Abstract
Purpose of the Review
Chronic total occlusions (CTOs) are found in about a third of patients with coronary artery disease (CAD) and can pose a significant challenge during percutaneous revascularization. However, advances in CTO percutaneous coronary intervention (PCI) strategies, devices, and algorithms have led to significant improvements in successful treatment of CTOs. This review summarizes current management of CTOs in the context of modern PCI techniques and current evidence.
Recent Findings
The hybrid algorithm now provides a standardized, teachable approach to CTO PCI, and success rates are approximately 90% in experienced hands. The first randomized controlled trial in patients with CTOs recently reported that patients with ST elevation myocardial infarction (STEMI) and a CTO in the non-culprit vessel showed an improvement in ejection fraction in patients undergoing CTO PCI of the LAD, but not other vessels. Updated data from the SYNTAX trial showed a benefit with complete revascularization in patients with coronary artery disease (CAD). Incomplete revascularization of CTOs in the PCI group may explain some of the benefit seen with CABG over PCI in patients with complex coronary disease. Contemporary CTO registries have reported success rates of approximately 90%, and the OPEN-CTO registry updates our understanding of CTO PCI complication rates and outcomes.
Summary
The available evidence highlights the potential benefits of CTO PCI in patients with an indication for revascularization. Technological advancements have paved the way for success rates approaching 90% at high-volume centers, but further studies evaluating outcomes following CTO PCI are needed, with several currently underway.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic total occlusions (CTOs) have posed the greatest challenge to percutaneous revascularization in patients with coronary artery disease (CAD). CTOs are defined as arteries with atherosclerotic luminal narrowing resulting in TIMI grade 0 flow for over 3 months based on angiographic findings or duration of patient symptoms and clinical presentation [1•]. These lesions are prevalent in approximately a third of patients with CAD [2•, 3•] and up to 10% of patients presenting with ST elevation myocardial infarction (STEMI) [4•]. Despite this, percutaneous coronary intervention (PCI) of CTO lesions comprises only 3.8% of attempted elective PCI cases in the National Cardiovascular Disease Registry (NCDR) CathPCI registry [5]. The presence of a CTO on coronary angiography affects therapeutic strategies, resulting in more frequent referral for coronary artery bypass grafting (CABG) or medical therapy compared to stenotic, non-occluded vessels [6]. This reflects the technical challenge of CTO PCI, with concerns of lower success rates and increased intra-procedural risk.
Over the last decade, vast strides in technologic advancements, dedicated research, organized algorithms, and the training of new operators in the field have led to exponential growth in the adoption and success of CTO PCI. The complexity of these lesions requires a specialized clinical approach and skill set. Here, we present an overview of the management of CTOs and discuss relevant updates to the literature.
Development of PCI for Coronary CTOs
The history of coronary CTO PCI provides an insight into current strategies and unmet challenges. Coronary angioplasty was reported in a series of CTO cases in 1985 by Kereiakes et al. [7], but much of the early progress of percutaneous intervention of chronic occlusions began in peripheral arterial disease. Subintimal angioplasty was first described in 1989 for chronic femoral-popliteal occlusions [8], but the practice was slow to expand out of concerns that it was too technically demanding and inferior to venous bypass grafting [9]. The morbidity and mortality associated with femoral bypass surgery prompted comparisons with contemporary endovascular interventions in PAD in the BASIL trial [10]. This randomized trial showed that short-term limb salvage outcomes were similar between surgery and endovascular intervention groups. With interval growth in CTO techniques, updated safety and outcome data became available [11], with endovascular-first strategy now gaining traction for the treatment of PAD [12, 13].
Percutaneous revascularization of coronary CTOs was pioneered in Japan and other parts of East Asia, where strong cultural preferences for PCI over CABG led to lower rates of CABG and created an environment for the development of devices and strategies to treat CTOs. At that time, an antegrade wire approach with serial escalation in wire stiffness was the primary strategy. This was augmented by the parallel wire [14] and intravascular ultrasound (IVUS)-guided [15] techniques to assist with intra-plaque crossing of wires into the distal true lumen. Technologic advancements in wire and microcatheter design facilitated novel approaches to this strategy [16], but success rates plateaued at approximately 60% [17,18,19,20,21].
Subsequently, retrograde wire crossing was performed in 1990 via saphenous vein grafts [22], followed by the use of septal collaterals a few years later [23]. Crossing the lesion via the distal cap has advantages, as it is often better defined and softer than an ambiguous proximal cap [24]. Practical and technical innovation in retrograde PCI led to further improvements in success rates to approximately 85% in Japan [25]. These tools and strategies then propagated worldwide through live case demonstrations and presentations [26]. Success rates with retrograde techniques vary considerably and impact overall CTO PCI success rates, affected by characteristics of collateral channels, operator experience, and volume [27].
Borrowing from techniques used in peripheral arterial occlusion, the subintimal tracking and re-entry (STAR) technique was adopted in coronary CTOs in 2005. This approach uses a knuckled wire to track around the CTO in the subintimal space before reentering the true lumen distally. Technical success rates rose above 90%, but long dissection planes with loss of side branches primarily limited use to RCA lesions. Long-term patency rates were unfortunately low due to poor distal flow, with approximately 50% of patients requiring repeat intervention due to significant restenosis [28].
