Abstract
Cardiac resynchronization therapy (CRT) has proven to improve quality of life, reduce heart failure hospitalization, and prolong life in selected heart failure patients with reduced ejection fraction, on optimal medical therapy and with electrical dyssynchrony. To ensure maximal benefit for CRT patients, optimization of care should be implemented. This begins with appropriate referring as well as selecting patients, knowing that the presence of left bundle branch block and QRS ≥ 150 ms is associated with the greatest reverse remodeling. The LV lead, preferably quadripolar, is best targeted in a postero-lateral position. After implantation, optimal device programming should aim for maximal biventricular pacing and in selected cases further electrical delay optimization might be of use. Even as important, is the implementation of thorough multidisciplinary heart failure care with medication uptitration, remote monitoring, rehabilitation, and patient education. The role of newer pacing strategies as endocardial or His-bundle pacing remains the subject of ongoing investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since its first introduction 25 years ago, cardiac resynchronization therapy (CRT) has become a fundamental part of heart failure (HF) therapy, supported by a large body of evidence. Current American and European guidelines recognize CRT as a class I life-saving therapy in HF with reduced ejection fraction (HFrEF) patients with a left bundle branch block (LBBB) and QRS ≥ 150 ms in sinus rhythm [1, 2]. In addition, most guidelines also suggest the use of CRT in patients with a less wide QRS (130–149 ms), a non-LBBB morphology, atrial fibrillation, or patients with moderate left ventricular (LV) dysfunction needing ventricular pacing. In approximately one third of CRT implants, a so-called non-response is reported, meaning that certain goals of improvement are not reached within the desired time frame [3]. Depending on the study, these goals can be either clinical improvement (measured as New York Heart Association class, quality of life questionnaires, 6-min walk test, and/or cardiopulmonary exercise test), reverse remodelingined as a decrease of 15% in LV end-systolic or end-diastolic volume or an increase in ejection fraction), or a reduction in events (HF hospitalizations and/or death). In addition to the large heterogeneity in defining non-response, the concept of non-progression has been the focus of more attention lately. Indeed, the natural history of HF is progressive of nature [4] and even stabilization should be regarded as treatment success in selected cases. For example, in 40 advanced HF patients with clinical deterioration after CRT implant, biventricular pacing (BiV) continued to provide hemodynamic benefits compared to a non-paced rhythm [5]. Also, the relevance of measuring the response to CRT therapy can be questioned, as it is not performed for other HF therapies such as ACE inhibitors or beta-blockers. Historically, CRT optimization has focused on the optimization of different pacing intervals to maximize hemodynamics. However, given the widespread underutilization of CRT and failing post-implant HF care, we propose that CRT optimization requires a more holistic approach (Table 1 and Fig. 1).
Different views on optimization. Left panel represents classic view on optimization, focusing on device programming. Right panel represents a holistic approach towards CRT optimization. The patient with his device is central. Pictograms represent patient selection (top), rehabilitation (following clockwise), patient education, remote monitoring, treating comorbidities, and heart failure medication uptitration.
Pre-implantation
Patient selection
A key issue and probably one of the most important aspects in optimization is patient referral. Historically, the number of HF patients eligible for CRT has been estimated at around 10% [1, 6]. More recent registries suggest that up to 27% of HFrEF patients have a class I–IIa indication according to current guidelines [7•, 8]. However, in reality, only a minority of these patients eventually receives a CRT device. In the ESC Heart Failure Long Term Registry, containing follow-up data from 7401 European HF patients between 2011 and 2013, CRT was indicated in around one fifth of cases, yet 40% of indicated patients were not implanted because of physician uncertainties or patient refusal [8]. The underutilization was even more pronounced in the Swedish HF registry where only 6.8% of HFrEF patients had a CRT, while 26.8% had a class I–IIa recommendation [9]. In the USA, underutilization has also been reported in the Get With The Guidelines Registry, where only 30% of eligible patients received a CRT [10]. The same holds true for large randomized controlled HF trials. For example, in the PARADIGM (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial, only 7% had a CRT [11]. Several reasons for underutilization can be given, including physician inertia, misinterpretation of benefit of CRT, perceived risks, need for referral to another center, cost issues, and patient reluctance [12]. If CRT optimization is interpreted on a population level, a better implementation of guidelines and not withholding patients from a lifesaving therapy should be a more important aspect of optimization. As such, the number one reason for a non-response is not getting the device. Education can facilitate better CRT adoption in clinical practice [13].
Contemporary CRT registries indicate that patients have a higher comorbidity burden and are significantly older than the landmark trial patients. For instance, in the ESC CRT-survey II [14], the median age was 70 years with 32% of total patients being ≥ 75 years. USA data are comparable with 23% of patients older than 80 years and an increasing comorbidity burden over the past 10 years [15•]. This might suggest that often CRT is postponed until later disease stages [9], despite data indicating that early implantation yields a higher benefit [16]. However, the presence of comorbidities does not affect the benefit from CRT [17••].
As CRT is aimed at treating dyssynchrony and given the varying rate of reverse remodeling in guideline indicated patients, some have advocated to add echocardiographic dyssynchrony parameters to the current ECG criteria to have a better patient selection. However, in the multicenter PROSPECT (Predictors of Response to CRT) trial, this hypothesis was refuted as no single parameter was neither sensitive nor specific enough to predict a significant decrease in LV end-systolic volume (LVESV) [18]. In addition, in HFrEF patients with normal conduction, but with echocardiographic signs of dyssynchrony, CRT increased mortality [19]. Thus, echocardiographic signs of dyssynchrony cannot be used to rule out or rule in CRT. Imaging prior to implantation should be reserved to the confirmation of HFrEF and can play a role in assessing the mechanism of dyssynchrony and the presence of structural heart disease or scar [20].
