Abstract
Amyloidosis is an infiltrative disease caused by the extracellular deposition of misfolded proteins in organs and tissues resulting in progressive functional and structural impairment. Cardiac amyloidosis is a leading cause of morbidity and mortality in primary light-chain amyloid (AL) and transthyretin-related cardiac amyloid (ATTR) and results in a restrictive cardiomyopathy, heart failure, and death. With the emergence of new subtype-specific pharmacological therapies targeting misfolded amyloid fibrils, the need for early recognition of a cardiac amyloid subtype is of major therapeutic and prognostic importance. Recently, nuclear scintigraphy with radioactive bone-seeking tracers has shown promising results in distinguishing ATTR amyloidosis from AL amyloidosis and may potentially be useful in following disease burden. This review begins with an overview of cardiac amyloidosis and then focuses on the current radioactive tracers involved in cardiac amyloid detection and concludes with future outlook.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amyloid deposition in the heart is termed as cardiac amyloidosis and is an underappreciated cause of heart failure with preserved ejection fraction (HFpEF) [1]. Predilection for the myocardium may occur with any amyloidogenic protein; however, the three most clinically relevant pathophysiologic substrates for cardiac amyloidosis are primary light-chain amyloid (AL) amyloidosis, familial mutant transthyretin-related cardiac amyloid (ATTRm) amyloidosis, and senile wild-type ATTR (ATTRwt) amyloidosis. Other forms of cardiac amyloidosis including the acquired serum amyloid A (AA) type rarely infiltrate the heart [2].
AL amyloidosis is caused by the deposition of monoclonal immunoglobulin light chains and has an estimated incidence of 2500 cases annually with cardiac involvement in up to 50 % of cases [3]. ATTRm amyloidosis, due to genetically abnormal transthyretin, leads to familial amyloidotic cardiomyopathy (FAC) or familial amyloidotic polyneuropathy (FAP) in an autosomal dominant fashion. In North America, up to 3.9 % of African-Americans are heterozygous carriers of the commonest mutation, a valine to isoleucine substitution (V122I), leading to cardiomyopathy in an age-dependent penetrant manner [1]. ATTRwt cardiomyopathy, due to genetically normal transthyretin, affects as many as 30 % of patients with HFpEF aged ≥75 years [4]. With the aging population, ATTRm amyloidosis and ATTRwt cardiomyopathy are becoming increasingly recognized and now account for >50 % of our referrals.
Diagnosis of cardiac amyloidosis is challenging on clinical grounds alone because symptoms are nonspecific and otherwise common among the elderly. Endomyocardial biopsy (EMB) combined with immunohistochemistry and/or sequence analysis by mass spectroscopy to identify the precursor protein remains to be the gold standard for diagnosis and has been given a class IIa recommendation by the American College of Cardiology (ACC) [5]. Although highly sensitive, EMB is limited to specialized centers and does not provide information on cardiac amyloid burden, disease progression, prognosis, or response to treatment. Complications are 6 % and include arrhythmia, perforation with pericardial tamponade, accidental arterial puncture, and pneumothorax [6], though in our experience, these complications, especially perforation, are less common.
Although cardiac involvement occurs in AL amyloidosis and ATTR amyloidosis, the two subtypes vastly differ in their therapeutic interventions and prognosis. For instance, the clinical course in AL cardiac amyloidosis is more rapidly progressive than that in ATTR amyloidosis [7–9] with median survival of 11 months in AL amyloidosis compared to 75 months in ATTRwt amyloidosis [9].
Treatment of AL amyloidosis is aimed at clonal plasma cells by chemotherapy. In contrast, therapies of ATTR amyloidosis include liver transplantation and, more recently, pharmacotherapies halting TTR misfolding and aggregation [10, 11]. One such drug, diflunisal, is a nonsteroidal anti-inflammatory drug (NSAID) that was recently shown to reduce the rate of progression in neurologic impairment and preserve the quality of life of patients with ATTRm-FAP [12••], and many of whom had cardiac involvement. Additional investigational drugs include tafamidis, a TTR stabilizer without NSAID side effects [13], and the use of antisense oligonucleotides [14, 15] and small interfering RNAs [16] to silence TTR expression.
