Abstract
Purpose of review
Chronic total occlusion (CTO) poses one of the greatest technical challenges to interventional cardiologists. Despite recent advancements in techniques and clinical trials showing significant benefits of CTO percutaneous coronary interventions (PCI), the proportion of patients with untreated CTOs remains high. We therefore aim to perform a comprehensive review of the various techniques available, recent advancements, benefits, and complications associated with CTO PCI.
Recent findings
Three randomized clinical trials examining the benefits of CTO PCI have recently been presented. Scoring systems have been developed to facilitate pre-procedural estimation of success and complications of CTO PCI. Technological enhancements in coronary wires and other interventional equipment along with dedicated training for CTO operators have improved the likelihood of successful recanalization of CTOs.
Summary
CTO PCI has been shown to improve patient symptoms and quality of life. It is therefore important to have an in-depth knowledge of the various CTO techniques, appropriate equipment, and complications when performing these complex procedures. Clinicians should weigh the risks and benefits and choose the appropriate patient population who may benefit from revascularization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A coronary chronic total occlusion (CTO) is a complete or nearly complete occlusion of an epicardial coronary vessel, usually secondary to heavy atherosclerotic plaque within the artery, resulting in thrombolysis in myocardial infarction flow (TIMI) of 0 [1, 2]. Defining the chronicity of obstruction can be challenging at times due to lack of recent angiograms, but per definition, the obstruction should be present at least for a period of 3 months or more. In patients where no prior angiograms are available, frequency and duration of symptoms and history of prior myocardial infarction may help to estimate the duration of occlusion. It is important to differentiate CTOs from recent infarct-related occluded arteries, between 3 to 30 days after a myocardial infarction (MI). These most recent occlusions typically supply infarcted muscle and do not benefit from percutaneous coronary revascularization (PCI) as shown in the occluded artery trial (OAT) [3].
Prevalence of CTOs
Previously published multicenter registries have shown that CTOs have a prevalence of 10–25% among patients undergoing diagnostic angiography [4,5,6, 7•]. Data from the Canadian chronic total occlusion study with 14,439 patients showed that the incidence of CTO was about 18% in patients with known coronary disease [8]. Also, in this registry, it was shown that female patients had a relatively lower prevalence of CTO and less likely to receive revascularization through coronary artery bypass grafting (CABG) [9]. The prevalence of CTOs in patients undergoing coronary angiography for acute ST segment elevation myocardial infarction is 21% in diabetics and 12% in non-diabetics [10].
Medical therapy and goals of revascularization in CTO
All patients with CTO should initially be treated with guideline-directed medical therapy (GDMT), including aspirin, beta-blockers, renin angiotensin blockers, and statin unless they have a contraindication. Antianginal medications, including nitrates, calcium-channel blockers, and ranolazine, should also be used to mitigate symptoms. Patients should be evaluated appropriately for revascularization with either percutaneous coronary intervention (PCI) or CABG. The objectives of revascularization in patients with CTO are to improve clinical outcomes, left ventricular function or symptoms, most commonly angina or dyspnea. Although observational studies have shown a negative association between the presence of a CTO and survival and a potential benefit of successful CTO PCI relative to unsuccessful attempts, data from well-designed randomized clinical trials are lacking.
Improvement in survival
In the HORIZONS-AMI trial, the presence of CTO in a non-IRA was shown to predict mortality at 3-year follow-up [11]. Another study showed that the presence of CTO was associated with increased mortality during long-term follow-up, after excluding early deaths [12]. Successful CTO was shown to reduce mortality by almost 48% in a meta-analysis of mostly observational studies comparing successful versus unsuccessful CTO PCI [13•]. A few other studies also showed improvement in mortality in patients with CTO receiving PCI, particularly when the target vessel was the left anterior descending artery [14]. A recent randomized trial comparing medical therapy to CTO PCI showed no significant mortality benefit [15]. Of note, this trial was powered to detect the difference in quality of life and health status and therefore had insufficient power for detecting a difference in major adverse clinical events (MACE). MACE occurred in 6.7% of patients in the medical arm and 5.2% in the CTO PCI arm.
Improvement in left ventricular function
In a previously published study, the presence of a CTO in non-IRA in STEMI patients was independently associated with reduced left ventricular ejection fraction [12]. Also, there was further deterioration in LVEF at 1-year follow-up. Another meta-analysis of PCI in CTO showed 4.44% increase in LVEF and reduced adverse remodeling of the ventricle [13•]. Numerous other studies have shown significant improvement in LVEF after treatment of CTO with PCI [16,17,18,19,20].
Improvements in quality of life
Successful CTO recanalization has been shown to reduce the need for antianginal medications and to improve exercise capacity, as demonstrated by longer 6-min walking distance [21]. The mechanism is probably secondary to reduced ischemic burden and anginal frequency with CTO PCI.
In a recent randomized clinical trial of 396 patients with CTO who were randomized to CTO PCI versus medical therapy, CTO PCI showed significant improvements on Seattle angina questionnaire (SAQ) in several domains including angina frequency (5.23, 95% CI = 1.75–8.71, p = 0.003) and quality of life (6.62, 95% CI = 1.78–11.46, p = 0.007). Complete relief from angina was also more common with CTO PCI relative to medical therapy (71.6% versus 57.8%, p = 0.008) [13•].
Current recommendations for CTO revascularization
The 2011-ACCF/AHA/SCAI Guideline for PCI recommended revascularization with PCI in patients with clinical indications and favorable anatomy, especially when performed by operators with adequate expertise (Class IIa, Level of Evidence [LOE] B) [22].