Peri-implantation
Implantation strategy
Anatomy guided
During a standard CRT implantation, the LV lead is preferably positioned in a suitable posterolateral or lateral branch of the coronary sinus vein, as these zones are latest activated in patients with a typical LBBB [1] thus allowing maximal resynchronization. This is further supported by post hoc analyses of landmark CRT randomized control trials [21,22,23] showing a reduction in HF hospitalization and mortality with a posterolateral or lateral LV lead position. Apical positions should be avoided because of an increased mortality [23•]. In general, it is recommended to keep the distance between RV and LV lead as far as possible [24, 25].
Electrical delay guided
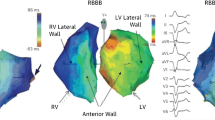
Because the activation pattern in LBBB and other forms of conduction delay are not always homogeneous in the HF population, the latest activated zone might not always be congruent with the posterolateral lead position [26]. As such, an alternative approach might be to target the latest electrically activated zone. During the procedure, the delay between the onset of the QRS complex on the surface ECG and the first activation at the tip of the LV lead can be measured and is called the QLV time. Longer QLV-times are associated with improved reverse remodeling and quality of life [27], as well as reduced HF hospitalization and death [28, 29]. However, the longest QLV is located in the posterolateral region in the large majority of cases, indicating that this is indeed the sweet spot. Moreover, since the introduction of quadripolar leads, more pacing options are easily available for the operator, even in case of few anatomical options, improving QLV feasibility. The current approach of many operators is to introduce a quadripolar lead in a suitable posterolateral vein, sequentially test the different poles and eventually select the pole with the longest QLV and the lowest pacing threshold without phrenic nerve stimulation. Two ongoing trials investigate whether a QLV targeted approach is beneficial compared to standard LV lead implantation (DANISH-CRT NCT03280862, ENHANCE CRT NCT01983293 [30]).
Imaging guided
Some investigators have advocated to primarily select the latest mechanically activated zone rather than the latest electrically activated zone for lead implantation. In the TARGET (Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronization Therapy) study [31], 220 patients were randomized to either targeted LV lead implantation, guided by 2D speckle tracking echocardiography identifying the latest zone of mechanical activation or routine non-guided LV placement in a lateral or posterolateral vein. After 6 months, there was a higher proportion of patients meeting the LVESV reduction threshold of ≥ 15% in the echocardiographic guided group compared to controls. Also, a higher clinical response and a reduction in combined all-cause mortality and HF-related hospitalization were noted. These results were confirmed by the STARTER (Speckle Tracking Assisted Resynchronization Therapy for Electrode Region) trial [32], where a similar approach in 187 patients led to a significant reduction in the combined end point of HF hospitalization or death in the echocardiography-guided group. However, despite these positive results, pre-implantation imaging to identify the target implantation site has not been widely adopted in routine clinical practice. Several reasons can be given. First, adequate echocardiographic imaging and especially speckle tracking is often not feasible because of poor acoustic windows, occurs off-line, and is time-consuming. Second, in the majority of guided cases, the LV lead is positioned in a lateral or posterolateral branch, which is also the main target of non-guided procedures. Third, technical issues including unsuitable anatomy, unstable lead position, high-pacing thresholds, and phrenic nerve stimulation might hamper the implantation success in targeted regions. Fourth, pacing from the latest mechanical activation also induces a different activation pattern. Moreover, in post hoc analyses it seems that avoiding scar zones is the main driver of outcome benefits rather than implantation in the zone of the latest mechanical activation [33, 34].
Other implantation strategies with the intention to avoid scar to improve CRT response have also been described. One single-center study suggested that implantation in non-scar regions guided by cardiac magnetic resonance reduces HF hospitalization or death [35]. However, these results need confirmation in larger randomized studies. Further, different forms of multimodality imaging to guide implantation have been introduced [36]. Although promising results have been published, these forms of imaging are laborious, time-consuming, and not applicable in routine clinical practice to date.
Lead and pacing configuration
Quadripolar leads
Quadripolar leads have a tip electrode and three ring electrodes, offering more programmable pacing vectors compared to classic bipolar leads. Given the often encountered implantation difficulties such as unfavorable anatomy, phrenic nerve stimulation, instable lead position, or high-pacing threshold, these quadripolar leads are currently the most used. In the MORE-CRT (More Options Available With a Quadripolar LV Lead Provide In-Clinic Solutions to CRT Challenges) trial [37••], the lead-related event rate was compared in 1074 patients randomized to either bipolar or quadripolar lead implantation. Quadripolar leads were associated with more than 50% reduction in intra-operative events, driven by a reduction in implant failure rate as well as less phrenic nerve stimulation, lower pacing thresholds, and less lead instability. Quadripolar leads have also been associated with reduced mortality, deactivation, and replacement need compared to bipolar leads [38]. If available, quadripolar leads are thus currently the first choice.
Multipoint pacing
Pacing from multiple sites simultaneously along a multipolar lead, or so called multipoint pacing (MPP), is a new option since the introduction of quadripolar leads. MPP can initiate larger activation wavefronts, possibly enhancing resynchronization and CRT response. Several studies have shown an improved acute hemodynamic response of MPP compared to conventional BiV [39, 40], and a large prospective Italian registry suggests a greater improvement in clinical composite score and LV ejection fraction [41]. However, the only randomized trial has failed to show benefit of MPP over conventional BiV in converting non-response to response, defined as a > 15% reduction in LVESV [42•]. Though other trials are ongoing, routine use of MPP cannot be advised, especially in the light of the higher battery use associated with MPP versus classic BiV.