While electrocardiogram, serum tests, echocardiographic findings, and cardiac magnetic resonance imaging (CMRI) can noninvasively aid in the diagnosis of cardiac amyloidosis, none can differentiate ATTR amyloidosis from AL amyloidosis. Recent advancements in cardiac nuclear imaging, specifically using bone-seeking tracers, can discriminate AL from ATTR. As new pharmacotherapies emerge to prevent TTR misfolding, the need to identify patients with TTR amyloid expeditiously is important. This review serves to highlight the current nuclear tracers in cardiac amyloid imaging.
Nuclear Imaging Modalities
The most clinically relevant nuclear tracers in cardiac amyloid detection are the bone-seeking tracers 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD) and 99mTc-pyrophosphate (99mTc-PYP) and the tracers that detect sympathetic denervation or 123I-metaiodobenzylguanidine (123I-MIBG) (Table 1). Recently, the bone-seeking tracers 99mTc-DPD and 99mTc-PYP have gained increasing recognition due to avid uptake in patients with ATTR with minimal uptake in those with AL.
SPECT Imaging
99mTc-DPD
The most studied bone-seeking tracer in cardiac amyloid detection is 99mTc-DPD. After the intravenous administration of 20 mCi of 99mTc-DPD, whole-body scans are taken at 5 min and 3 h (anterior and posterior projection) using a dual-head gamma camera with low-energy and high-resolution collimators. If there is positive myocardial uptake, chest SPECT is performed and images are acquired with an acquisition time of 25 s per frame using a 128 × 128 matrix.
In an initial small study by Perugini et al. of 25 patients with cardiac amyloidosis (10 with ATTRm, 5 ATTRwt, and 10 AL) confirmed with biopsy and immunohistochemistry or by genotyping with typical echocardiographic appearance, strong myocardial uptake of 99mTc-DPD was found in all 15 patients with ATTR amyloidosis compared to absent uptake in 10 patients with AL amyloidosis, suggesting the discriminatory ability of 99mTc-DPD for ATTR detection with 100 % sensitivity and 100 % specificity [17]. Additional studies of elderly patients with unexplained left ventricular hypertrophy and non-dilated left ventricles who had positive myocardial uptake of 99mTc-DPD showed that all 46 patients had a biopsy-proven ATTR [18], confirming the affinity of 99mTc-DPD for ATTR.
The accuracy of 99mTc-DPD to selectively bind to ATTR over AL was found in larger studies to be lower than previously thought due to unexpected tracer uptake in one third of patients with AL. In a cohort study of 79 patients (28 with ATTRm, 17 ATTRwt, and 34 AL) where tracer retention was calculated using a heart-to-whole body ratio (H/WB), sensitivity was 100 % and specificity was 88 %, using moderate to strong uptake as cutoff. Using visual scoring (0 = no uptake, 1 = mild uptake, 2 = moderate uptake, 3 = strong uptake), the positive predictive value (PPV) and negative predictive value (NPV) for a visual score (VS) of ≥1 were 80 and 100 %, respectively, compared to 100 and 68 % for a VS of ≥3. Using a VS of ≥2, 99mTc-DPD had a NPV of 100 % for excluding AL while positive cardiac uptake of 99mTc-DPD had a PPV of 88 % for ATTR [19••].
In addition to differentiating ATTR from cardiac AL, heart retention (HR) of 99mTc-DPD has a positive correlation with interventricular septal thickness and severity of cardiac amyloid deposition assessed by impaired longitudinal function [e.g., mitral annular plane systolic excursion (MAPSE)/tricuspid annular plane systolic excursion (TAPSE)] in patients with ATTRwt amyloidosis [20]. Furthermore, 99mTc-DPD myocardial uptake has a prognostic value in predicting major adverse cardiac events (MACEs), either alone or in combination with left ventricular (LV) wall thickness with highest event rates when LV wall thickness is >12 mm and H/WB is >7.5 [17].