The 2014 European Society of Cardiology and the European Association for Cardiothoracic Surgery guidelines recommend CTO PCI to be considered in patients in whom ischemia reduction is expected in a corresponding myocardial territory and/or angina relief (Class IIa, LOE B). The guidelines recommend an initial anterograde approach to CTO followed by consideration of a retrograde approach if anterograde approach fails or a primary retrograde approach in appropriately selected patients (Class IIb, LOE C) [23•].
In the recent 2017-ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS Appropriate Use Criteria for revascularization in patients with stable ischemic heart disease, there are no separate criteria for CTO lesions as was the case back in the 2012 guidelines. Currently, the indications for revascularization in stable ischemic heart disease patients are determined irrespective of whether the lesion is a CTO or not [24]. The indication for revascularization of a coronary artery lesion is now purely based on symptoms, the extent of antianginal medications, and the risk of ischemia and not whether the lesion is a CTO or not.
Frequency of CTO intervention and risk-benefit analysis
True benefit derived from CTO PCI depends on patient symptoms, the likelihood of achieving success, and complications including periprocedural and long-term complications. It is generally not recommended to intervene on a CTO in an asymptomatic patient. In such patients, an ischemic work-up including a treadmill stress testing or quality of life questionnaire could help to define the area of ischemia and potential limitations in terms of quality of life. If the myocardial territory is large enough to warrant intervention, PCI of the CTO should be considered. In a study by Safley et al. [25], it was shown that patients with > 12.5% ischemic burden in the CTO territory are found to experience significant improvement in their ischemic burden post CTO PCI.
CTO PCI is associated with higher procedural complications relative to non-CTO PCI, and despite the potential benefits, CTO PCI attempt rates are significantly lower than non-CTO PCI rates [26•]. Before the The SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) trial [27], one of the major exclusion criteria in trials comparing PCI versus coronary artery bypass graft (CABG) surgery was the presence of a CTO, which eventually led to referral for CABG surgery. Over the past decade or so, there has been a significant improvement in the treatment options available for CTO PCI and this has led to a remarkable increase in success rates with CTO PCI. In a previous analysis from the National Cardiovascular Data Registry (NCDR), procedural success of CTO PCI was only 59% between 2009 and 2013 [26•]. Another meta-analysis reported success rates of 70–80% [28]. With improvement in treatment strategies, including the use of the hybrid algorithm and dedicated interventional equipment, the success rate of CTO has improved significantly to 85–90% in experienced centers [29,30,31,32,33]. Thus, it appears that dedicated CTO operators have been able to overcome many of the challenges encountered during CTO PCI and that may explain the gap between experienced CTO centers versus the non-experienced ones.
Scoring systems for CTO
There are numerous scores used for estimating the success of a PCI procedure for CTO. The initial scoring system for PCI in CTO came from a Japanese CTO registry and is called the J-CTO score [34]. The score uses five variables including blunt stump, occlusion length > 20 mm, CTO tortuosity, CTO calcification, and prior failed attempt. The score helps to predict successful guidewire crossing within the first 30 min of the procedure.
A second scoring system which is more commonly used is the prospective global registry for the study of chronic total occlusion intervention (PROGRESS-CTO) [35•]. The score includes proximal cap ambiguity, circumflex CTO, moderate severe tortuosity, and the absence of interventional collaterals. The four-point system appears to predict technical success and long-term outcomes better than the other existing scores [36].
There are other scoring systems that exist including the ORA score (ostial location, collateral filling of Rentrop < 2, age over 75 years) [31], RECHARGE (REgistry of Crossboss and Hybrid Procedures in FrAnce, the NetheRlands, BelGium and UnitEd Kingdom), the CL scoring systems [37], and the scoring system by Ellis et al. [38] using proximal cap ambiguity that are infrequently used in clinical practice. Scoring systems perform well in antegrade CTO approaches.
Published randomized and non-randomized trials on CTO
There have been three randomized controlled trials (RCT) of CTO PCI versus medical therapy that have been presented at scientific conferences or in peer-reviewed publications (Table 1). The EXPLORE (Evaluating Xience and Left Ventricular Function in Percutaneous Coronary Intervention on Occlusions After ST-Elevation Myocardial Infarction) trial randomized 304 patients with non-IRA CTO in patients admitted for acute ST elevation myocardial infarction [39]. Patients were randomized to PCI of the non-IRA CTO versus medical therapy. The study did not show any difference in ejection fraction or LV dimensions at 4-month follow-up. They reported a 73% success rate for CTO PCI [39].
The DECISION-CTO (NCT01078051) is another RCT with 834 patients comparing CTO PCI plus optimal medical therapy to optimal medical therapy alone and was terminated early. Clinical outcomes were similar between the two groups at a medial follow-up of 3.1 years. There were major criticisms of the trial including suboptimal primary end point selection, early termination, and high crossover rates (reported as high as 18% switch from medical therapy to intervention group) and the presence of only mild symptoms at baseline in both groups. This trial was presented at the American College of Cardiology 2017 Annual Scientific Session but has not been published yet.
The EuroCTO (A Randomized Multicentre Trial to Evaluate the Utilization of Revascularization or Optimal Medical Therapy for the Treatment of Chronic Total Coronary Occlusions) trial (NCT01760083) was terminated after enrollment of 396 patients; it was originally designed to enroll 1200 patients [15]. Despite being underpowered, the trial showed that patients who received CTO PCI had significant improvements in physical limitation, angina frequency, and quality of life at 1 year when compared to medical therapy alone.
