84. https://doi.org/10.1016/j.jacc.2005.05.073.
Galat A, Rosso J, Guellich A, Van Der Gucht A, Rappeneau S, Bodez D, et al. Usefulness of (99m)Tc-HMDP scintigraphy for the etiologic diagnosis and prognosis of cardiac amyloidosis. Amyloid. 2015;22(4):210–20. https://doi.org/10.3109/13506129.2015.1072089.
Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–12. https://doi.org/10.1161/CIRCULATIONAHA.116.021612.
Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M, et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019;12(9):e006075. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006075.
Fine NM, Arruda-Olson AM, Dispenzieri A, Zeldenrust SR, Gertz MA, Kyle RA, et al. Yield of noncardiac biopsy for the diagnosis of transthyretin cardiac amyloidosis. Am J Cardiol. 2014;113(10):1723–7. https://doi.org/10.1016/j.amjcard.2014.02.030.
Rapezzi C, Quarta CC, Guidalotti PL, Pettinato C, Fanti S, Leone O, et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging. 2011;4(6):659–70. https://doi.org/10.1016/j.jcmg.2011.03.016.
Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R, et al. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol. 2016;1(8):880–9. https://doi.org/10.1001/jamacardio.2016.2839.
Musumeci MB, Cappelli F, Russo D, Tini G, Canepa M, Milandri A, et al. Low sensitivity of bone scintigraphy in detecting Phe64Leu mutation-related transthyretin cardiac amyloidosis. JACC Cardiovasc Imaging. 2020;13(6):1314–21. https://doi.org/10.1016/j.jcmg.2019.10.015.
Waddington-Cruz M, Schmidt H, Botteman MF, Carter JA, Stewart M, Hopps M, et al. Epidemiological and clinical characteristics of symptomatic hereditary transthyretin amyloid polyneuropathy: a global case series. Orphanet J Rare Dis. 2019;14(1):34. https://doi.org/10.1186/s13023-019-1000-1.
Lee HJ, Im DJ, Youn JC, Chang S, Suh YJ, Hong YJ, et al. Myocardial extracellular volume fraction with dual-energy equilibrium contrast-enhanced cardiac CT in nonischemic cardiomyopathy: a prospective comparison with cardiac MR imaging. Radiology. 2016;280(1):49–57. https://doi.org/10.1148/radiol.2016151289.
• Scully PR, Patel KP, Saberwal B, Klotz E, Augusto JB, Thornton GD, et al. Identifying cardiac amyloid in aortic stenosis: ECV quantification by CT in TAVR patients. JACC Cardiovasc Imaging. 2020;13(10):2177–89. https://doi.org/10.1016/j.jcmg.2020.05.029 Discusses the potential for CCTA to evaluate for cardiac amyloidosis in patients being imaged for TAVR planning.
McVeigh ER, Pourmorteza A, Guttman M, Sandfort V, Contijoch F, Budhiraja S, et al. Regional myocardial strain measurements from 4DCT in patients with normal LV function. J Cardiovasc Comput Tomogr. 2018;12(5):372–8. https://doi.org/10.1016/j.jcct.2018.05.002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dennis Toy, Lauren K. Groner, Joanna G. Escalon, Devrim Ersahin, Stacey V. Weisman, Alan C. Legasto and David M. Naeger declare that they have no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Imaging
Rights and permissions
About this article
Cite this article
Toy, D., Groner, L.K., Escalon, J.G. et al. Updates on the Role of Imaging in Cardiac Amyloidosis. Curr Treat Options Cardio Med 23, 11 (2021). https://doi.org/10.1007/s11936-020-00890-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s11936-020-00890-2