Due to its affinity for ATTR compared to AL and prognostic value, 99mTc-DPD scintigraphy has been used throughout Europe for cardiac amyloid detection. Since this tracer is not available in the US market, a similar bone-seeking tracer, 99mTc-PYP, has recently shown similar promising results.
99mTc-PYP
After the intravenous administration of 15–25 mCi of 99mTc-PYP, whole-body planar images are taken 1 h post injection over 8 min using a dual-head SPECT/CT camera with low-energy and high-resolution collimators. If there was positive myocardial uptake, SPECT images were performed using a 64 × 64 matrix, a 1.46 zoom factor, and a Butterworth filter.
Many case reports dated from the early 1980s demonstrated myocardial uptake of 99mTc-PYP in patients with amyloid. In 1982, Wizeberg et al. reported diffuse cardiac uptake of 99mTc-PYP in all 10 patients with tissue-proven amyloid of unknown subtype, suggesting a possible role of 99mTc-PYP imaging in patients with biopsy-proven amyloid with suggestive echocardiographic features [21]. However, in a larger study of 34 patients with biopsy-proven amyloid (undefined subtype) and echocardiographic features, only 20 patients had positive uptake, a finding which was also noted in 15 out of 20 controls without amyloid heart disease [22]. These early studies casted doubt on the utility of 99mTc-PYP and warranted it as an insensitive method in cardiac amyloid detection.
Recent studies have found that 99mTc-PYP preferentially binds to ATTR with less affinity for AL, a finding that can explain the varying sensitivities of earlier studies which did not define an amyloid subtype. In 2012, Yamamoto et al. used a quantitative method, the “PYP score,” to assess the utility of 99mTc-PYP in differentiating subjects with heart failure due to amyloid (one with AL, one AA, three ATTRm, eight ATTRwt) from heart failure due to non-amyloid etiologies (n = 37). PYP score, defined as the ratio of myocardial mean counts to ventricular cavity mean counts, was found to have 84.6 % sensitivity and 94.5 % specificity for distinguishing cardiac amyloidosis from non-amyloid heart failure [23•].
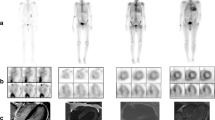
In a study by Bokhari et al. that enrolled 45 subjects (12 with AL, 16 ATTRwt, and 17 ATTRm) with cardiac amyloidosis (37 EMB proven), 99mTc-PYP SPECT was able to selectively bind to ATTR with good sensitivity and specificity. Cardiac retention was assessed using both a semiquantitative visual score (see Fig. 1) in relation to bone uptake (0 = no cardiac uptake to 3 = high uptake greater than bone) and a quantitative analysis by drawing a region of interest (ROI) over the heart corrected for contralateral counts and calculating a heart-to-contralateral ratio (H/CL). Although a cohort with AL had some tracer uptake, patients with ATTR had significantly higher semiquantitative cardiac VS as well as quantitative scores. H/CL ≥ 1.5 consistent with intensely diffuse myocardial tracer retention had 97 % sensitivity and 100 % specificity for identifying ATTR cardiac amyloidosis [24••]. In addition, the degree of cardiac tracer retention correlated with LV thickness and mass similar to prior studies with 99mTc-DPD [19••].
a–c Quantitative method of calculating the distribution of 99mTc-PYP uptake. Raw images of (a) a representative negative subject and (b) a positive subject are shown 1 h after radiotracer infusion. ROI circles are depicted in green, and the contralateral comparison circle is depicted in blue. SPECT image of (c) a patient with ATTR amyloidosis showing predominantly apical septal uptake. ROI region of interest, CL contralateral, cts counts, Std Dev standard deviation
Several points are notable in this study. First, a simple nuclear scan can distinguish ATTR from cardiac AL with good sensitivity and specificity using a quantitative method (H/CL ≥ 1.5), eliminating potential reader bias from semiquantitative methods. Second, although subjects with ATTRwt had greater LV mass and wall thickness, those with AL and ATTRm did not, and yet a H/CL was significantly higher in patients with ATTRm, suggesting that 99mTc-PYP has a unique affinity for ATTR. Finally, several limitations are worth noting. This was a small single-center study wherein no control group (left ventricular hypertrophy without amyloid) was included for comparison, so the absence of uptake may mean AL or non-amyloid heart disease. Many patients had severe phenotypes with thickened LV walls. In addition, a large percentage of patients with ATTRm had the V122I mutation which is the commonest mutation in the USA. Despite these limitations, 99mTc-PYP shows promise in detecting ATTR and is readily available in the USA.