The major difference between the trials is the timing and management of severe non-CTO lesions. In DECISION-CTO, 77% of patients had multivessel disease (non-CTO lesions) and were treated after randomization and baseline assessment in the medical arm, effectively diluting the potential beneficial effect of the intervention in the CTO PCI arm. In contrast in EuroCTO, all significant non-CTO lesions were treated prior to randomization and baseline assessment.
Technical aspects of CTO PCI
CTO operators should be familiar with the available interventional equipment and understand their strengths and limitations. There should also be a good understanding on the complications that may arise during CTO PCI and how to effectively manage them. The importance of operator experience cannot be overstated as it relates to procedural success. In unselected populations, the success rate of CTO PCI remains low at 59–61% [26•, 40] whereas in experienced centers, procedural success rates of > 85% are the norm [29, 32].
It is advisable to use longer sheaths for CTO PCI and most hybrid centers routinely use bilateral femoral 45-cm sheaths. Consider higher than the usual ACTs (> 300 s) for retrograde CTO PCIs to prevent thrombosis of the donor vessel. Heparin is the anticoagulant of choice for CTO PCI as this can be easily reversed in case of coronary perforation. For radial access, the 7 Fr Slender sheath allows the use of a 7 Fr guiding catheter. Smaller size sheaths are not preferred especially because of weaker support and inability to accommodate coronary equipment.
In the USA, the dual 8 Fr systems are preferred, whereas in Europe, 7 Fr systems are favored, although 8 Fr femoral access has been shown to be associated with more vascular complications [41] and the use of fluoroscopic guidance has shown to reduce complications [42]. A study which compared outcomes of radial versus femoral CTO PCI showed that the technical success with both approaches was similar, except in complex CTO PCIs defined as J-CTO score of ≥ 3, where radial access was associated with a lower technical success rate [43]. Though radial operators are increasingly adopting radial access for CTO PCI, this may be associated with lower success and efficiency, especially in complex CTO PCIs [44]. More recently, CTO operators are using an 8 Fr femoral guide for the antegrade guiding catheter and a 6 Fr radial access for the retrograde guiding catheter especially for antegrade cases.
The use of shorter guiding catheters (90 cm) facilitates the retrograde approach as it minimizes the distance which the retrograde wire needs to travel before externalization. With the availability of long externalization guidewires including RG3 and RG350, guide catheter shortening is not mandatory. A Y connector with hemostatic valve can help to minimize blood loss associated with CTO PCI. Guide catheter extensions including Guidezila II by Boston Scientific, Trapliner by Vascular Solutions, GuideLiner V3 (Vascular Solutions), or Giodion (interventional Medical Device Solutions) are routinely used to increase support and facilitate reverse controlled antegrade dissection (reverse CART) and retrograde tracking. An over-the-wire system, usually over a microcatheter or over-the-wire (OTW) balloon, is always preferred for CTO crossing because it allows reshaping of the guidewire tip and facilitates guidewire exchanges. Microcatheters are preferred to OTW balloons because the marker for a microcatheter is located conveniently at the tip and provides better visualization. Many microcatheters are available on the market including Corsair Pro (Asahi Intecc), Corsair, Spiral and Gold (Vascular Solutions), Turnpike, Turnpike LP, and Finecross (Terumo) and support catheters including MultiCross and CenterCross (Roxwood) are also used. Recommended guidewires for CTO PCI and its principal applications include Fighter (Boston Scientific) or Fielder XT (Asahi) for crossing micro-channels and knuckle formation. For penetration, the Gaia guidewire and the Confianza Pro 12 (Asahi) have stiff tapered tip and the Pilot 200 (Abbott Vascular) is a moderately stiff non-tapered wire. In particular, the Confianza Pro 12 can be very useful for puncturing a calcified CTO cap (proximal or distal). For crossing collateral channels, the hydrophilic Sion (Asahi) wire is preferred as it is highly torquable with excellent shape retention properties. Fielder FC, XT-R could be used as well. Finally, for wire externalization, the RG3 (Asahi, 330 cm long) and R350 (Vascular Solutions, 350 cm long) are preferred.
Dual coronary injection is one of the most important aspects of CTO PCI as it allows to accurately define the proximal cap, estimate lesion length, location of distal cap, and presence of interventional collaterals. This is done with a low magnification without panning and with long cine acquisition. The donor vessel is injected first followed by the injection of the occluded vessel after collaterals have filled the distal vessel. It is advisable to avoid a side-hole catheter. According to the hybrid algorithm, four key elements should be studied in detail before CTO PCI: (1) proximal cap ambiguity, (2) lesion length, (3) collateral circulation and the presence of “interventional collaterals,” and (4) location and quality of distal target vessel. The fluoroscopy rate is usually set at 7.5 frames per second to minimize radiation exposure.
CTO crossing techniques
According to the hybrid algorithm, there are three major techniques to perform CTO PCI: (1) antegrade wire escalation, (2) antegrade dissection and reentry, and (3) retrograde.
