Abstract
Purpose of review
Spontaneous coronary artery dissection (SCAD) is a non-iatrogenic and non-traumatic separation of the coronary arterial wall. While SCAD represents an important cause of myocardial infarction, optimal diagnostic and therapeutic options remain challenging. We sought to review recent studies and provide an update on diagnosis and management of SCAD.
Recent findings
Coronary angiography is the first-line diagnostic modality for SCAD, with three angiographic features commonly observed in SCAD: type 1 (pathognomonic angiographic appearance with contrast staining of the arterial wall), type 2 (long coronary stenosis), and type 3 (focal tubular stenosis). In addition, adjunctive intracoronary imaging can aid in identifying coronary dissections. Conservative management with beta-blockers and aspirin remains the mainstay of therapy. However, patients with high-risk features and recurrent symptoms may require revascularization. Several techniques have been reported, such as long stents to seal the entire length of the dissection, stepwise stenting starting at the distal edge followed by proximal edge stenting, use of bioabsorbable stents, and cutting balloon angioplasty. Furthermore, cardiac rehabilitation appears to be safe and offers significant benefits for patients with SCAD.
Summary
Coronary angiographic classification contributed to the increased recognition of SCAD in recent years. Selecting the most suitable and appropriate therapy based on accurate diagnosis is the cornerstone of management in SCAD. Further studies are needed to establish optimal treatment of SCAD depending on anatomical and/or clinical features.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spontaneous coronary artery dissection (SCAD) is defined as a non-iatrogenic and non-traumatic separation of the coronary arterial wall. Once thought to be a rare condition, SCAD is garnering more attention as an important cause of acute coronary syndromes (ACS). Specifically, SCAD is the most common cause of myocardial infarction (MI) during pregnancy or in the postpartum period [1]. The precise mechanism of SCAD is yet to be fully understood, but two proposed hypotheses suggest intimal tear or medial hemorrhage that leads to the intramural hematoma (IMH), which then creates a false lumen with subsequent true lumen compression and myocardial ischemia [2]. Fibromuscular dysplasia (FMD) is one of the most common predisposing conditions, and it has been observed in 52 to 86% of patients who present with SCAD [3,4,5]. There is frequently an underlying arteriopathy with fragile arterial wall architecture in FMD and connective tissue disorders, which plays a role in the propagation of the dissection that has been seen during diagnostic or therapeutic procedures. The majority of patients with SCAD present with ACS and the awareness and recognition of SCAD continue to grow [6, 7]. In this review, we will discuss recent data regarding the diagnosis and management of SCAD.
Diagnosis
High clinical index of suspicion is important to accurately and promptly diagnose SCAD. SCAD should be considered in young women, especially during pregnancy or postpartum periods, presenting with ACS. We will discuss some of the conventional diagnostic tools, which can help to make an accurate diagnosis of SCAD.
Coronary anatomy in SCAD
SCAD can affect any coronary artery and may involve multiple coronary arteries. In a retrospective study of 189 patients, left anterior descending (LAD) artery was the most frequently affected vessel (32.3%), followed by left circumflex artery (13.2%) and right coronary artery (13.2%) [8]. Left main artery is rarely involved (2.1%), but dissections can occur in multiple arteries (7.9% of patients who present with SCAD) [8]. Majority of dissections occur in mid to distal segments of coronary arteries [4, 8]. However, in pregnancy-associated SCAD, left main artery is more frequently involved (24–36%) with a greater proportion of multivessel dissections (33–40%). Pregnancy-associated SCAD patients present more commonly with high-risk clinical features (e.g., ST-segment elevation MI [STEMI] and lower left ventricular ejection fraction) than non-pregnancy-associated SCAD [9•, 10]. In addition, ostial or proximal segments of the coronary arteries are more frequently involved (62.5 vs. 10.1%) in pregnancy-associated SCAD [8, 10].