123I-MIBG
123I-MIBG is a norepinephrine analog that undergoes little enzymatic breakdown and therefore provides an objective measure of cardiac sympathetic denervation due to amyloid deposition. After the intravenous administration of 5 mCi of 123I-MIBG, static anterior chest images are taken at 15 min (early) and 4 h (delayed) using a gamma camera with a medium-energy low-penetration parallel-hole collimator. After ROI is set in the heart and mediastinum, cardiac 123I-MIBG uptake is quantified via a heart-to-mediastinum ratio (H/M) and a washout rate.
A number of case reports [25–28] in patients with ATTRm-FAP have shown absent myocardial uptake of 123I-MIBG, indicating a lack of sympathetic activity due to amyloid deposition. In a study of 17 patients with FAP confirmed by rectal and nerve biopsy, cardiac 123I-MIBG uptake was significantly decreased in patients with FAP with no difference in washout rates despite preserved left ventricle systolic function and cardiac perfusion. In addition, the severity of polyneuropathy had a negative correlation with MIBG uptake at 4 h [28], suggesting that 123I-MIBG imaging may detect early cardiac amyloid specifically in FAP, which is characterized by early autonomic nervous system involvement. The utility of 123I-MIBG in patients with ATTRwt and ATTRm-FAC who do not present with predominant manifestations of autonomic dysfunction requires additional studies.
123I-MIBG was also used to examine a cohort of 25 patients with AL, and it was found that myocardial uptake and turnover of 123I-MIBG is heterogeneous and dependent on the presence or absence of heart failure and cardiac autonomic dysfunction [29]. In a more recent study of 61 patients (39 with AL, 11 AA, and 11 ATTRm), late MIBG H/M was significantly lower with higher washout rates irrespective of the amyloid subtype compared to healthy controls in patients who had echocardiographic features of amyloidosis. Furthermore, patients with ATTRm without echocardiographic signs of amyloidosis had lower H/M compared to the other subtypes (AL and AA), a finding that may be related to concomitant neuropathic involvement. The author concluded that 123I-MIBG scintigraphy can detect cardiac denervation in patients with ATTRm before signs of amyloidosis appear on echocardiogram [30•].
The prompt detection of cardiac denervation with 123I-MIBG in patients with ATTRm-FAP is important as sympathetic denervation occurs early in the disease, and all current therapies under investigation, including liver transplantation [31], are aimed at halting disease progression and not at removing a preexisting amyloid. 123I-MIBG myocardial imaging has been used in Europe and Japan, and recently, the US Food and Drug Administration (FDA) has approved this tracer for the assessment of myocardial sympathetic innervation in patients with New York Heart Association (NYHA) class II or class III heart failure and left ventricular ejection fraction (LVEF) <35 %.
Positron Emission Tomography Imaging
There is limited data on the use of positron emission tomography (PET) for cardiac amyloid detection. Pittsburgh compound B (11C-PIB), a tracer designed for β-amyloid in Alzheimer disease, is believed to bind to amyloid fibrils of any type. In a study of 10 patients with amyloid (seven with AL, two ATTRm, and one ATTRwt) with cardiac involvement (five biopsy proven), 11C-PIB uptake was seen in all patients 15–25 min after injection of the tracer compared to absent uptake in five controls, suggesting its possible role in cardiac amyloid imaging [32]. Other PET tracers include 11C-BF-227, which has shown significant cardiac retention compared to a control in a patient with ATTRm amyloidosis [46], and 124I-m11-1F4, a murine amyloid-reactive monoclonal antibody, which are currently in phase I clinical trial developed for passive immunotherapy in patients with AL [33].