Antegrade wire escalation
This is the most commonly utilized technique for crossing the CTO and is very useful in short occlusions < 20 mm in length, in stent restenosis, and longer occlusions of straight segments. In this technique, wires of increasing stiffness are used usually over a microcatheter or OTW balloon in an attempt to cross the CTO. As mentioned previously, microcatheters are preferred over OTW balloons as the marker is at the tip for microcatheter. Initially, we prefer to use a polymer-jacketed tapered guidewire such as the Fielder XT (Asahi Intecc) but if this fails, still polymer-jacketed wire such as pilot 200 (Abbot Vascular) can be used, especially when the course of the occluded vessel is unclear. If the occluded vessel course is known, then a stiff wire such as the Confianza Pro 12 (Asahi Intecc) is preferred. Crossing the CTO into the distal lumen involves sliding which is the first step followed by drilling with controlled rotation of guidewire and finally penetration with forward guidewire advancement steering the wire but not blindly rotating. The family of Asahi Gaia guidewires (first, second, and third) is increasingly used for antegrade crossing as they have increased tip load (1.7, 3.5, and 4.5 gf).
Antegrade dissection/reentry
The basic principle of dissection/reentry is to use the subintimal space as a conduit to gain access to true lumen of the vessel distal to the CTO, bypassing the CTO itself. The term subintimal space could refer to the tissue plane within or beyond the occlusion and it could be as follows: (1) subintimal, (2) intra plaque, and (3) intra-adventitial or their combinations. Subintimal space is usually reached with a “knuckle wire,” formed by advancing the wire Fielder XT (Asahi Intecc) to form a small loop in subintimal space. Large wire loops should be avoided as the dissection plane can grow significantly, which makes reentry more difficult. A blunt-tipped microcatheter (CrossBoss, Boston Scientific) can also be used. Once the microcatheter or the knuckle wire reaches the subintimal space distal to the CTO, reentry can be achieved with a Stingray balloon (BSCI) or less preferably a guidewire.
Several reentry techniques have been described. First, the Subintimal Tracking and Re-entry (STAR) refers to spontaneous reentry of the guidewire into the true lumen after the CTO [45]. A modified mini-STAR [46] or Limited Antegrade Subintimal Tracking (LAST) was introduced to minimize the size of subintimal crossing. Fielder XT or FC guidewire is used in mini-STAR, whereas in LAST, pilot 200 or Confianza Pro 12 are used. A deflectable tip venture catheter by Vascular Solutions can assist in distal true lumen reentry [47]. The preferred technique for most CTO operators is the Stingray balloon guided reentry. The Stingray is a 1-mm balloon with three ports all connected to one common lumen. One port is used to stabilize the balloon in position while the other two ports (one proximal and one distal) are facing 180° away, one facing the true lumen while the other facing the adventitia. A Stingray wire is a high gram force wire which is inserted and guided out through the port for the true lumen. This is guided by retrograde injections. The wire is angulated at the tip with a distal tapering probe that helps to grab the tissue while the operator advances the wire through the port facing the desired lumen. Usually, a pilot 200 is used for exchange. The CrossBoss system has been shown to have a 77% success rate in the FAST CTO trial (Facilitated Antegrade Steering Technique in Chronic Total Occlusions) [48]. Another technique named microcatheter knuckle technique (MKT) has been introduced by operators where significant resistance is experienced restricting further guidewire advancement in antegrade or retrograde dissection [49].
The retrograde approach
This was initially introduced in Japan and has been widely adopted worldwide [50]. The technique involves using the distal cap of the CTO, which is not exposed to arterial pressure and therefore it is softer and easier to cross in comparison to the proximal cap [51]. Any of the collaterals, including septal perforators, bypass grafts, or any other epicardial collateral can be used if deemed appropriate. Werner collateral channel classification (WCC) includes CC1 (invisible collaterals), CC1 (thread-like continuous connection), and CC2 (side branch–like connection) but CCo may be visible on selective collateral injection through a microcatheter. The suitability of using a collateral for a retrograde approach (called “interventional collaterals”) depends on wiring difficulty, tortuosity, perforation risk, and ability to dilate the vessel. Septal collaterals are preferred as they can be easily crossed using a “surfing” technique, can be dilated, and perforations are usually contained. The risk of coronary perforation is higher when epicardial collaterals are used. A bypass graft is the second most ideal retrograde conduit as it allows manipulation of wire and accommodates essential interventional equipment. Another advantage is that they are less tortuous and there is a lower risk of tamponade with perforation secondary to pericardial scarring after a CABG. Despite these advantages, a multicenter registry has shown that bypass grafts were used only in 8% of the patient population with CTO and septal perforators were preferred. The reported procedural success rate was 80% with a complication rate of 2.6% [52]. Collateral crossing technique involves using of a soft polymer-jacketed guidewire (pilot 50 or Fielder FC) or Sion blue (Asahi Intecc) with a 45° bend at its distal tip. The wire is inserted over a Corsair catheter (Asahi Intecc), which has a hydrophilic coating and stainless-steel braiding design which helps to serve for both wire support and collateral dilatation. After, the retrograde wire is lodged in the distal cap and wire escalation or dissection reentry technique is used to enter the true lumen but reported success rate is around 40% [53•] and more often the reverse CART technique is required. The technique involves advancing a balloon over the antegrade wire and inflating in the subintimal space to expand it and simultaneously advancing the retrograde wire into the false lumen created. In the CART technique, balloon inflation is done retrograde to facilitate antegrade wire entry. More recently, Matsuno et al. proposed a new classification system for reverse CART techniques. According to them, reverse CART can be classified as follows: (1) conventional reverse CART (a large balloon on antegrade wire to help retrograde wire entry within CTO segment), (2) directed reverse CART (small antegrade balloon and intentional vessel tracking and entry with retrograde wire within CTO segment), (3) extended reverse CART (reentry outside the CTO segment by extending intimal/subintimal dissection outside the CTO segment) [54]. Once reverse CART is achieved, the guidewire is advanced into the antegrade guide catheter and then trapped. This allows the advancement of microcatheter over the guidewire followed by wire exchange with a longer guidewire with externalization. The RG3 (330 cm long, Asahi) and R350 (350 cm long, Vascular Solutions) are preferred for wire externalization. Donor vessel injury may occur during wire externalization and active guide manipulation may be required to prevent it.