While STAR primarily exists as a last resort when antegrade wire crossing fails and collaterals do not support a retrograde approach, this sparked an interest in utilizing the subintimal space and refining control over the extent of the dissection plane and point of reentry into the true lumen. Multiple variations developed to attempt to limit the morbidity of the antegrade dissection and reentry (ADR) strategy, but were difficult to reproduce and complicated by high rates of perforation and restenosis [29]. With the advent of the blunt-tipped CrossBoss catheter (Boston Scientific) to minimize tissue trauma during dissection and the Stingray balloon (Boston Scientific) to control lumen reentry, ADR became predictable and reproducible [30].
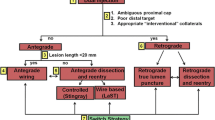
These strategies were organized through the development of the “hybrid algorithm” in 2012 [31] (Fig. 1) Use of this systematic approach led to improvement in procedural success rates to approximately 90% [32].
The “hybrid” CTO algorithm provides a structured approach to CTO PCI. Reprinted from JACC Cardiovascular Interventions [31] with permission from Elsevier, copyright 2012 American College of Cardiology Foundation (license 3982640452149)
The CTO Patient Population
Compared to patients undergoing non-CTO PCI, CTO patients are generally older with more comorbidities and impaired left ventricular (LV) function [4•, 5, 6, 33], placing them at increased risk for adverse events. CTO lesions also have a higher prevalence of 54% in patients with prior coronary artery bypass graft (CABG) surgery [4•]. This is an important and vulnerable subset of the CTO population with a high prevalence in part because the presence of a CTO is the highest predictor of referral for CABG [6]. Additionally, proximal atherosclerotic plaque appears to progress following bypass grafting due to decreased flow in the native coronary artery, and this may accelerate the process to complete occlusion [34].
Saphenous vein graft (SVG) occlusion is observed in at least 40% of vein grafts at 10 years [35, 36], and repeat CABG surgery is less desirable in this inherently high-risk population, with operative mortality reported at 3–10% [37,38,39]. Adverse outcomes of repeat CABG are high [40]; so, SVG PCI in diseased but non-occluded SVGs is pursued. However, this carries a risk of distal embolization, low long-term patency rates, and increased mortality as compared to non-SVG PCI [41] and is contraindicated in chronically occluded SVGs (class III recommendation) [42].
Analysis from the NCDR showed that 62.5% of post-CABG patients undergoing PCI received native vessel PCI. However, only 5% were CTO PCI [43], suggesting that many patients found to have CTOs post-CABG did not undergo PCI and may have been treated medically. In the contemporary Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion (OPEN CTO) registry, 37% of patients undergoing CTO PCI had undergone prior CABG (unpublished data from the OPEN CTO registry, NCT02026466). Given the poor outcomes of repeat CABG, native vessel CTO PCI is often the best treatment option for post-CABG patients with CTOs and an indication for revascularization.
Pre-Procedural Evaluation
Patient evaluation remains the most challenging and important aspect of managing patients with CAD. It begins with initial symptom assessment followed by appropriate stress testing and coronary angiography. An adequate trial of medical therapy, noninvasive risk stratification, and assessment of anatomical and patient factors are of particular importance in the CTO population prior to choosing surgical or percutaneous revascularization options.
Despite an emphasis on symptoms for indications of PCI, a patient’s angina burden is often underestimated [44]. A quantitative tool such as the Seattle Angina Questionnaire [45] may help to assess patients before and after revascularization. Patients with CTO lesions may demonstrate a high frequency of atypical angina and dyspnea [46], and a careful history is needed to sort out the contribution of heart failure or other comorbidities. Given higher rates of restenosis and lesion complexity with CTO PCI, any concern over medication adherence or contraindications to dual anti-platelet therapy must be evaluated prior to referral.
Myocardial viability is often considered prior to CTO PCI in patients with multivessel disease and LV dysfunction, with multiple studies linking viability to improvement in regional function following PCI [47,48,49,50]. While its use is controversial in the face of two negative trials [51, 52], criticisms regarding significant crossover and lack of adherence to viability results highlight the complexity of this issue. While the jury is still out on whether myocardium without evidence of viability will improve after CTO PCI, we believe that it is beneficial to demonstrate viability in segments subtended by CTOs given the increased adverse event rates and complexity of the procedure.
Ad hoc CTO PCI is strongly discouraged [53] as extensive pre-procedural planning is needed and deferring PCI is usually safe [54]. This allows time for a heart team (HT) approach [55], where multidisciplinary services give patients the best chance of a successful outcome [56, 57], irrespective of whether surgical, percutaneous, or hybrid (left internal mammary artery bypass surgery to the LAD and PCI of other vessels) revascularization strategies are pursued. Other pre-procedure considerations include determining a vascular access plan, interventional strategy, and placing limits on case duration, radiation, and contrast dose.
Appropriate Use Criteria for CTO PCI
The 2012 appropriate use criteria (AUC) update [58] classifies CTO PCI as inappropriate when there is concomitant left main disease, and uncertain with 3 vessel CAD, where CABG is the preferred revascularization strategy. In our practice, most patients referred for CTO PCI have persistent CCS class III–IV angina despite two or more antianginal medications. For patients with a single vessel CTO, this would classify as “uncertain” by the AUC, but intermediate or high-risk findings on stress testing would upgrade the indication to “appropriate.”