Recently, the PET tracer 18F-florbetapir was FDA approved and used to visualize beta-amyloid plaques in the brain in Alzheimer patients. In an open-label, multicenter brain imaging study of 18F-florbetapir PET imaging on 32 patients (16 Alzheimer patients and 16 controls), mean cortical standardized uptake value ratios (SUVRs) were significantly higher in Alzheimer patients compared to healthy controls [34]. The role of 18F-florbetapir PET imaging for cardiac amyloidosis is currently in clinical trial (NCT01683825).
Non-nuclear Imaging Modalities
Echocardiography is universally available and is one of the first tests utilized in suspected cardiac amyloid. Classic echocardiographic features are neither sensitive nor specific and commonly present in advanced stages of disease [35, 36]. Patients with cardiac amyloid are more likely to have bi-atrial dilation in addition to biventricular, valvular, and septal thickening. Ultrasound may show granular echogenicity, diastolic dysfunction, and preserved ejection fraction. Advanced echocardiographic techniques including tissue Doppler imaging and strain and strain rate measurements may detect early cardiac changes [37].
CMRI is highly reproducible and may provide functional and morphological information of cardiac amyloid. Deposition of amyloid proteins in the myocardium results in changes in tissue composition and architecture and can be visualized as late gadolinium enhancement (LGE) on T1-weighted images differentiating it from other infiltrative cardiomyopathies [38]. The presence of qualitative global and subendocardial LGE was associated with increased ventricular mass and impaired left ventricular systolic function [39]. In addition to LGE, patients with cardiac amyloid have faster gadolinium washout from the blood. More recently, the use of noncontrast T1 mapping in patients with AL offers promise in patients with severe renal impairment in which gadolinium is contraindicated [40].
Conclusion
Cardiac amyloidosis is a challenging diagnosis. While EMB remains to be the gold standard for cardiac amyloid detection, it is performed at specialized centers, but it is not a minimal-risk procedure and does not provide information on the extent of myocardial infiltration, disease progression, or response to treatment. With the emergence of innovative pharmacotherapies aimed at halting ATTR misfolding and aggregation, the need to diagnose cardiac amyloid expeditiously becomes crucial. Recent advancements in cardiac nuclear imaging have shown promising results, specifically the bone-seeking tracers (99mTc-DPD and 99mTc-PYP) which can discriminate ATTR from AL with good accuracy.
There remain many questions that need attention before the widespread adaptation of nuclear imaging modalities for cardiac amyloid detection. First, the role of 123I-MIBG imaging for early cardiac amyloid detection before echocardiographic features was studied mainly in patients with FAP who present with primary autonomic dysfunction. Whether this can be generalized to patients with FAC who do not have predominant autonomic dysfunction needs further investigation. Second, 99mTc-PYP cardiac imaging was studied in patients who had advanced cardiac involvement. Can 99mTc-PYP be used to detect a subclinical cardiac amyloid in patients with less severe phenotypes? Finally, the role of the bone-seeking tracers in following disease progression and response to anti-TTR therapies needs investigation.
There has been great progress in the field of nuclear imaging for myocardial amyloid detection providing hope for better outcomes in patients with amyloid. The role of PET tracers for potential cardiac imaging is under trial. Studies to identify the role of bone-seeking tracers for early cardiac amyloid detection and to quantify the amount of amyloid burden with serial scans remain to be investigated. Do bone-seeking tracers for cardiac amyloid detection have widespread use in the foreseeable future?
Abbreviations
- ATTRm:
-
Mutant transthyretin
- ATTRwt:
-
Wild-type transthyretin
- EMB:
-
Endomyocardial biopsy
- FAC:
-
Familial amyloidotic cardiomyopathy
- FAP:
-
Familial amyloidotic polyneuropathy
- HFpEF:
-
Heart failure with preserved ejection fraction
- MIBG:
-
Metaiodobenzylguanidine
- PYP:
-
Pyrophosphate
- DPD:
-
Diphosphono-1,2-propanodicarboxylic acid
- ROI:
-
Region of interest
- H/M:
-
Heart-to-mediastinum ratio
- H/CL:
-
Heart-to-contralateral ratio
- 11C-PIB:
-
Pittsburgh compound B
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dharmarajan K, Maurer MS. Transthyretin cardiac amyloidoses in older North Americans. J Am Geriatr Soc. 2012;60(4):765–74.