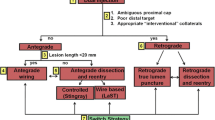
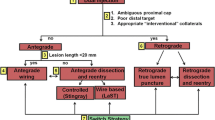
CTO crossing algorithm
An algorithm for CTO crossing was proposed by Brilakis et al. in 2012 (Fig. 1). The technique starts with the initial vessel assessment for proximal cap ambiguity (ambiguous or identifiable), occlusion length (less than or greater than 20 mm), size and location of the distal vessel (> 2-mm vessel without disease and distal cap at bifurcation), and availability of interventional collaterals. The initial strategy is selected based on the abovementioned parameters and the strategy is modified accordingly avoiding prolonged crossing attempts with one strategy [55•].
Stent selection
Drug eluting stents are preferred over bare metal stents for CTO interventions. Data from the National Cardiovascular Data Registry showed that DES are associated with overall low mortality with a lower incidence of myocardial infarction and repeat coronary revascularization [56]. Meta-analyses have also showed lower incidence of restenosis with DES [57, 58]. Another randomized trial showed that sirolimus eluting stent had lower target vessel revascularization when compared to bare metal stents [59]. CIBELES showed that everolimus eluting stents (EES) and sirolimus eluting stents (SES) have similar outcomes in CTO interventions [60] whereas ACE-CTO showed higher rates of stent strut malapposition and incomplete stent coverage after CTO PCI with EES [61].
Complications of CTO PCI
In a study from 12 expert CTO centers from the USA with 1000 consecutive procedures, the overall reported complication rate was around 10% and was more commonly seen with the retrograde approach [62•]. Most common events were perforation (8.8%), periprocedural myocardial infarction (2.6%), arrhythmia (1.2%), cardiogenic shock (1.1%), and in hospital death (0.9%). Investigators from the PROGRESS-CTO registry reported a complication rate of 2.8% in 1569 CTO PCI procedures. Three factors were independent predictors of complications: patient age > 65 years, lesion length ≥ 23 mm, and the use of the retrograde approach [63].
Another propensity matched cohort study with 4014 patients compared CTO PCI to non-CTO PCI and showed similar rates of death, myocardial infarction, urgent CABG, and combined MACE [64]. A few other studies also showed similar outcomes between CTO and non-CTO PCI [65, 66]. As perforation of coronaries is a dreaded complication, pre-procedural planning is essential and coils and covered stents should be readily available before the procedure [67]. As CTO procedures are time-consuming, there is risk of radiation injury, and therefore it is advisable to use fluoroscopy at 7.5 frames per second rather than 15 frames or higher. Recently, a preliminary experience for a low-dose protocol was suggested for CTO PCI [67]. Also, it is important to switch angiographic views frequently and maximize the distance between the X-ray source and the patient to avoid radiation-induced skin damage.
Conclusion
In experienced centers, successful outcomes of CTO PCI can be achieved in 80–90% of patients. Optimal patient selection requires a combination of clinical evaluation, functional testing, dual angiography, and procedural expertise. Complications of CTO PCI are higher than for non-CTO PCI with serious complications occurring in ~ 3% of patients. The main clinical benefit of CTO PCI is that it improves symptoms of angina. Training and proctoring are important to achieve high success rates and deal with complications of CTO PCI effectively.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Garcia S, Abdullah S, Banerjee S, Brilakis ES. Chronic total occlusions: patient selection and overview of advanced techniques. Curr Cardiol Rep. 2013;15(2):334.
Shah PB. Management of coronary chronic total occlusion. Circulation. 2011;123(16):1780–4.
Hochman JS, Lamas GA, Buller CE, Dzavik V, Reynolds HR, Abramsky SJ, et al. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355(23):2395–407.
George S, Cockburn J, Clayton TC, Ludman P, Cotton J, Spratt J, et al. Long-term follow-up of elective chronic total coronary occlusion angioplasty: analysis from the U.K. Central Cardiac Audit Database. J Am Coll Cardiol. 2014;64(3):235–43.
Ladwiniec A, Allgar V, Thackray S, Alamgir F, Hoye A. Medical therapy, percutaneous coronary intervention and prognosis in patients with chronic total occlusions. Heart. 2015;101(23):1907–14.
Tomasello SD, Boukhris M, Giubilato S, Marzà F, Garbo R, Contegiacomo G, et al. Management strategies in patients affected by chronic total occlusions: results from the Italian Registry of Chronic Total Occlusions. Eur Heart J. 2015;36(45):3189–98.
• Råmunddal T, Hoebers LP, Henriques JP, Dworeck C, Angerås O, Odenstedt J, et al. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc Interv. 2016;9(15):1535-44. The Swedish registry studied 14, 441 patients with CTO and reported higher mortality with CTO interventions. This data highlighted the importance of appropriate patient selection and operator expertise in CTO PCIs.
Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59(11):991–7.
Wolff R, Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Sparkes JD, et al. Gender differences in the prevalence and treatment of coronary chronic total occlusions. Catheter Cardiovasc Interv. 2016;87(6):1063–70.