Asymptomatic patients are classified as “inappropriate” in many scenarios, but may be appropriate in the setting of high-risk findings on noninvasive testing, including severe LV dysfunction. The ACCF/AHA/SCAI and ESC PCI guidelines provide a class IIa recommendation for CTO PCI for patients with these indications [42, 59]. Given these recommendations, we advise caution in pursuing CTO PCI in asymptomatic patients, with careful review of indications and an individualized assessment.
Commonly Presented Arguments Against CTO PCI
1. Robust Collaterals Indicate that the Myocardium in the CTO vessel’s Distribution Is Not Ischemic
Werner et al. evaluated the coronary flow reserve across the CTO lesion in 107 patients without prior MI and found an inadequate increase in coronary flow reserve in 93% of patients, suggesting that the myocardium subtended by the CTO was ischemic despite adequate collateral flow at rest [60].
2. Opening an Occluded Vessel Does Not Improve Outcomes
The Occluded Artery Trial (OAT) [61] evaluated PCI in occluded culprit arteries 3 to 28 days after an acute MI. No benefit was found with PCI compared to optimal medical therapy at 4-year follow-up. These lesions were, by definition, not CTOs and the subtended myocardium may not have been viable, limiting the application of these results to patients with CTOs.
The COURAGE trial [62] failed to show a difference in death or MI in patients with CAD that had stable angina and myocardial ischemia randomized to an initial strategy of PCI versus medical therapy. While CTO patients were excluded from the study, angina was improved in the PCI group. There was also significant crossover in the medical therapy arm with 32.6% of patients undergoing revascularization with PCI or CABG at 4.6 years for symptom relief.
While PCI has not led to an improvement in death or MI in patients with stable CAD, including CTOs, there has been significant improvement in patient-reported outcomes such as angina and quality of life with revascularization. Therefore, as reflected in the AUC [58], patients with refractory angina despite optimal medical therapy warrant revascularization including CTO PCI to improve symptoms. This reflects the population undergoing CTO PCI, where over 70% of referrals were for symptom relief (unpublished data, OPEN-CTO registry).
3. CABG Is Better for CTO Patients
Several trials have compared CABG versus PCI in stable CAD patients. The SYNTAX trial randomized patients with left main or three vessel CAD to CABG versus PCI, using first generation, TAXUS drug-eluting stents [63]. PCI resulted in a higher rate of adverse events at 12 months, mainly driven by repeat revascularization, and a higher rate of MI-related death at 5-year follow up [64]. Stratifying for complexity of anatomy using the SYNTAX score, there was no difference in MACE between groups in patients with less complex anatomy (with low and intermediate SYNTAX scores). However, there was a significant difference noted in patients with high SYNTAX scores ≥33. Further analyses demonstrated that this may have been due to a lower proportion of complete revascularization in the PCI group (43.1%) as compared to the CABG group (56.4%) [65].
Among those excluded from the trial, 22% were due to a CTO that the investigators felt could not be successfully revascularized. Of those included, CTOs were present in 23% of patients, and these were successfully treated by PCI in 49% compared to 68% by CABG [66]. The presence of a CTO was identified as the strongest predictor for incomplete revascularization in those undergoing PCI. Additionally, patients with a post-PCI SYNTAX score reduced to <8 have improved outcomes, irrespective of pre-PCI SYNTAX score [67, 68].
These data suggest that completeness of revascularization in patients with multivessel CAD is much higher with CABG compared to PCI, mainly due to the inability to successfully perform CTO PCI and may have led to the differences in MACE noted in patients with the highest SYNTAX scores [64, 66]. In patients that are at high risk for CABG or inoperable, PCI may be the preferred alternative when complete revascularization, including CTO PCI, can be achieved.
Assessment of the CTO Lesion
Coronary angiography dedicated to characterization of the CTO lesion and visualizing collaterals for retrograde techniques is ideally performed at the time of diagnostic coronary angiography. A prolonged cine run without panning is imperative to completely visualize the target vessel and collaterals for pre-procedural planning. In select cases, dedicated CT angiography can be used when lesion length is unclear and can help to characterize calcification and tortuosity [69].
The Japan-CTO (J-CTO) score (Fig. 2) is a widely used scoring tool to characterize CTO lesions [70]. This point-based score uses tortuosity within the lesion, calcification, blunt vs tapered proximal cap, lesion length > 20 mm, and prior failed attempts to describe complexity. This was validated for successful guidewire crossing within 30 min but did not predict ultimate angiographic success [71]. The PROGRESS CTO Score was derived from a cohort of 781 CTO PCIs to predict technical success. Absence of interventional collaterals, circumflex artery CTO and proximal cap ambiguity were identified as predictors for unsuccessful PCI using the hybrid algorithm [32] and were included in the 4 point score [72]. The PROGRESS investigators found their score slightly better predicted technical success compared to the J-CTO score, with a C-statistic of 0.746 versus 0.720. These tools provide a guide for anticipating procedure time, contrast, and radiation exposure [71] with reasonable prediction of successful PCI [70, 72].
A guide to calculating the J-CTO (Multicenter CTO Registry of Japan) score. Reprinted from JACC: Cardiovascular Interventions, 4(2), Morino, Y et al. “Predicting Successful Guidewire Crossing Through Chronic Total Occlusion of Native Coronary Lesions Within 30 Minutes: The J-CTO(Multicenter CTO Registry in Japan) Score as a Difficulty Grading and Time Assessment Tool,” pg 213–21, copyright 2011 American College of Cardiology Foundation, published with permission from Elsevier (License 4010400868803)
The hybrid algorithm is used for planning CTO PCI strategies by characterization of the proximal cap, lesion length, and the distal target vessel [32]. The four strategies include antegrade wire escalation (AWE), antegrade dissection and reentry (ADR), retrograde wire escalation (RWE), and retrograde dissection and reentry. Review of the angiogram identifies potential options for successful CTO PCI and rules out options when anatomy renders an approach impractical, such as lack of interventional collaterals for a retrograde approach.