Desai HV, Aronow WS, Peterson SJ, Frishman WH. Cardiac amyloidosis: approaches to diagnosis and management. Cardiol Rev. 2010;18(1):1–11.
Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91(2):141–57.
Sultan AM, Edwards WD, Mohammed SF, et al. Cardiac amyloid deposition is common in elderly patients with heart failure and preserved ejection fraction. Circulation. 2010;122.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013.
Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116(19):2216–33.
Dubrey SW, Cha K, Skinner M, LaValley M, Falk RH. Familial and primary (AL) cardiac amyloidosis: echocardiographically similar diseases with distinctly different clinical outcomes. Heart. 1997;78(1):74–82.
Gertz MA, Kyle RA, Thibodeau SN. Familial amyloidosis: a study of 52 North American-born patients examined during a 30-year period. Mayo Clin Proc. 1992;67(5):428–40.
Ng B, Connors LH, Davidoff R, Skinner M, Falk RH. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Arch Intern Med. 2005;165(12):1425–9.
Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52(4):347–61.
Macario AJ, Conway de Macario E. Sick chaperones and ageing: a perspective. Ageing Res Rev. 2002;1(2):295–311.
Berk JL, Suhr OB, Obici L, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310(24):2658–67. This is a multicenter, randomized double-blind trial showing that the use of diflunisal in patients with ATTRm-FAP decreased progression of neurological impairment and improved quality of life after a 2-year follow-up compared to placebo. This trial reinforces the need to diagnose cardiac amyloidosis early as potential therapies may halt progression of disease.
Coelho T, Maia LF, Martins da Silva A, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79(8):785–92.
Sah D. Phase I safety, pharmacokinetic and pharmacodynamics results for ALN-TTR01, a novel RNAi therapeutic for the treatment of transthyretin amyloidosis. VIIIth International Symposium on Familial Amyloid Polyneuropathy. 2011.
Benson MD, Pandey S, Witchell D, et al. Antisense oligonucleotide therapy for TTR amyloidosis. Amyloid. 2011;18 Suppl 1:60.
Coelho T, Adams D, Silva A, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369(9):819–29.
Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46(6):1076–84.
Quarta CC, Guidalotti PL, Longhi S, et al. Defining the diagnosis in echocardiographically suspected senile systemic amyloidosis. JACC Cardiovasc Imaging. 2012;5(7):755–8.
Rapezzi C, Quarta CC, Guidalotti PL, et al. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2011;38(3):470–8. This is the largest trial validating the role of 99m Tc-DPD in cardiac amyloid imaging. Although with less accuracy than previously thought, 99m Tc-DPD can detect a cardiac amyloid with overall good sensitivity and specificity.
Kristen AV, Haufe S, Schonland SO, et al. Skeletal scintigraphy indicates disease severity of cardiac involvement in patients with senile systemic amyloidosis. Int J Cardiol. 2011.
Wizenberg TA, Muz J, Sohn YH, et al. Value of positive myocardial technetium-99m-pyrophosphate scintigraphy in the non-invasive diagnosis of cardiac amyloidosis. Am Heart J. 1982;103(4 Pt 1):468–73.
Gertz MA, Brown ML, Hauser MF, Kyle RA. Utility of technetium Tc 99m pyrophosphate bone scanning in cardiac amyloidosis. Arch Intern Med. 1987;147(6):1039–44.
Yamamoto Y, Onoguchi M, Haramoto M, et al. Novel method for quantitative evaluation of cardiac amyloidosis using (201)TlCl and (99m)Tc-PYP SPECT. Ann Nucl Med. 2012;26(8):634–43. This was the first trial using a quantitative method, “PYP score,” to distinguish heart failure due to amyloid etiologies from heart failure due to non-amyloid etiologies.
Bokhari S, Castaño A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. 99mTc-Pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6(2):195–201. This is the first trial using an objective quantitative method for distinguishing ATTR from AL. 99m Tc-PYP is widely available in the USA and was found to have good sensitivity and specificity for identifying ATTR.