Coronary angiography
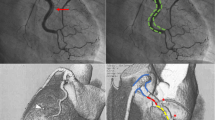
Coronary angiography remains the first-line diagnostic modality for SCAD. There are three distinct types of angiographic descriptions of SCAD, proposed by Saw et al. (Fig. 1) [11]. Type 1 is characterized by the pathognomonic angiographic appearance: contrast dye staining of the arterial wall and multiple radiolucent lumens, with or without the presence of contrast hang-up or delayed clearing. Type 2 represents long (typically > 20 mm in length) coronary stenosis with severity varying between mild stenosis and complete occlusion. This diffuse angiographic narrowing of coronary lumen commonly affects mid to distal segments of coronary arteries and corresponds to the extent of IMH. Proximally, a transition point where the normal caliber of coronary artery abruptly narrows can commonly be appreciated. Distally, the narrowing can either be bordered by normal artery segments (type 2A) or extend to the tip of the artery (type 2B). Intracoronary nitroglycerin should be administered to exclude potential coronary vasospasm. If the narrowing persists after nitroglycerin and the diagnosis remains uncertain, intracoronary imaging (optical coherence tomography [OCT] or intravascular ultrasound [IVUS]) can be pursued to confirm the diagnosis. If there is a concern that adjunctive imaging will cause propagation of the dissection and further compromise of coronary blood flow, then repeat coronary angiography with/without intracoronary imaging can be considered in 4 to 6 weeks. Although type 2 SCAD is often missed, interventionalists need to gain familiarity with its unique features. Type 3 SCAD is characterized by a focal tubular stenosis, which can be challenging to distinguish from an atherosclerotic disease by angiography. If there is a high suspicion for SCAD, operators should utilize intracoronary imaging modalities to confirm the diagnosis, particularly given the challenges with type 3 SCAD. Lack of atherosclerotic changes in other coronary arteries should also raise the suspicion of SCAD. In addition, a case-control study demonstrated an association between SCAD and coronary tortuosity, defined by the presence of ≥ 3 consecutive curvatures of 90° to 180° measured at end-diastole in a major epicardial coronary artery ≥ 2 mm in diameter [12]. Tortuosity was prevalent in SCAD (78 vs. 17% in controls; p < 0.0001). Based on the described coronary angiographic classification of SCAD, type 2 was the most common (67.5–69.8%), followed by type 1 (25.6–29.1%) and type 3 (3.4–4.7%) in retrospective studies [4, 13••]. Angiographic classification of SCAD has greatly increased recognition of SCAD in recent years as operators gained familiarity with the various angiographic appearances of SCAD [14].
Coronary angiographic types of spontaneous coronary artery dissection. Type 1 has the pathognomonic appearance with contrast media staining of arterial wall. Type 2A shows long coronary stenosis bordered by normal artery segments. Type 2B has long coronary stenosis that extends to the tip of the artery. Type 3 shows focal or tubular stenosis that is challenging to distinguish from atherosclerosis.
It is noteworthy that corresponding ventricular regional wall motion abnormality on ventriculography can be another clue for the diagnosis of SCAD. A case series of 22 patients with SCAD involving 25 coronary arteries demonstrated wall motion abnormalities (hypokinesis or akinesis) corresponding to the dissected arteries in all but one case [15]. This finding is particularly instrumental in distinguishing angiographic type 2B SCAD from “normal vessel tapering.”
Intracoronary imaging
While coronary angiography is excellent in diagnosing luminal narrowing, its two-dimensional luminal imaging has limitations in assessing the pathological process affecting the coronary arterial wall. IVUS and OCT can aid with confirmation of SCAD features by providing visualization of vessel wall tear, especially when the diagnosis is uncertain by angiography.
IVUS produces images by utilizing backscattering of emitted ultrasonic waves, which are then converted to electrical signals. Maehara et al. first described five patients with SCAD who were diagnosed with IVUS without characteristic angiographic features, emphasizing the role of IVUS in the diagnosis of SCAD [2]. IVUS can confirm SCAD by demonstrating the presence of IMH and/or false lumen. With greater vessel wall penetration, IVUS allows deeper vessel visualization and potentially better evaluation of the extent of IMH than OCT [16, 17]. However, the utility of IVUS is limited by its inferior resolution compared to OCT and its ability to accurately locate the intimal rupture site. Paulo et al. compared IVUS and OCT in patients who presented with SCAD. The OCT technology was found to be superior to IVUS in demonstrating intimal rupture sites [16].