Multisite pacing
Multisite pacing (MSP) is an alternative to MPP and was already introduced before the availability of multipolar leads. Using two bipolar LV leads in addition to the conventional RV apical and right atrial lead, different LV sites can be stimulated simultaneously. Small studies have suggested that MSP can increase CRT response [43, 44] and possibly reduce ventricular arrhythmias and mortality [45]. In contrast, in the only randomized study, there was no benefit of MSP over BiV in terms of clinical or echocardiographic improvement, but MSP was associated with a higher peri-procedural complication rate [46]. Since quadripolar leads are a lot easier to implant, have a shorter procedural and fluoroscopy time, require less hardware, and are implanted according to the same principle of maximizing the activation wavefront, future developments will probably mainly focus on MPP.
Post-implantation
Device optimization
AV and VV optimization
CRT is a treatment of dyssynchrony in HF, which is composed of intraventricular, interventricular (VV), and atrioventricular (AV) dyssynchrony. After device implantation, programming should be aimed at maximizing the resynchronization of these different components. Various techniques for AV and VV optimization, using echocardiography, electrocardiography, or invasive hemodynamical evaluation, have been described [47,48,49]. Despite evidence of hemodynamic improvement with AV optimization [50], the routine implementation of this strategy did not yield the expected benefit in trials. In the SMART-AV (The SmartDelay Determined AV Optimization: A Comparison to Other AV Delay Methods Used in Cardiac Resynchronization Therapy) [51] and FREEDOM (Frequent Optimization Study Using the QuickOpt Method) trial [52] echocardiographic optimization of the AV interval and an automated device algorithm were not better than an “out-of-the-box” setting of 100–120 ms in terms of reverse remodeling and clinical composite score, respectively. The role of VV optimization is even less clear, as evidence is limited to small trials with conflicting results [53,54,55]. In addition, observational data indicate that AV and VV optimization are not performed in the majority of CRT implants, due to its time-consuming nature [56]. Current guidelines thus do not recommend routine AV and VV optimization and suggest to reserve this strategy for initial non-responders [1, 2].
As the optimal AV and VV interval at rest can change over time and during exercise, newer automated device algorithms that address this dynamic issue have recently been developed. The AdaptivCRT algorithm (Medtronic, Inc., Mounds View, Minnesota) provides automatic adjustments to AV and VV intervals and selects between BiV or LV only pacing according to the measured intrinsic AV conduction and heart rate. This algorithm was non-inferior to echocardiographic optimization in a recent study in patients with normal AV conduction and LBBB and resulted in a 44% reduction of RV pacing [57]. Whether AdaptivCRT can reduce mortality and HF hospitalization is the subject of an ongoing prospective trial [58]. Another new automated algorithm uses the SonR sensor (LivaNova, Paris, France), which is a micro-accelerometer embedded in the tip of the right atrial lead. The sensor measures cardiac muscle vibrations, correlated with dP/dt and thus reflecting contractility. The device uses these measurements to optimize AV and VV intervals on a weekly basis, at rest, and during exercise. In the RESPOND CRT (SonRtip lead and automatic AV-VV optimization) trial, the use of this algorithm was non-inferior to echocardiographic optimization in the rate of clinical response after 12 months [59•]. Of note, after a mean follow-up of 548 days, there was a 35% risk reduction in HF hospitalization.
Biventricular pacing optimization
A key issue and probably the most important step towards effective CRT programming is maximizing the amount of BiV to as close to 100% as possible [1]. Several studies have consistently shown that BiV > 92% [60] or > 98% [61] is associated with decreased mortality and HF hospitalization. Therefore, at every follow-up, the device counter should be checked and effective BiV should be electrocardiographically confirmed. In case of low BiV, special attention should be given to inadequate AV delay programming, loss of Biv during exercise, or the presence of arrhythmias, as these are the most common causes [62]. However, ineffective sensing or pacing, also leading to loss of BiV, might not influence the counters and should be kept in mind as well. In case of atrial fibrillation, AV junctional ablation should strongly be considered, especially if other rate or rhythm control strategies fail to improve the percentage of BiV [1, 2].
Heart failure care optimization
Heart failure therapy uptitration
As CRT only treats a piece of HF pathophysiology (i.e., dyssynchrony), after implantation, the focus should not only be on the device, but also on HF therapy optimization. Data from the IMPROVE-HF (Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting) registry indicate that prior to CRT implant, most HF patients are treated with sub-optimal doses of neurohormonal blockers [63]. CRT can often reduce several reasons why further uptitration of neurohormonal blockers was not possible before CRT implant, such a bradycardia, AV conduction disorders, or low blood pressures. Uptitration of neurohormonal blockade was indeed feasible in more than half of CRT patients and was associated with a reduction in mortality and HF hospitalization in a retrospective analysis of 650 patients [64•]. In addition, loop diuretic downtitration could often be performed, especially in patients with non-ischemic cardiomyopathy and improved LVEF, and was also associated with improved outcomes [65]. Despite being on maximal tolerated doses prior to CRT implantation, hemodynamic improvements after CRT might reduce the needs for diuretics and facilitate medication uptitration. Other retrospective studies have also shown the association between higher doses of neurohormonal blockade and survival free of HF hospitalization after CRT [66,67,68]. The implementation of a post-implant HF protocol incorporating primary care physicians might overcome the shortcomings of current practice as this approach, combining medication uptitration, device optimization, arrhythmia management, and HF education in a multidisciplinary setting led to improved reverse remodeling and fewer adverse events in a single center study [69].
Rehabilitation
Exercise training reduces HF hospitalization and mortality [70] and is a class I indication according to current European HF guidelines [71]. Several studies evaluated the value of exercise training after CRT implantation. Exercise training not only improves symptoms, exercise capacity, and quality of life [72,73,74], but might also improve outcomes [75]. In contrast, a subanalysis of the randomized HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial could not show any benefit in a large (non-CRT) HF patient population on HF hospitalization or death, possibly due to insufficient power [74]. Nevertheless, exercise training itself after CRT is feasible, safe, and might improve patient outcome.