Reviewing the CTO PCI strategies with the catheterization lab technicians and nurses prior to the case ensures organization once the procedure is underway. While these measures aid in planning a safe procedure, these cases should always be performed with additional operators and surgical backup available.
Intraprocedural Considerations
The highest predictor of failure of CTO PCI is lack of dual angiography [73]. It helps to map the true length of the CTO lesion, evaluate collaterals, and plan for PCI and stenting of the distal vessel. Retrograde angiography is also critical for confirmation of distal wire position when antegrade techniques are used. We commonly use an 8F guide via the femoral artery for the target vessel and a 6F guide via the radial artery for the retrograde donor vessel, except in the case of post-CABG patients, where we often use bi-femoral 8F sheathes for additional guide support. Several tools facilitate equipment delivery, including guide extensions, microcatheters, short balloons, and atherectomy, but details of their use are beyond the scope of this review.
Operators must be prepared to manage all PCI complications with perforation being the most common life-threatening complication in CTO PCI. Comfort with emergent pericardiocentesis and methods to limit extravasation, including coils, beads, emulsified fat, thrombin, or covered stents, must be readily available [24]. Contrary to the common belief that there is low risk of tamponade in CABG patients, it can instead present with localized hemopericardium and focal compression, requiring surgical washout for management in unstable patients. Comfort with managing vascular access complications is also imperative, owing to larger sheaths and aggressive anti-coagulation utilized during CTO PCI.
Outcomes of CTO PCI
The CTO population is an inherently difficult population to study, given heterogenous anatomy, overlap with complex coronary disease, and variable clinical presentations. No randomized trials have evaluated the clinical outcomes after CTO PCI, but recent interest has prompted multiple important observational and registry studies that shed light on potential benefits.
In the NCDR CathPCI database, success rate of CTO PCI was only 59% compared to 96% in non-CTO PCI, with twice the incidence of major adverse cardiac events (MACE) at 1.6 versus 0.8% [5]. Interestingly, procedural success of CTO PCI improved over the study period from 2009 to 2013 and was significantly higher for operators performing >10 procedures at 75 versus 53% for operators performing fewer than 5 annually. Complication rates were also lower than those in CTO PCI registries. The Canadian multicenter registry reported a procedural success rate of 70% in CTO PCI [4•] while the RECHARGE registry reported an overall success rate of 74% using the hybrid algorithm [74]. Success of retrograde CTO PCI is more dependent on operator experience [75] and is expected to improve as adoption of retrograde techniques continues to expand [76].
Procedural MACE in CTO PCI with experienced providers was previously reported at 1.8% [32]. Coronary perforation and MI were the most common adverse events in a meta-analysis of over 18,000 patients undergoing CTO PCI [77]. Coronary perforations leading to tamponade, emergency CABG, and death were reported at <1% each [5, 77] with tamponade occurring more frequently in retrograde cases [76, 77]. However, in the recent OPEN-CTO registry of experienced CTO operators, the adverse event rate was 7%, including 4% periprocedural MI and death [78].
Long-term MACE at 1 year was evaluated in an Italian registry and found to be 2.6% driven by target lesion revascularization [79]. A Korean cohort of elective CTO PCI patients showed a rate of cardiac death of 5% and repeat revascularization of 8.6% in successful cases at a median of 4.6 years of follow-up [80]. Successful CTO PCI has also been associated with a reduction in symptoms [81,82,83,84], sustained improvement in quality of life [80], improved left ventricular function [49], reduced need for subsequent CABG [85], and improved survival [21, 86].
Recent Updates and Future Directions
-
1.
The short duration of follow-up, lack of adjudicated and patient reported outcomes in the observational studies mentioned above led to the development of the OPEN CTO registry. This is an adjudicated, prospective cohort of over 1000 consecutive CTO patients that underwent PCI at 14 high-volume centers in the USA with 30-day, 6-month, and 1-year patient follow-up (NCT02026466). Success rates have been reported at 89% with 7.2% MACE, including 1.4% in-hospital death [78]. This registry which includes post-CABG and inoperable patients will continue to provide real-world data regarding outcomes of CTO PCI.
-
2.
The EXPLORE-CTO trial randomized 300 patients presenting with STEMI and found to have a CTO in a non-culprit vessel to CTO PCI within 7 days versus no CTO PCI. The primary outcome was improvement in LVEF, of which no difference was observed between groups at 4-month follow-up. However, subgroup analyses showed a significant increase in LVEF (40.4 to 47.2%; p = 0.02) when the non-culprit CTO lesion was in the LAD [87].
-
3.
The STITCH trial [88] randomized patients with multivessel CAD and severe LV dysfunction (EF <35%) to revascularization with CABG versus medical therapy. It failed to show a difference in adverse events at 5 years. Recently, 10-year results were published and showed an improvement in all-cause mortality in the CABG group (58.9 vs 66.1%, p = 0.02) [89]. While this trial did not evaluate CTO PCI, it suggests that patients with ischemic cardiomyopathy benefit from complete revascularization. In patients that are inoperable due to comorbidities, complete revascularization with PCI including CTO PCI may also offer benefit, but needs further study.