Claessen BE, Hoebers LP, van der Schaaf RJ, Kikkert WJ, Engstrom AE, Vis MM, et al. Prevalence and impact of a chronic total occlusion in a non-infarct-related artery on long-term mortality in diabetic patients with ST elevation myocardial infarction. Heart. 2010;96(24):1968–72.
Claessen BE, Dangas GD, Weisz G, Witzenbichler B, Guagliumi G, Möckel M, et al. Prognostic impact of a chronic total occlusion in a non-infarct-related artery in patients with ST-segment elevation myocardial infarction: 3-year results from the HORIZONS-AMI trial. Eur Heart J. 2012;33(6):768–75.
Claessen BE, van der Schaaf RJ, Verouden NJ, Stegenga NK, Engstrom AE, Sjauw KD, et al. Evaluation of the effect of a concurrent chronic total occlusion on long-term mortality and left ventricular function in patients after primary percutaneous coronary intervention. JACC Cardiovasc Interv. 2009;2(11):1128–34.
• Hoebers LP, Claessen BE, Elias J, Dangas GD, Mehran R, Henriques JP. Meta-analysis on the impact of percutaneous coronary intervention of chronic total occlusions on left ventricular function and clinical outcome. Int J Cardiol. 2015;187:90–6 Meta analysis of 34 PCI CTO studies showed improvement in left ventricular ejection fraction and improvement in survival, which has not been proven in randomized trials yet.
Choi IJ, Koh YS, Lim S, Choo EH, Kim JJ, Hwang BH, et al. Impact of percutaneous coronary intervention for chronic total occlusion in non-infarct-related arteries in patients with acute myocardial infarction (from the COREA-AMI Registry). Am J Cardiol. 2016;117(7):1039–46.
Werner GS, Martin-Yuste V, Hildick-Smith D, Boudou N, Sianos G, Gelev V, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39(26):2484–93.
Kirschbaum SW, Baks T, van den Ent M, Sianos G, Krestin GP, Serruys PW, et al. Evaluation of left ventricular function three years after percutaneous recanalization of chronic total coronary occlusions. Am J Cardiol. 2008;101(2):179–85.
Baks T, van Geuns RJ, Duncker DJ, Cademartiri F, Mollet NR, Krestin GP, et al. Prediction of left ventricular function after drug-eluting stent implantation for chronic total coronary occlusions. J Am Coll Cardiol. 2006;47(4):721–5.
Sirnes PA, Myreng Y, Mølstad P, Bonarjee V, Golf S. Improvement in left ventricular ejection fraction and wall motion after successful recanalization of chronic coronary occlusions. Eur Heart J. 1998;19(2):273–81.
Piscione F, Galasso G, De Luca G, Marrazzo G, Sarno G, Viola O, et al. Late reopening of an occluded infarct related artery improves left ventricular function and long term clinical outcome. Heart. 2005;91(5):646–51.
Melchior JP, Meier B, Urban P, Finci L, Steffenino G, Noble J, et al. Percutaneous transluminal coronary angioplasty for chronic total coronary arterial occlusion. Am J Cardiol. 1987;59(6):535–8.
Rossello X, Pujadas S, Serra A, Bajo E, Carreras F, Barros A, et al. Assessment of inducible myocardial ischemia, quality of life, and functional status after successful percutaneous revascularization in patients with chronic total coronary occlusion. Am J Cardiol. 2016;117(5):720–6.
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013;82(4):E266–355.
• Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541–619 The European guieline document given a class II a indication for CTO PCI in appropriately chosen patient population.
Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69(17):2212–41.
Safley DM, Koshy S, Grantham JA, Bybee KA, House JA, Kennedy KF, et al. Changes in myocardial ischemic burden following percutaneous coronary intervention of chronic total occlusions. Catheter Cardiovasc Interv. 2011;78(3):337–43.
• Brilakis ES, Banerjee S, Karmpaliotis D, Lombardi WL, Tsai TT, Shunk KA, et al. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2015;8(2):245–53 The authors evaluated the national cardiovascular data registry including 594,510 PCIs for stable angina and reported underutilization of CTO interventions, which led to further trials to evalute its safety and efficacy of CTO intervnetions.
Kappetein AP, Dawkins KD, Mohr FW, Morice MC, Mack MJ, Russell ME, et al. Current percutaneous coronary intervention and coronary artery bypass grafting practices for three-vessel and left main coronary artery disease. Insights from the SYNTAX run-in phase. Eur J Cardiothorac Surg. 2006;29(4):486–91.
Patel VG, Brayton KM, Tamayo A, Mogabgab O, Michael TT, Lo N, et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv. 2013;6(2):128–36.
Christopoulos G, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, Wyman RM, et al. Application and outcomes of a hybrid approach to chronic total occlusion percutaneous coronary intervention in a contemporary multicenter US registry. Int J Cardiol. 2015;198:222–8.
Maeremans J, Walsh S, Knaapen P, Spratt JC, Avran A, Hanratty CG, et al. The hybrid algorithm for treating chronic total occlusions in Europe: the RECHARGE registry. J Am Coll Cardiol. 2016;68(18):1958–70.
Galassi AR, Boukhris M, Azzarelli S, Castaing M, Marzà F, Tomasello SD. Percutaneous coronary revascularization for chronic total occlusions: a novel predictive score of technical failure using advanced technologies. JACC Cardiovasc Interv. 2016;9(9):911–22.