Remote monitoring
With remote monitoring, close monitoring of the outpatient’s amount of BiV, presence of arrhythmias, impeding pulmonary fluid accumulation, and device integrity is possible. Whether early intervention triggered by remote monitoring information of contemporary devices might lead to improved outcomes was tested in different trials. The IN-TIME (Influence of Home Monitoring on Mortality and Morbidity in Heart Failure Patients with Impaired Left Ventricular Function) trial [76] randomized 716 NYHA class II–III patients undergoing ICD or CRT-D implantation to either remote monitoring with daily data transmission or routine follow-up. After 1 year, remote monitoring reduced the composite clinical score as well as all-cause mortality. The recent MORE-CARE trial with 865 randomized CRT-D patients could not reproduce these results but showed a significant reduction in healthcare resource utilization [77]. In addition, home telemonitoring may be considered for patients with HF in order to reduce the risk of recurrent cardiovascular and HF hospitalizations and cardiovascular death [78]. Awaiting results of new trials, remote monitoring is a valuable tool in HF care and attempts should be made to maximally adopt it in CRT patients.
Comorbidity treatment
HF patients in general suffer from an increasing number of comorbidities [79, 80]. Treatment should thus not only focus on HF, but also on patient’s comorbidities as they might impair prognosis [81, 82]. Of these comorbidities, iron deficiency is of specific interest. The importance of iron deficiency and its treatment in HFrEF to improve symptoms and exercise capacity is now well established [71]. Interestingly, iron deficiency was present in up to 56% of CRT patients and was also associated with a reduced clinical response as well as less reverse remodeling in a retrospective cohort [83]. As iron is a co-factor in energy proteins and involved in reverse remodeling [84], its defiency might hamper CRT response. An ongoing randomized trial is investigating the effect of IV iron on reverse remodeling and rate-dependent cardiac contractility in iron-deficient CRT patients (NCT03380520).
Alternative pacing strategies
Because of the sometimes encountered technical issues during implantation such as unsuitable anatomy, venous occlusion, high-pacing thresholds, or phrenic nerve stimulation and the fact that the latest activated LV site does not always coincide with the epicardial lateral or posterolateral position, new approaches for LV lead placement are under investigation [85]. These include endocardial pacing and His-bundle pacing.
Endocardial pacing
Endocardial pacing has several potential advantages such as access to all regions of the LV, a faster propagation of the activation wavefront than during epicardial stimulation, a more physiologic endocardial to epicardial LV activation, and avoidance of technical issues specific to the transvenous approach [86]. Analyses of the acute hemodynamic response to different epicardial and endocardial pacing sites in 35 patients with non-ischemic cardiomyopathy suggested that there is a high inter-individual variability in optimal pacing site and that endocardial pacing may improve diastolic function [87]. A small study compared multisite, multipolar, and endocardial pacing and found that the best overall hemodynamic response was achieved with endocardial pacing [88]. A multicenter safety study in 138 patients with non-response or failed implant showed that implantation, using an atrial trans-septal approach, was successful in 89% of cases with a clinical or echocardiographic response after 6 months in more than half [89•]. However, complications rates were high, especially transient ischemic attacks and strokes that occurred in 6.8% and 3.8% of patients, respectively, despite oral anticoagulation.
Following the recent developments of leadless pacing, a leadless LV pacing electrode (WiSe-CRT, EBR Systems, Sunnyvale, CA, USA) has been introduced. The SELECT-LV (Safety and Performance of Electrodes implanted in the Left Ventricle) feasibility study evaluated the safety and performance of the WiSE-CRT system in 35 patients with failed transvenous LV lead implantation [90]. The procedure was successful in all but one patient with a clinical response of 85% after 6 months. Currently, the SOLVE-CRT (Stimulation of the Left Ventricular Endocardium for Cardiac Resynchronization Therapy in Non-Responders and Previously Untreatable Patients) study (NCT02922036) is ongoing, investigating the safety and efficacy of this system in 350 non-responders and failed transvenous implant patients. In summary, the potential role of endocardial pacing in CRT remains unclear to date.
His-bundle pacing
His-bundle pacing is an emerging form of more physiological pacing, possibly also applicable in CRT eligible patients. As fibers from the LBB and RBB are already separated in the AV node [91], a LBBB might be overcome with His-bundle pacing in case of a proximal block. Moreover, His-bundle pacing does not require anticoagulation or transseptal puncture in contrast to endocardial pacing. In 23 patients with LBBB, His-bundle pacing resulted in an increased resynchronization and greater hemodynamic response than BiV [92]. Very recently, the His-SYNC (His Corrective Pacing or Biventricular Pacing for Cardiac Resynchronization in Heart Failure) study [93••] randomized 41 patients with CRT indication to either His-bundle pacing or conventional CRT. In the intention-to-treat analysis, there was no difference in terms of reverse remodeling with low event rates and no lead issues. Because of high cross-over rate, a subsequent per-protocol analysis was performed. However, besides a superior electrical resynchronization with His-bundle pacing, there was only a non-significant trend towards improved reverse remodeling, possibly explained by insufficient statistical power [94]. Of note, 48% of patients assigned to His-bundle pacing had cross-over, half of whom were due to a not correctable intraventricular conduction delay. This illustrates the importance of patient selection in future trials. For now, His-bundle pacing is promising, but more data are needed before it can be widely adopted in CRT eligible patients.
Conclusion
CRT improves survival and reduces HF hospitalization in HFrEF patients with electrical dyssynchrony. A holistic approach to CRT optimization as a multifaceted process with better referral and optimal patient selection, implantation targeting the latest activated zone, optimal device programming, and rigorous follow-up with multidisciplinary HF care might further improve patient benefit from CRT.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34(29):2281–329.
Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NA 3rd, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. [corrected]. Circulation. 2012;126(14):1784–800.
Daubert C, Behar N, Martins RP, Mabo P, Leclercq C. Avoiding non-responders to cardiac resynchronization therapy: a practical guide. Eur Heart J. 2017;38(19):1463–72.
McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441–6.
Mullens W, Verga T, Grimm RA, Starling RC, Wilkoff BL, Tang WHW. Persistent hemodynamic benefits of cardiac resynchronization therapy with disease progression in advanced heart failure. J Am Coll Cardiol. 2009;53(7):600–7.
McAlister FA, Tu JV, Newman A, Lee DS, Kimber S, Cujec B, et al. How many patients with heart failure are eligible for cardiac resynchronization? Insights from two prospective cohorts. Eur Heart J. 2006;27(3):323–9.
• Lund LH, Svennblad B, Dahlstrom U, Stahlberg M. Effect of expanding evidence and evolving clinical guidelines on the prevalence of indication for cardiac resynchronization therapy in patients with heart failure. Eur J Heart Fail. 2018;20(4):769–77 Good overview on evolution of guidelines and effect on CRT eligibility.
Maggioni AP, Anker SD, Dahlstrom U, Filippatos G, Ponikowski P, Zannad F, et al. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2013;15(10):1173–84.
Lund LH, Braunschweig F, Benson L, Stahlberg M, Dahlstrom U, Linde C. Association between demographic, organizational, clinical, and socio-economic characteristics and underutilization of cardiac resynchronization therapy: results from the Swedish Heart Failure Registry. Eur J Heart Fail. 2017;19(10):1270–9.
Pun PH, Sheng S, Sanders G, DeVore AD, Friedman D, Fonarow GC, et al. Prescription of guideline-recommended implantable cardioverter defibrillator and cardiac resynchronization therapy among patients hospitalized with heart failure and varying degrees of renal function. Am J Cardiol. 2017;119(6):886–92.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004.
Bank AJ, Gage RM, Olshansky B. On the underutilization of cardiac resynchronization therapy. J Card Fail. 2014;20(9):696–705.
Wilcox JE, Fonarow GC, Zhang Y, Albert NM, Curtis AB, Gheorghiade M, et al. Clinical effectiveness of cardiac resynchronization and implantable cardioverter-defibrillator therapy in men and women with heart failure: findings from IMPROVE HF. Circ Heart Fail. 2014;7(1):146–53.
Dickstein K, Normand C, Auricchio A, Bogale N, Cleland JG, Gitt AK, et al. CRT Survey II: a European Society of Cardiology survey of cardiac resynchronisation therapy in 11 088 patients-who is doing what to whom and how? Eur J Heart Fail. 2018;20(6):1039–51.
• Hosseini SM, Moazzami K, Rozen G, Vaid J, Saleh A, Heist KE, et al. Utilization and in-hospital complications of cardiac resynchronization therapy: trends in the United States from 2003 to 2013. Eur Heart J. 2017;38(27):2122-8– Very contemporary data on real world CRT use.
Steffel J, Ruschitzka F. Superresponse to cardiac resynchronization therapy. Circulation. 2014;130(1):87–90.
•• Zeitler EP, Friedman DJ, Daubert JP, Al-Khatib SM, Solomon SD, Biton Y, et al. Multiple comorbidities and response to cardiac resynchronization therapy: MADIT-CRT long-term follow-up. J Am Coll Cardiol. 2017;69(19):2369–79 This post-hoc analysis of the landmark MADIT-CRT trial, illustrates how comorbidities influence survival and heart failure hospitalizations after CRT. A higher comorbity burden impaires outcome in general. However when compared according to the number of comorbidities CRT improves outcome in every category.
Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117(20):2608–16.
Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013;369(15):1395–405.
Mullens W, Tang WH, Grimm RA. Using echocardiography in cardiac resynchronization therapy. Am Heart J. 2007;154(6):1011–20.
Saxon LA, Olshansky B, Volosin K, Steinberg JS, Lee BK, Tomassoni G, et al. Influence of left ventricular lead location on outcomes in the COMPANION study. J Cardiovasc Electrophysiol. 2009;20(7):764–8.
Thebault C, Donal E, Meunier C, Gervais R, Gerritse B, Gold MR, et al. Sites of left and right ventricular lead implantation and response to cardiac resynchronization therapy observations from the REVERSE trial. Eur Heart J. 2012;33(21):2662–71.
• Kutyifa V, Kosztin A, Klein HU, Biton Y, Nagy VK, Solomon SD, et al. Left ventricular lead location and long-term outcomes in cardiac resynchronization therapy patients. JACC Clin Electrophysiol. 2018;4(11):1410–20 Recent long-term follow-up data on patient outcomes according to lead location from the landmark MADIT-CRT trial.
Heist EK, Fan D, Mela T, Arzola-Castaner D, Reddy VY, Mansour M, et al. Radiographic left ventricular-right ventricular interlead distance predicts the acute hemodynamic response to cardiac resynchronization therapy. Am J Cardiol. 2005;96(5):685–90.
Stabile G, D’Onofrio A, Pepi P, De Simone A, Santamaria M, Caico SI, et al. Interlead anatomic and electrical distance predict outcome in CRT patients. Heart Rhythm. 2015;12(11):2221–9.
Fung JW, Yu CM, Yip G, Zhang Y, Chan H, Kum CC, et al. Variable left ventricular activation pattern in patients with heart failure and left bundle branch block. Heart. 2004;90(1):17–9.
Gold MR, Birgersdotter-Green U, Singh JP, Ellenbogen KA, Yu Y, Meyer TE, et al. The relationship between ventricular electrical delay and left ventricular remodelling with cardiac resynchronization therapy. Eur Heart J. 2011;32(20):2516–24.