-
4.
The Euro CTO (NCT01760083) and Korean-based Decision CTO (NCT01078051) trials are underway and randomizing patients with CTOs and chronic stable angina to PCI versus medical therapy. Their primary outcomes are MACE and quality of life at 3 to 5 years (unpublished trials, clinicaltrials.gov.)
Conclusion
Interventional cardiology has experienced a recent renaissance in PCI with renewed interest in revascularization of complex anatomy and high-risk patients. This has been facilitated by the advent of additional tools for hemodynamic support, formation of patient-centered multidisciplinary Heart Teams, and the development of organized strategies and algorithms to tackle challenging lesions such as CTOs. Courses and fellowships to acquire the necessary skills for CTO PCI are available and foster relationships between CTO operators across institutions. As CTO PCI becomes more available, it is imperative that the culture of scientific investigation and collaboration continue to push the field forward to minimize variation and ensure that all patients receive appropriate and necessary therapies.
References
Papers of Particular Interest, Published recently, Have Been Highlighted as: • Of importance
• Stone GW, Kandzari DE, Mehran R, et al (2005) Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part I. In: Circulation. American Heart Association, Inc, pp 2364–2372
• Kahn JK. Angiographic suitability for catheter revascularization of total coronary occlusions in patients from a community hospital setting. Am Heart J. 1993;126:561–4.
• Werner GS, Gitt AK, Zeymer U, Juenger C, Towae F, Wienbergen H, Senges J. Chronic total coronary occlusions in patients with stable angina pectoris: impact on therapy and outcome in present day clinical practice. Clin Res Cardiol. 2009;98:435–41.
• Fefer P, Knudtson ML, Cheema AN, et al. Current perspectives on coronary chronic total occlusions: the Canadian multicenter chronic Total occlusions registry. J Am Coll Cardiol. 2012;59:991–7.
Brilakis ES, Banerjee S, Karmpaliotis D, Lombardi WL, Tsai TT, Shunk KA, Kennedy KF, Spertus JA, Holmes DR, Grantham JA. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2015;8:245–53.
Christofferson RD, Lehmann KG, Martin GV, Every N, Caldwell JH, Kapadia SR. Effect of chronic total coronary occlusion on treatment strategy. AJC. 2005;95:1088–91.
Kereiakes DJ, Selmon MR, McAuley BJ, McAuley DB, Sheehan DJ, Simpson JB. Angioplasty in total coronary artery occlusion: experience in 76 consecutive patients. JACC. 1985;6:526–33.
Bolia A, Brennan J, Bell PR. Recanalisation of femoro-popliteal occlusions: improving success rate by subintimal recanalisation. Clin Radiol. 1989;40:325.
Ascher E, Hingorani A (2007) Subintimal angioplasty. J Cardiovasc Surg
Bradbury AW, Ruckley CV, Fowkes F, Forbes JF (2005) Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. doi: 10.1016/S0140-6736(05)67704-5
Bown MJ, Bolia A, Sutton AJ. Subintimal angioplasty: meta-analytical evidence of clinical utility. European Journal of Vascular & Endovascular Surgery. 2009;38:323–37.
Klein AJ, Ross CB. Endovascular treatment of lower extremity peripheral arterial disease. Trends in Cardiovascular Medicine. 2016;26:495–512.
Markose G, Bolia A. Subintimal angioplasty in the management of lower limb ischaemia. J Cardiovasc Surg. 2006;47:399–406.
Mitsudo K, Yamashita T, Asakura Y, Muramatsu T, Doi O, Shibata Y, Morino Y. Recanalization strategy for chronic total occlusions with tapered and stiff-tip guidewire. The results of CTO new techniQUE for STandard procedure (CONQUEST) trial. J Invasive Cardiol. 2008;20:571–7.
Galassi AR, Sumitsuji S, Boukhris M, et al. Utility of intravascular ultrasound in percutaneous revascularization of chronic Total occlusions. JACC Cardiovasc Interv. 2016;9:1979–91.
Sumitsuji S, Inoue K, Ochiai M, Tsuchikane E, Ikeno F. Fundamental wire technique and current standard strategy of percutaneous intervention for chronic total occlusion with histopathological insights. JACC Cardiovasc Interv. 2011;4:941–51.
Holmes DR, Vlietstra RE, Reeder GS, Bresnahan JF, Smith HC, Bove AA, Schaff HV. Angioplasty in total coronary artery occlusion. JACC. 1984;3:845–9.
Safian RD, McCabe CH, Sipperly ME, McKay RG, Baim DS. Initial success and long-term follow-up of percutaneous transluminal coronary angioplasty in chronic total occlusions versus conventional stenoses. AJC. 1988;61:23G–8G.
Stone GW, Rutherford BD, McConahay DR, Johnson WL, Giorgi LV, Ligon RW, Hartzler GO. Procedural outcome of angioplasty for total coronary artery occlusion: an analysis of 971 lesions in 905 patients. JACC. 1990;15:849–56.
Hoye A, van Domburg RT, Sonnenschein K, Serruys PW. Percutaneous coronary intervention for chronic total occlusions: the Thoraxcenter experience 1992-2002. Eur Heart J. 2005;26:2630–6.
Mehran R, Claessen BE, Godino C, et al. Long-term outcome of percutaneous coronary intervention for chronic total occlusions. JACC Cardiovasc Interv. 2011;4:952–61.