Nakata T, Shimamoto K, Yonekura S, et al. Cardiac sympathetic denervation in transthyretin-related familial amyloidotic polyneuropathy: detection with iodine-12-MIBG. J Nucl Med. 1995;36(6):1040–2.
Arbab AS, Koizumi K, Toyama K, et al. Scan findings of various myocardial SPECT agents in a case of amyloid polyneuropathy with suspected myocardial involvement. Ann Nucl Med. 1997;11(2):139–41.
Tanaka M, Hongo M, Kinoshita O, et al. Iodine-123 metaiodobenzylguanidine scintigraphic assessment of myocardial sympathetic innervation in patients with familial amyloid polyneuropathy. J Am Coll Cardiol. 1997;29(1):168–74.
Delahaye N, Dinanian S, Slama MS, et al. Cardiac sympathetic denervation in familial amyloid polyneuropathy assessed by iodine-123 metaiodobenzylguanidine scintigraphy and heart rate variability. Eur J Nucl Med. 1999;26(4):416–24.
Hongo M, Urushibata K, Kai R, et al. Iodine-123 metaiodobenzylguanidine scintigraphic analysis of myocardial sympathetic innervation in patients with AL (primary) amyloidosis. Am Heart J. 2002;144(1):122–9.
Noordzij W, Glaudemans AW, Rheenen RW, et al. (123)I-Labelled metaiodobenzylguanidine for the evaluation of cardiac sympathetic denervation in early stage amyloidosis. Eur J Nucl Med Mol Imaging. 2012;39(10):1609–17. This trial demonstrates the potential of 123 I-MIBG in detecting early cardiac amyloid, specifically in patients with ATTRm. This will be important with the emergence of new pharmacotherapies available for cardiac amyloid.
Delahaye N, Rouzet F, Sarda L, et al. Impact of liver transplantation on cardiac autonomic denervation in familial amyloid polyneuropathy. Med (Baltimore). 2006;85(4):229–38.
Antoni G, Lubberink M, Estrada S, et al. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J Nucl Med. 2012.
Wall JS, Kennel SJ, Stuckey AC, et al. Radioimmunodetection of amyloid deposits in patients with AL amyloidosis. Blood. 2010;116(13):2241–4.
Wong DF, Rosenberg PB, Zhou Y, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F18). J Nucl Med. 2010;51(6):913–20.
Cueto-Garcia L, Tajik AJ, Kyle RA, et al. Serial echocardiographic observations in patients with primary systemic amyloidosis: an introduction to the concept of early (asymptomatic) amyloid infiltration of the heart. Mayo Clin Proc. 1984;59(9):589–97.
Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112(13):2047–60.
Sun JP, Stewart WJ, Yang XS, et al. Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am J Cardiol. 2009;103(3):411–5.
Penugonda N. Cardiac MRI in infiltrative disorders: a concise review. Curr Cardiol Rev. 2010;6(2):134–6.
Kwong RY, Falk RH. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111(2):122–4.
Karamitsos TD, Piechnik SK, Banypersad SM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6(4):488–97.
Compliance with Ethics Guidelines
Conflict of Interest
Sabahat Bokhari and Reehan Shahzad declare that they have no conflict of interest.
Dr. Maurer reports grants from Pfizer, Inc, grants from Alnylam Pharmaceuticals, Inc, during the conduct of the study; and Dr. Maurer's institution has received fees for serving on the Steering Committee of a clinical trial being conducted by Pfizer Inc (ATTR-ACT) and for consulting for Alnylam Pharmaceuticals, Inc and ISIS Pharmaceuticals, Inc.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Cardiac Nuclear Imaging
Rights and permissions
About this article
Cite this article
Bokhari, S., Shahzad, R. & Maurer, M. Radionuclide Imaging in Cardiac Amyloidosis: Are Nuclear Bone Tracers a Foreseeable Future?. Curr Cardiovasc Imaging Rep 8, 3 (2015). https://doi.org/10.1007/s12410-014-9317-6
Published:
DOI: https://doi.org/10.1007/s12410-014-9317-6