Kandala J, Upadhyay GA, Altman RK, Parks KA, Orencole M, Mela T, et al. QRS morphology, left ventricular lead location, and clinical outcome in patients receiving cardiac resynchronization therapy. Eur Heart J. 2013;34(29):2252–62.
Roubicek T, Wichterle D, Kucera P, Nedbal P, Kupec J, Sedlakova J, et al. Left ventricular lead electrical delay is a predictor of mortality in patients with cardiac resynchronization therapy. Circ Arrhythm Electrophysiol. 2015;8(5):1113–21.
Singh JP, Berger RD, Doshi RN, Lloyd M, Moore D, Daoud EG, et al. Rationale and design for ENHANCE CRT: QLV implant strategy for non-left bundle branch block patients. ESC Heart Fail. 2018;5(6):1184–90.
Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O'Halloran D, Elsik M, et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59(17):1509–18.
Saba S, Marek J, Schwartzman D, Jain S, Adelstein E, White P, et al. Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy: results of the Speckle Tracking Assisted Resynchronization Therapy for Electrode Region trial. Circ Heart Fail. 2013;6(3):427–34.
Kydd AC, Khan FZ, Watson WD, Pugh PJ, Virdee MS, Dutka DP. Prognostic benefit of optimum left ventricular lead position in cardiac resynchronization therapy: follow-up of the TARGET Study Cohort (Targeted Left Ventricular Lead Placement to guide Cardiac Resynchronization Therapy). JACC Heart Fail. 2014;2(3):205–12.
Sade LE, Saba S, Marek JJ, Onishi T, Schwartzman D, Adelstein EC, et al. The association of left ventricular lead position related to regional scar by speckle-tracking echocardiography with clinical outcomes in patients receiving cardiac resynchronization therapy. J Am Soc Echocardiogr. 2014;27(6):648–56.
Leyva F, Foley PW, Chalil S, Ratib K, Smith RE, Prinzen F, et al. Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:29.
Gorcsan J 3rd. Multimodality lead positioning for cardiac resynchronization therapy: how much imaging do we need? Eur J Heart Fail. 2016;18(11):1383–5.
•• Boriani G, Connors S, Kalarus Z, Lemke B, Mullens W, Osca Asensi J, et al. Cardiac resynchronization therapy with a quadripolar electrode lead decreases complications at 6 months: results of the MORE-CRT randomized trial. JACC Clin Electrophysiol. 2016;2(2):212–20 First large randomized trial on quadripolar leads, indicating superiority in terms of complication rate compared to bipolar leads.
Turakhia MP, Cao M, Fischer A, Nabutovsky Y, Sloman LS, Dalal N, et al. Reduced mortality associated with quadripolar compared to bipolar left ventricular leads in cardiac resynchronization therapy. JACC Clin Electrophysiol. 2016;2(4):426–33.
Thibault B, Dubuc M, Khairy P, Guerra PG, Macle L, Rivard L, et al. Acute haemodynamic comparison of multisite and biventricular pacing with a quadripolar left ventricular lead. Europace. 2013;15(7):984–91.
Rinaldi CA, Kranig W, Leclercq C, Kacet S, Betts T, Bordachar P, et al. Acute effects of multisite left ventricular pacing on mechanical dyssynchrony in patients receiving cardiac resynchronization therapy. J Card Fail. 2013;19(11):731–8.
Forleo GB, Santini L, Giammaria M, Potenza D, Curnis A, Calabrese V, et al. Multipoint pacing via a quadripolar left-ventricular lead: preliminary results from the Italian registry on multipoint left-ventricular pacing in cardiac resynchronization therapy (IRON-MPP). Europace. 2017;19(7):1170–7.
• Leclercq C, Burri H, Curnis A, Delnoy PP, Rinaldi CA, Sperzel J, et al. Cardiac resynchronization therapy non-responder to responder conversion rate in the more response to cardiac resynchronization therapy with MultiPoint Pacing (MORE-CRT MPP) study: results from Phase I. Eur Heart J. 2019. First large randomizedtrial comparing multipoint pacing to conventional bipolar pacing. The programming was at the physician’s discretion in this first phase. Multipoint pacing did not increase reverse remodeling in initial CRT non-responders. In phase 2, a standardized programming protocol will be implemented.
Lenarczyk R, Kowalski O, Kukulski T, Szulik M, Pruszkowska-Skrzep P, Zielinska T, et al. Triple-site biventricular pacing in patients undergoing cardiac resynchronization therapy: a feasibility study. Europace. 2007;9(9):762–7.
Leclercq C, Gadler F, Kranig W, Ellery S, Gras D, Lazarus A, et al. A randomized comparison of triple-site versus dual-site ventricular stimulation in patients with congestive heart failure. J Am Coll Cardiol. 2008;51(15):1455–62.
Providencia R, Rogers D, Papageorgiou N, Ioannou A, James A, Babu G, et al. Long-term results of triventricular versus biventricular pacing in heart failure: a propensity-matched comparison. JACC Clin Electrophysiol. 2016;2(7):825–35.
Bordachar P, Gras D, Clementy N, Defaye P, Mondoly P, Boveda S, et al. Clinical impact of an additional left ventricular lead in cardiac resynchronization therapy nonresponders: the V(3) trial. Heart Rhythm. 2018;15(6):870–6.
Barold SS, Ilercil A, Herweg B. Echocardiographic optimization of the atrioventricular and interventricular intervals during cardiac resynchronization. Europace. 2008;10(Suppl 3):iii88–95.
Pujol-Lopez M, San Antonio R, Mont L, Trucco E, Tolosana JM, Arbelo E, et al. Electrocardiographic optimization techniques in resynchronization therapy. Europace. 2019.