Kahn JK, Hartzler GO. Retrograde coronary angioplasty of isolated arterial segments through saphenous vein bypass grafts. Catheter Cardiovasc Interv. 1990;20:88–93.
Surmely J-F, Tsuchikane E, Katoh O, Nishida Y, Nakayama M, Nakamura S, Oida A, Hattori E, Suzuki T. New concept for CTO recanalization using controlled antegrade and retrograde subintimal tracking: the CART technique. J Invasive Cardiol. 2006;18:334–8.
Brilakis E (2013) Manual of Coronary Chronic Total Occlusion Interventions. Academic Press
Rathore S, Katoh O, Matsuo H, et al. Retrograde percutaneous recanalization of chronic total occlusion of the coronary arteries: procedural outcomes and predictors of success in contemporary practice. Circ Cardiovasc Interv. 2009;2:124–32.
Kandzari DE. Import and export of interventional technique: something to declare at the border. JACC Cardiovasc Interv. 2009;2:843–5.
Karmpaliotis D, Karatasakis A, Alaswad K, et al. Outcomes with the use of the retrograde approach for coronary chronic Total occlusion interventions in a contemporary multicenter US registry. Circ Cardiovasc Interv. 2016;9:e003434.
Colombo A, Mikhail GW, Michev I, et al (2005) Treating chronic total occlusions using subintimal tracking and reentry: the STAR technique. Catheter Cardiovasc Interv 64:407–11– discussion 412
Carlino M, Godino C, Latib A, Moses JW, Colombo A. Subintimal tracking and re-entry technique with contrast guidanc: a safer approach. Catheter Cardiovasc Interv. 2008;72:790–6.
Wilson W, Spratt JC. Advances in procedural techniques--antegrade. Curr Cardiol Rev. 2014;10:127–44.
Brilakis ES, Grantham JA, Rinfret S, et al. A percutaneous treatment algorithm for crossing coronary chronic Total occlusions. JACC Cardiovasc Interv. 2012;5:367–79.
• Christopoulos G, Menon RV, Karmpaliotis D, Alaswad K, Lombardi W, Grantham A, Patel VG, Rangan BV, Brilakis E. The efficacy and safety of the “hybrid” approach to coronary chronic total occlusions: insights from a contemporary multicenter US registry and comparison with prior studies. J Invasive Cardiol. 2014;26:427–32. The organization of multiple, complex techniques in the Hybrid algorithm provides a structure to approaching CTO PCI that increases accessibility for learning operators and standardizes the process to aid further study in this field.
Jeroudi OM, Alomar ME, Michael TT, et al. Prevalence and management of coronary chronic total occlusions in a tertiary veterans affairs hospital. Catheter Cardiovasc Interv. 2013;84:637–43.
Bourassa MG, Campeau L, Lesperance J, Grondin CM. Changes in grafts and coronary arteries after saphenous vein aortocoronary bypass surgery: results at repeat angiography. Circulation. 1982;65:90–7.
Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. JACC. 2004;44:2149–56.
Sabik III JF, Lytle BW, Blackstone EH, Houghtaling PL, Cosgrove DM. Comparison of saphenous vein and internal thoracic artery graft patency by coronary system. Ann Thorac Surg. 2005;79:544–51.
Loop FD, Lytle BW, Cosgrove DM, Woods EL, Stewart RW, Golding LA, Goormastic M, Taylor PC (1990) Reoperation for coronary atherosclerosis. Changing practice in 2509 consecutive patients. Annals of Surgery 212:378–85– discussion 385–6
Yap C-H, Sposato L, Akowuah E, et al. Contemporary results show repeat coronary artery bypass grafting remains a risk factor for operative mortality. Ann Thorac Surg. 2009;87:1386–91.
Freeman M, Farouque HMO, Shardey GC, Smith JA, Andrianopoulos N, Reid C, Ajani AE, Duffy SJ, Brennan A, Clark DJ. Percutaneous coronary intervention (PCI) versus redo coronary artery bypass grafting (CABG) in “protected” left main (PLM) coronary disease. Heart, Lung and Circulation. 2009;18:S69–70.
Morrison DA, Sethi G, Sacks J, Henderson WG, Grover F, Sedlis S, Esposito R. Percutaneous coronary intervention versus repeat bypass surgery for patients with medically refractory myocardial ischemia: AWESOME randomized trial and registry experience with post-CABG patients. JACC. 2002;40:1951–4.
Lee MS, Park S-J, Kandzari DE, et al. Saphenous vein graft intervention. JACC Cardiovasc Interv. 2011;4:831–43.
Levine GN, Bates ER, Blankenship JC, et al (2011) 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. In: Circulation. American Heart Association, Inc, pp e574–651
• Brilakis ES, Banerjee S, Karmpaliotis D, Lombardi WL, Tsai TT, Shunk KA, Kennedy KF, Spertus JA, Holmes DR, Grantham JA. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2015;8:245–253. The NCDR’s report is the only current real-world evaluation of procedural outcomes that includes CTO PCI patients. The reported complication and success rates likely underestimate both as compared to CTO-specific registries; look for OPEN CTO registry results to give us better answers regarding these questions.