Wilson WM, Walsh SJ, Yan AT, Hanratty CG, Bagnall AJ, Egred M, et al. Hybrid approach improves success of chronic total occlusion angioplasty. Heart. 2016;102(18):1486–93.
Habara M, Tsuchikane E, Muramatsu T, Kashima Y, Okamura A, Mutoh M, et al. Comparison of percutaneous coronary intervention for chronic total occlusion outcome according to operator experience from the Japanese retrograde summit registry. Catheter Cardiovasc Interv. 2016;87(6):1027–35.
Morino Y, Abe M, Morimoto T, Kimura T, Hayashi Y, Muramatsu T, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (multicenter CTO registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4(2):213–21.
• Christopoulos G, Kandzari DE, Yeh RW, Jaffer FA, Karmpaliotis D, Wyman MR, et al. Development and validation of a novel scoring system for predicting technical success of chronic total occlusion percutaneous coronary interventions: the PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) Score. JACC Cardiovasc Interv. 2016;9(1):1–9 The trial introduced a novel scoring system for interventions in CTO and is one of the scores being widely used worldwide for CTO interventions.
Forouzandeh F, Suh J, Stahl E, Ko YA, Lee S, Joshi U, et al. Performance of J-CTO and PROGRESS CTO scores in predicting angiographic success and long-term outcomes of percutaneous coronary interventions for chronic total occlusions. Am J Cardiol. 2018;121(1):14–20.
Alessandrino G, Chevalier B, Lefèvre T, Sanguineti F, Garot P, Unterseeh T, et al. A clinical and angiographic scoring system to predict the probability of successful first-attempt percutaneous coronary intervention in patients with total chronic coronary occlusion. JACC Cardiovasc Interv. 2015;8(12):1540–8.
Ellis SG, Burke MN, Murad MB, Graham JJ, Badawi R, Toma C, et al. Predictors of successful hybrid-approach chronic total coronary artery occlusion stenting: an improved model with novel correlates. JACC Cardiovasc Interv. 2017;10(11):1089–98.
Henriques JP, Hoebers LP, Råmunddal T, Laanmets P, Eriksen E, Bax M, et al. Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: the EXPLORE trial. J Am Coll Cardiol. 2016;68(15):1622–32.
Hannan EL, Zhong Y, Jacobs AK, Stamato NJ, Berger PB, Walford G, et al. Patients with chronic total occlusions undergoing percutaneous coronary interventions: characteristics, success, and outcomes. Circ Cardiovasc Interv. 2016;9(5):e003586.
Kinnaird T, Anderson R, Ossei-Gerning N, Gallagher S, Large A, Strange J, et al. Vascular access site and outcomes among 26,807 chronic total coronary occlusion angioplasty cases from the British Cardiovascular Interventions Society National Database. JACC Cardiovasc Interv. 2017;10(7):635–44.
Fairley SL, Lucking AJ, McEntegart M, Shaukat A, Smith D, Chase A, et al. Routine use of fluoroscopic-guided femoral arterial puncture to minimise vascular complication rates in CTO intervention: multi-centre UK experience. Heart Lung Circ. 2016;25(12):1203–9.
Tanaka Y, Moriyama N, Ochiai T, Takada T, Tobita K, Shishido K, et al. Transradial coronary interventions for complex chronic total occlusions. JACC Cardiovasc Interv. 2017;10(3):235–43.
Alaswad K, Menon RV, Christopoulos G, Lombardi WL, Karmpaliotis D, Grantham JA, et al. Transradial approach for coronary chronic total occlusion interventions: insights from a contemporary multicenter registry. Catheter Cardiovasc Interv. 2015;85(7):1123–9.
Colombo A, Mikhail GW, Michev I, Iakovou I, Airoldi F, Chieffo A, et al. Treating chronic total occlusions using subintimal tracking and reentry: the STAR technique. Catheter Cardiovasc Interv. 2005;64(4):407–11 discussion 12.
Galassi AR, Tomasello SD, Costanzo L, Campisano MB, Barrano G, Ueno M, et al. Mini-STAR as bail-out strategy for percutaneous coronary intervention of chronic total occlusion. Catheter Cardiovasc Interv. 2012;79(1):30–40.
Badhey N, Lombardi WL, Thompson CA, Brilakis ES, Banerjee S. Use of the venture wire control catheter for subintimal coronary dissection and reentry in chronic total occlusions. J Invasive Cardiol. 2010;22(9):445–8.
Whitlow PL, Burke MN, Lombardi WL, Wyman RM, Moses JW, Brilakis ES, et al. Use of a novel crossing and re-entry system in coronary chronic total occlusions that have failed standard crossing techniques: results of the FAST-CTOs (facilitated antegrade steering technique in chronic total occlusions) trial. JACC Cardiovasc Interv. 2012;5(4):393–401.
Carlino M, Demir OM, Colombo A, Azzalini L. Microcatheter knuckle technique: a novel technique for negotiating the subintimal space during chronic total occlusion recanalization. Catheter Cardiovasc Interv. 2018;92:1256–60.
Rathore S, Katoh O, Matsuo H, Terashima M, Tanaka N, Kinoshita Y, et al. Retrograde percutaneous recanalization of chronic total occlusion of the coronary arteries: procedural outcomes and predictors of success in contemporary practice. Circ Cardiovasc Interv. 2009;2(2):124–32.
Ozawa N. A new understanding of chronic total occlusion from a novel PCI technique that involves a retrograde approach to the right coronary artery via a septal branch and passing of the guidewire to a guiding catheter on the other side of the lesion. Catheter Cardiovasc Interv. 2006;68(6):907–13.