Whinnett ZI, Francis DP, Denis A, Willson K, Pascale P, van Geldorp I, et al. Comparison of different invasive hemodynamic methods for AV delay optimization in patients with cardiac resynchronization therapy: implications for clinical trial design and clinical practice. Int J Cardiol. 2013;168(3):2228–37.
Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The pacing therapies for congestive heart failure study group. The Guidant congestive heart failure research group. Circulation. 1999;99(23):2993–3001.
Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, Mittal S, Waggoner AD, et al. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122(25):2660–8.
Abraham WT, Gras D, Yu CM, Guzzo L, Gupta MS, Committee FS. Rationale and design of a randomized clinical trial to assess the safety and efficacy of frequent optimization of cardiac resynchronization therapy: the Frequent Optimization Study Using the QuickOpt Method (FREEDOM) trial. Am Heart J. 2010;159(6):944–8e1.
Leon AR, Abraham WT, Brozena S, Daubert JP, Fisher WG, Gurley JC, et al. Cardiac resynchronization with sequential biventricular pacing for the treatment of moderate-to-severe heart failure. J Am Coll Cardiol. 2005;46(12):2298–304.
Boriani G, Biffi M, Muller CP, Seidl KH, Grove R, Vogt J, et al. A prospective randomized evaluation of VV delay optimization in CRT-D recipients: echocardiographic observations from the RHYTHM II ICD study. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S120–5.
Abraham WT, Leon AR, St John Sutton MG, Keteyian SJ, Fieberg AM, Chinchoy E, et al. Randomized controlled trial comparing simultaneous versus optimized sequential interventricular stimulation during cardiac resynchronization therapy. Am Heart J. 2012;164(5):735–41.
Gras D, Gupta MS, Boulogne E, Guzzo L, Abraham WT. Optimization of AV and VV delays in the real-world CRT patient population: an international survey on current clinical practice. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S236–9.
Martin DO, Lemke B, Birnie D, Krum H, Lee KL, Aonuma K, et al. Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm. 2012;9(11):1807–14.
Filippatos G, Birnie D, Gold MR, Gerritse B, Hersi A, Jacobs S, et al. Rationale and design of the AdaptResponse trial: a prospective randomized study of cardiac resynchronization therapy with preferential adaptive left ventricular-only pacing. Eur J Heart Fail. 2017;19(7):950–7.
• Brugada J, Delnoy PP, Brachmann J, Reynolds D, Padeletti L, Noelker G, et al. Contractility sensor-guided optimization of cardiac resynchronization therapy: results from the RESPOND-CRT trial. Eur Heart J. 2017;38(10):730–8 Large randomized trial illustrating the non-inferiority of the SonR algorithm compared to echo guided electrical delay optimization.
Koplan BA, Kaplan AJ, Weiner S, Jones PW, Seth M, Christman SA. Heart failure decompensation and all-cause mortality in relation to percent biventricular pacing in patients with heart failure: is a goal of 100% biventricular pacing necessary? J Am Coll Cardiol. 2009;53(4):355–60.
Hayes DL, Boehmer JP, Day JD, Gilliam FR 3rd, Heidenreich PA, Seth M, et al. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm. 2011;8(9):1469–75.
Cheng A, Landman SR, Stadler RW. Reasons for loss of cardiac resynchronization therapy pacing: insights from 32 844 patients. Circ Arrhythm Electrophysiol. 2012;5(5):884–8.
Heywood JT, Fonarow GC, Yancy CW, Albert NM, Curtis AB, Gheorghiade M, et al. Comparison of medical therapy dosing in outpatients cared for in cardiology practices with heart failure and reduced ejection fraction with and without device therapy: report from IMPROVE HF. Circ Heart Fail. 2010;3(5):596–605.
• Martens P, Verbrugge FH, Nijst P, Bertrand PB, Dupont M, Tang WH, et al. Feasibility and association of neurohumoral blocker up-titration after cardiac resynchronization therapy. J Card Fail. 2017;23(8):597–605 This retrospective analysis indicates that neurohromonal uptitration after CRT implantation is often feasable and is associated with improved outcomes.
Martens P, Verbrugge FH, Nijst P, Dupont M, Mullens W. Changes in loop diuretic dose and outcome after cardiac resynchronization therapy in patients with heart failure and reduced left ventricular ejection fractions. Am J Cardiol. 2017;120(2):267–73.
Schmidt S, Hurlimann D, Starck CT, Hindricks G, Luscher TF, Ruschitzka F, et al. Treatment with higher dosages of heart failure medication is associated with improved outcome following cardiac resynchronization therapy. Eur Heart J. 2014;35(16):1051–60.
Mantziari L, Guha K, Khalique Z, McDonagh T, Sharma R. Relation of dosing of the renin-angiotensin system inhibitors after cardiac resynchronization therapy to long-term prognosis. Am J Cardiol. 2012;109(11):1619–25.
Witt CT, Kronborg MB, Nohr EA, Mortensen PT, Gerdes C, Nielsen JC. Optimization of heart failure medication after cardiac resynchronization therapy and the impact on long-term survival. Eur Heart J Cardiovasc Pharmacother. 2015;1(3):182–8.
Mullens W, Kepa J, De Vusser P, Vercammen J, Rivero-Ayerza M, Wagner P, et al. Importance of adjunctive heart failure optimization immediately after implantation to improve long-term outcomes with cardiac resynchronization therapy. Am J Cardiol. 2011;108(3):409–15.
O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–50.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200.
Conraads VM, Vanderheyden M, Paelinck B, Verstreken S, Blankoff I, Miljoen H, et al. The effect of endurance training on exercise capacity following cardiac resynchronization therapy in chronic heart failure patients: a pilot trial. Eur J Cardiovasc Prev Rehabil. 2007;14(1):99–106.