Arnold SV, Grodzinsky A, Gosch KL, Kosiborod M, Jones PG, Breeding T, Towheed A, Beltrame J, Alexander KP, Spertus JA (2016) Predictors of Physician Under-Recognition of Angina in Outpatients With Stable Coronary Artery Disease. Circ Cardiovasc Qual Outcomes CIRCOUTCOMES.116.002781
Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle angina questionnaire: a new functional status measure for coronary artery disease. JACC. 1995;25:333–41.
Galassi AR, Brilakis ES, Boukhris M, et al (2015a) Appropriateness of percutaneous revascularization of coronary chronic total occlusions: an overview. European Heart Journal ehv391
Werner GS, Emig U, Bahrmann P, Ferrari M, Figulla HR. Recovery of impaired microvascular function in collateral dependent myocardium after recanalisation of a chronic total coronary occlusion. Heart. 2004;90:1303–9.
Baks T, van Geuns R-J, Duncker DJ, Cademartiri F, Mollet NR, Krestin GP, Serruys PW, de Feyter PJ. Prediction of left ventricular function after drug-eluting stent implantation for chronic Total coronary occlusions. J Am Coll Cardiol. 2006;47:721–5.
Kirschbaum SW, Baks T, van den Ent M, Sianos G, Krestin GP, Serruys PW, de Feyter PJ, van Geuns R-JM. Evaluation of left ventricular function three years after percutaneous recanalization of chronic Total coronary occlusions. Am J Cardiol. 2008;101:179–85.
Sirnes PA, Myreng Y, Mølstad P, Bonarjee V, Golf S. Improvement in left ventricular ejection fraction and wall motion after successful recanalization of chronic coronary occlusions. Eur Heart J. 1998;19:273–81.
Bonow RO, Maurer G, Lee KL, et al. Myocardial Viability and Survival in Ischemic Left Ventricular Dysfunction. 2011;364:1617–1625. doi:10.1056/NEJMoa1100358
Beanlands RSB, Nichol G, Huszti E, et al. F-18-Fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease. J Am Coll Cardiol. 2007;50:2002–12.
Banning AP, Baumbach A, Blackman D, et al. Percutaneous coronary intervention in the UK: recommendations for good practice 2015. Heart. 2015;101(Suppl 3):1–13.
Fang H-Y, Lee W-C, Hussein H, et al. In-hospital and 3-year clinical outcomes following ad hoc versus staged percutaneous coronary interventions in chronic total occlusion — a real world practice. IJC Heart & Vessels. 2014;4:73–80.
Writing Committee Members, Levine GN, Bates ER, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–651.
Feit F, Brooks MM, Sopko G, et al. Long-term clinical outcome in the bypass angioplasty revascularization investigation registry: comparison with the randomized trial. BARI Investigators Circulation. 2000;101:2795–802.
King III SB, Barnhart HX, Kosinski AS, et al. Angioplasty or surgery for multivessel coronary artery disease: comparison of eligible registry and randomized patients in the EAST trial and influence of treatment selection on outcomes. Am J Cardiol. 1997;79:1453–9.
Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update. JACC. 2012;59:857–81.
Members ATF, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2014;35:278–2619.
Werner GS, Surber R, Ferrari M, Fritzenwanger M, Figulla HR. The functional reserve of collaterals supplying long-term chronic total coronary occlusions in patients without prior myocardial infarction. Eur Heart J. 2006;27:2406–12.
Hochman JS, Lamas GA, Buller CE, et al. Coronary Intervention for Persistent Occlusion after Myocardial Infarction. 2009;355:2395–2407. doi:10.1056/NEJMoa066139.
Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16.
Serruys PW, Morice M-C, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–72.
Milojevic M, Head SJ, Parasca CA, et al. Causes of death following PCI versus CABG in complex CAD: 5-year follow-up of SYNTAX. J Am Coll Cardiol. 2016;67:42–55. Understanding the nuances and patient characteristics in the SYNTAX trial is imperative when counseling patients, particularly those who are at high risk for CABG and PCI.
Head SJ, Mack MJ, Holmes DR, Mohr FW, Morice M-C, Serruys PW, Kappetein AP. Incidence, predictors and outcomes of incomplete revascularization after percutaneous coronary intervention and coronary artery bypass grafting: a subgroup analysis of 3-year SYNTAX data. Eur J Cardiothorac Surg. 2012;41:535–41.
• Farooq V, Serruys PW, Garcia-Garcia HM, et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with Total occlusions. J Am Coll Cardiol. 2013;61:282–94. Complete revascularization is an emerging clinical endpoint that promises to be pertinent as a quality benchmark and predictor for prognosis, which is described in this paper evaluating the SYNTAX cohort.
Loutfi M, Wagdy S, Sobhy M (2016) Impact of the Residual SYNTAX Score on Outcomes of Revascularization in Patients with ST-Segment Elevation Myocardial Infarction and Multivessel Disease. CMC 29–7
Park KW, Kang J, Kang S-H, et al. The impact of residual coronary lesions on clinical outcomes after percutaneous coronary intervention: residual SYNTAX score after percutaneous coronary intervention in patients from the efficacy of Xience/Promus versus cypher in rEducing late loss after stENTing (EXCELLENT) registry. Am Heart J. 2014;167:384–92.
Hoe J. CT coronary angiography of chronic total occlusions of the coronary arteries: how to recognize and evaluate and usefulness for planning percutaneous coronary interventions. Int J Cardiovasc Imaging. 2009;25:43–54.
Morino Y, Abe M, Morimoto T, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (multicenter CTO registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4:213–21.