Karmpaliotis D, Michael TT, Brilakis ES, Papayannis AC, Tran DL, Kirkland BL, et al. Retrograde coronary chronic total occlusion revascularization: procedural and in-hospital outcomes from a multicenter registry in the United States. JACC Cardiovasc Interv. 2012;5(12):1273–9.
• Brilakis ES, Grantham JA, Thompson CA, DeMartini TJ, Prasad A, Sandhu GS, et al. The retrograde approach to coronary artery chronic total occlusions: a practical approach. Catheter Cardiovasc Interv. 2012;79(1):3–19 An important manuscript that highlights the importance of the retrograde approach to CTO PCI and provides practical recopmmendations.
Matsuno S, Tsuchikane E, Harding SA, Wu EB, Kao HL, Brilakis ES, et al. Overview and proposed terminology for the reverse controlled antegrade and retrograde tracking (reverse CART) techniques. EuroIntervention. 2018;14(1):94–101.
• Brilakis ES, Grantham JA, Rinfret S, Wyman RM, Burke MN, Karmpaliotis D, et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc Interv. 2012;5(4):367–79 This article is the first description of the hybrid algorithm for CTO PCI.
Patel MR, Marso SP, Dai D, Anstrom KJ, Shunk KA, Curtus JP, et al. Comparative effectiveness of drug-eluting versus bare-metal stents in elderly patients undergoing revascularization of chronic total coronary occlusions: results from the National Cardiovascular Data Registry, 2005–2008. JACC Cardiovasc Interv. 2012;5(10):1054–61.
Saeed B, Kandzari DE, Agostoni P, Lombardi WL, Rangan BV, Banerjee S, et al. Use of drug-eluting stents for chronic total occlusions: a systematic review and meta-analysis. Catheter Cardiovasc Interv. 2011;77(3):315–32.
Colmenarez HJ, Escaned J, Fernández C, Lobo L, Cano S, del Angel JG, et al. Efficacy and safety of drug-eluting stents in chronic total coronary occlusion recanalization: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(17):1854–66.
Suttorp MJ, Laarman GJ, Rahel BM, Kelder JC, Bosschaert MA, Kiemeneij F, et al. Primary Stenting of Totally Occluded Native Coronary Arteries II (PRISON II): a randomized comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions. Circulation. 2006;114(9):921–8.
Moreno R, García E, Teles R, Rumoroso JR, Cyrne Carvalho H, Goicolea FJ, et al. Randomized comparison of sirolimus-eluting and everolimus-eluting coronary stents in the treatment of total coronary occlusions: results from the chronic coronary occlusion treated by everolimus-eluting stent randomized trial. Circ Cardiovasc Interv. 2013;6(1):21–8.
Sherbet DP, Christopoulos G, Karatasakis A, Danek BA, Kotsia A, Navara R, et al. Optical coherence tomography findings after chronic total occlusion interventions: insights from the “AngiographiC evaluation of the everolimus-eluting stent in chronic total occlusions” (ACE-CTO) study (NCT01012869). Cardiovasc Revasc Med. 2016;17(7):444–9.
• Riley RF, Sapontis J, Kirtane AJ, Karmpaliotis D, Kalra S, Jones PG, et al. Prevalence, predictors, and health status implications of periprocedural complications during coronary chronic total occlusion angioplasty. EuroIntervention. 2018. In this study, retrograde approach, increasing age, and increasing lesion complexity are associated with poor outcomes in CTO PCI, thus highlighting the importance of learning curve, adequate planning and choosing the right patient population.
Suero JA, Marso SP, Jones PG, Laster SB, Huber KC, Giorgi LV, et al. Procedural outcomes and long-term survival among patients undergoing percutaneous coronary intervention of a chronic total occlusion in native coronary arteries: a 20-year experience. J Am Coll Cardiol. 2001;38(2):409–14.
Safley DM, House JA, Marso SP, Grantham JA, Rutherford BD. Improvement in survival following successful percutaneous coronary intervention of coronary chronic total occlusions: variability by target vessel. JACC Cardiovasc Interv. 2008;1(3):295–302.
Di Mario C, Werner GS, Sianos G, Galassi AR, Büttner J, Dudek D, et al. European perspective in the recanalisation of chronic total occlusions (CTO): consensus document from the EuroCTO Club. EuroIntervention. 2007;3(1):30–43.
Brilakis ES, Karmpaliotis D, Patel V, Banerjee S. Complications of chronic total occlusion angioplasty. Interv Cardiol Clin. 2012;1(3):373–89.
Ge L, Zhong X, Ma J, Fan B, Lu H, Qian J, et al. Safety and feasibility of a low frame rate protocol for percutaneous coronary intervention to chronic total occlusions: preliminary experience. EuroIntervention. 2018;14(5):e538–e45.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mahesh Anantha Narayanan declares no potential conflicts of interest. Santiago Garcia is a consultant for Surmodics, Osprey medical, Medtronic, Edwards Lifesciences, Abbott and Boston Scientific. Research grants from Edwards Life Sciences, Minnesota Veterans Research Foundation and VA Office of Research and Development.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Coronary Artery Disease
Rights and permissions
About this article
Cite this article
Anantha-Narayanan, M., Garcia, S. Contemporary Approach to Chronic Total Occlusion Interventions. Curr Treat Options Cardio Med 21, 1 (2019). https://doi.org/10.1007/s11936-019-0704-9
Published:
DOI: https://doi.org/10.1007/s11936-019-0704-9