Patwala AY, Woods PR, Sharp L, Goldspink DF, Tan LB, Wright DJ. Maximizing patient benefit from cardiac resynchronization therapy with the addition of structured exercise training: a randomized controlled study. J Am Coll Cardiol. 2009;53(25):2332–9.
Zeitler EP, Piccini JP, Hellkamp AS, Whellan DJ, Jackson KP, Ellis SJ, et al. Exercise training and pacing status in patients with heart failure: results from HF-ACTION. J Card Fail. 2015;21(1):60–7.
Martens P, Jacobs G, Dupont M, Mullens W. Effect of multidisciplinary cardiac rehabilitation on the response to cardiac resynchronization therapy. Cardiovasc Ther. 2018;36(6):e12467.
Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384(9943):583–90.
Boriani G, Da Costa A, Quesada A, Ricci RP, Favale S, Boscolo G, et al. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail. 2017;19(3):416–25.
Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan B-A, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392(10152):1047–57.
van Deursen VM, Urso R, Laroche C, Damman K, Dahlstrom U, Tavazzi L, et al. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014;16(1):103–11.
Sharma A, Zhao X, Hammill BG, Hernandez AF, Fonarow GC, Felker GM, et al. Trends in noncardiovascular comorbidities among patients hospitalized for heart failure: insights from the get with the guidelines-heart failure registry. Circ Heart Fail. 2018;11(6):e004646.
Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42(7):1226–33.
Verbrugge FH, Dupont M, Rivero-Ayerza M, de Vusser P, Van Herendael H, Vercammen J, et al. Comorbidity significantly affects clinical outcome after cardiac resynchronization therapy regardless of ventricular remodeling. J Card Fail. 2012;18(11):845–53.
Martens P, Verbrugge F, Nijst P, Dupont M, Tang WH, Mullens W. Impact of iron deficiency on response to and remodeling after cardiac resynchronization therapy. Am J Cardiol. 2017;119(1):65–70.
Agnetti G, Kaludercic N, Kane LA, Elliott ST, Guo Y, Chakir K, et al. Modulation of mitochondrial proteome and improved mitochondrial function by biventricular pacing of dyssynchronous failing hearts. Circ Cardiovasc Genet. 2010;3(1):78–87.
Galand V, Singh JP, Leclercq C. Alternative left ventricular pacing approaches for optimal cardiac resynchronization therapy. Heart Rhythm. 2019.
Bordachar P, Derval N, Ploux S, Garrigue S, Ritter P, Haissaguerre M, et al. Left ventricular endocardial stimulation for severe heart failure. J Am Coll Cardiol. 2010;56(10):747–53.
Derval N, Steendijk P, Gula LJ, Deplagne A, Laborderie J, Sacher F, et al. Optimizing hemodynamics in heart failure patients by systematic screening of left ventricular pacing sites: the lateral left ventricular wall and the coronary sinus are rarely the best sites. J Am Coll Cardiol. 2010;55(6):566–75.
Shetty AK, Sohal M, Chen Z, Ginks MR, Bostock J, Amraoui S, et al. A comparison of left ventricular endocardial, multisite, and multipolar epicardial cardiac resynchronization: an acute haemodynamic and electroanatomical study. Europace. 2014;16(6):873–9.
• Morgan JM, Biffi M, Geller L, Leclercq C, Ruffa F, Tung S, et al. ALternate Site Cardiac ResYNChronization (ALSYNC): a prospective and multicentre study of left ventricular endocardial pacing for cardiac resynchronization therapy. Eur Heart J. 2016;37(27):2118–27 Prospective study describing safety of an endocardial LV lead system in patients with failed conventional implant. There was a significant incidence of transient ischemic attack and stroke despite oral anticoagulant thereapy.
Reddy VY, Miller MA, Neuzil P, Sogaard P, Butter C, Seifert M, et al. Cardiac resynchronization therapy with wireless left ventricular endocardial pacing: the SELECT-LV study. J Am Coll Cardiol. 2017;69(17):2119–29.
Narula OS. Longitudinal dissociation in the His bundle. Bundle branch block due to asynchronous conduction within the His bundle in man. Circulation. 1977;56(6):996–1006.
Arnold AD, Shun-Shin MJ, Keene D, Howard JP, Sohaib SMA, Wright IJ, et al. His resynchronization versus biventricular pacing in patients with heart failure and left bundle branch block. J Am Coll Cardiol. 2018;72(24):3112–22.
•• Upadhyay GA, Vijayaraman P, Nayak HM, Verma N, Dandamudi G, Sharma PS, et al. His corrective pacing or biventricular pacing for cardiac resynchronization in heart failure. J Am Coll Cardiol. 2019. First small randomized controlled trial comparing His-bundle pacing to conventional CRT in first implants. In the intention to treat analysis, there was no difference in terms of reverse remodelling or heart failure hospitalization or death after median of 6.2 months fo follow-up. However, there was a high cross-ove rrate.
Upadhyay GA, Vijayaraman P, Nayak HM, Verma N, Dandamudi G, Sharma PS, et al. On-treatment comparison between corrective his bundle pacing and biventricular pacing for cardiac resynchronization: a secondary analysis of his-sync. Heart Rhythm 2019.
Grant support
Jeroen Dauw, Pieter Martens, and Wilfried Mullens are researchers for the Limburg Clinical Research Center (LCRC) UHasselt-ZOL-Jessa, supported by the foundation Limburg Sterk Merk (LSM), province of Limburg, Flemish government, Hasselt University, Ziekenhuis Oost-Limburg, and Jessa Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Heart Failure
Rights and permissions
About this article
Cite this article
Dauw, J., Martens, P. & Mullens, W. CRT Optimization: What Is New? What Is Necessary?. Curr Treat Options Cardio Med 21, 45 (2019). https://doi.org/10.1007/s11936-019-0751-2
Published:
DOI: https://doi.org/10.1007/s11936-019-0751-2