Nombela-Franco L, Urena M, Jerez-Valero M, Nguyen CM, Ribeiro HB, Bataille Y, Rodes-Cabau J, Rinfret S. Validation of the J-chronic Total occlusion score for chronic Total occlusion percutaneous coronary intervention in an independent contemporary cohort. Circ Cardiovasc Interv. 2013;6:635–43.
Christopoulos G, Kandzari DE, Yeh RW, et al. Development and validation of a novel scoring system for predicting technical success of chronic Total occlusion percutaneous coronary interventions: the PROGRESS CTO (prospective global registry for the study of chronic Total occlusion intervention) score. JACC Cardiovasc Interv. 2016;9:1–9.
Singh M, Bell MR, Berger PB, Holmes DR. Utility of bilateral coronary injections during complex coronary angioplasty. J Invasive Cardiol. 1999;11:70–4.
Maeremans J, Walsh S, Knaapen P, et al. The hybrid algorithm for treating chronic Total occlusions in Europe: the RECHARGE registry. J Am Coll Cardiol. 2016;68:1958–70.
Thompson CA, Jayne JE, Robb JF, Friedman BJ, Kaplan AV, Hettleman BD, Niles NW, Lombardi WL. Retrograde techniques and the impact of operator volume on percutaneous intervention for coronary chronic total occlusions an early U.S. experience. JACC Cardiovasc Interv. 2009;2:834–42.
Galassi AR, Sianos G, Werner GS, et al. Retrograde recanalization of chronic Total occlusions in Europe: procedural, In-hospital, and long-term outcomes from the multicenter ERCTO registry. J Am Coll Cardiol. 2015b;65:2388–400.
Patel VG, Brayton KM, Tamayo A, et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic Total occlusion interventions. JACC Cardiovasc Interv. 2013;6:128–36.
Riley R, Sapontis J, Jones P, Kirtane A, Yeh R, Karmpaliotis D, Grantham JA, Lombardi W, Brilakis E, McCabe J (2016) TCT-282 Risk Factors Associated with Adverse Events during Chronic Total Occlusion Percutaneous Coronary Interventions: A sub-Analysis of the OPEN-CTO Study. JACC 68:B116
Tomasello SD, Boukhris M, Giubilato S, et al (2015) Management strategies in patients affected by chronic total occlusions: results from the Italian Registry of Chronic Total Occlusions. European Heart Journal 36:ehv450–3198
Lee PH, Lee S-W, Park H-S, et al. Successful recanalization of native coronary chronic Total occlusion is not associated with improved long-term survival. JACC Cardiovasc Interv. 2016;9:530–8.
Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic Total occlusion recanalization: results from the FlowCardia's approach to chronic Total occlusion recanalization (FACTOR) trial. Circ Cardiovasc Qual Outcomes. 2010;3:284–90.
Valenti R, Migliorini A, Signorini U, Vergara R, Parodi G, Carrabba N, Cerisano G, Antoniucci D. Impact of complete revascularization with percutaneous coronary intervention on survival in patients with at least one chronic total occlusion. Eur Heart J. 2008;29:2336–42.
Ivanhoe RJ, Weintraub WS, Douglas JS, Lembo NJ, Furman M, Gershony G, Cohen CL, King SB. Percutaneous transluminal coronary angioplasty of chronic total occlusions. Primary success, restenosis, and long-term clinical follow-up. Circulation. 1992;85:106–15.
Olivari Z, Rubartelli P, Piscione F, et al. Immediate results and one-year clinical outcome after percutaneous coronary interventions in chronic total occlusions. J Am Coll Cardiol. 2003;41:1672–8.
Joyal D, Afilalo J, Rinfret S. Effectiveness of recanalization of chronic total occlusions: a systematic review and meta-analysis. Am Heart J. 2010;160:179–87.
Jones DA, Weerackody R, Rathod K, et al. Successful recanalization of chronic Total occlusions is associated with improved long-term survival. JACC Cardiovasc Interv. 2012;5:380–8.
Henriques JPS, Hoebers LP, Råmunddal T, et al. Percutaneous intervention for concurrent chronic Total occlusions in patients with STEMI. J Am Coll Cardiol. 2016;68:1622–32.
Velazquez EJ, Lee KL, Deja MA, Jain A. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–16.
Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Kathleen Kearney declares no conflicts of interest.
Ravi S. Hira declares personal fees from Abiomed for speaking.
Robert F. Riley declares personal fees from Abiomed and Spectranetics.
Arun Kalyanasundaram declares personal fees from Boston Scientific, Abbot Vascular, and Asahi Intecc for consultant work.
William L. Lombardi declares personal fees from Spectranetics (his wife is an employee); personal fees from Shockwave for a one-time agreement, technical advisor TCT 10/2016; personal fees from Vascular Solutions for consultant/technical advisor work; and declares stock equity with Corindus, and possible upcoming roll as consultant/technical advisor.
Human and Animal Rights and Informed Consent
All studies by William Lombardi involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
Additional information
This article is part of the Topical Collection on Coronary Heart Disease
Rights and permissions
About this article
Cite this article
Kearney, K., Hira, R.S., Riley, R.F. et al. Update on the Management of Chronic Total Occlusions in Coronary Artery Disease. Curr Atheroscler Rep 19, 19 (2017). https://doi.org/10.1007/s11883-017-0655-0
Published:
DOI: https://doi.org/10.1007/s11883-017-0655-0