OCT provides high-resolution images by using near-infrared light and optical scattering. The spatial resolution of OCT (10–20 μm) is tenfold greater than that of IVUS (150 μm) and enables better characterizations of intimal tear site, false lumens, and IMH in the diagnosis of SCAD [16, 18]. Alfonso et al. reported 11 cases of OCT-confirmed SCAD. Intimal tear was identified in seven patients, IMH was visualized in eight patients, and false lumen was easily recognized in the majority of cases [19]. Furthermore, OCT allows detailed assessment of various features of SCAD including the length of the affected vessel dissection, a thickness of the flap, associated thrombus formation, extent of luminal compromise, and side-branch involvement, all of which can guide with the planning of coronary intervention [20]. However, OCT has several limitations: its poor depth of penetration (2–3 mm) limits the diameter of vessel that can be assessed with OCT and left main coronary artery SCAD is often poorly visualized [21]. In addition, residual blood from suboptimal flushing can cause signal attenuation, especially when the lumen is collapsed, and blood clearance can be difficult [22]. Nonetheless, OCT offers valuable information to confirm the diagnosis of SCAD particularly for type 2 and type 3 SCAD lesions. If needed, a combination of OCT and IVUS could minimize the inherent limitations of each modality in the diagnosis of SCAD [16, 17].
A number of arteriopathies, such as FMD, are associated with fragile arterial wall structure and susceptibility to iatrogenic trauma [3]. An observational study from the Vancouver General Hospital SCAD registries demonstrated a higher incidence of iatrogenic coronary artery dissection during diagnostic angiography or percutaneous coronary intervention (PCI) in patients with SCAD than in general population (3.4 vs. 0.2%) [23]. Therefore, the use of intracoronary imaging modalities should be limited to when the benefit of additional data clearly outweighs the risks of the procedure given the possibility of iatrogenic dissection of coronary artery by guide catheter, and possible propagation of dissection by coronary wire, imaging catheter or hydraulic pressure from forceful OCT contrast media injection.
Cardiac computed tomography angiography
Cardiac computed tomography angiography (CCTA) is a potential, novel modality in diagnosing SCAD [24, 25]. CT offers a noninvasive way to assess for the presence of SCAD without the risk of iatrogenic complications and propagation of dissection flap. However, a negative CCTA results should not exclude a diagnosis of SCAD [26]. Most cases of SCAD involve mid to distal segments of coronary arteries, and limited spatial resolution of CCTA may not allow proper evaluation of lumen and walls of small coronary arteries in these distal segments. Therefore, CCTA is not recommended as a first-line diagnostic modality for SCAD. However, CCTA can be an effective noninvasive modality to evaluate and follow the resolution of SCAD. Roura et al. analyzed 34 SCAD patients and reported complete healing of the coronary dissection in 83% of cases visualized by CCTA [27].
Management of SCAD
The current management strategy is largely based on observational studies given the lack of evidence from randomized controlled trials to guide the treatment of this complex disease. However, most of the studies suggest that treatment of SCAD differs from the traditional approach to atherosclerotic lesions.
Medical management
Beta-blockers
Beta-blockers reduce heart rate, blood pressure, and myocardial oxygen consumption, which then lead to decreased local arterial shear stress [28, 29]. Moreover, beta-blockers have been shown to decrease the infarct size and risk of ventricular fibrillation in patients with acute myocardial infarction (AMI) [30, 31]. Accordingly, beta-blockers remain a mainstay of acute medical management of SCAD. Importantly, SCAD patients are at risk of developing ventricular fibrillation/tachycardia, particularly in those with pregnancy-associated SCAD being at the highest risk (occurred in ~ 16% of patients) [4, 10, 13••]. Thus, beta-blockers may play an important role in reducing the risk of such lethal arrhythmias. Of note, Saw et al. recently reported 10.4% recurrence rate of de novo SCAD observed during median follow-up of 3.1 years [13••]. Importantly, chronic beta-blocker use was independently associated with 64% reduction of risk of recurrence (hazard ratio 0.36, p = 0.004), which is striking considering the frequent recurrence of SCAD [13••]. This evidence supports the importance of acute and chronic utilization of beta-blockers in the management of SCAD.
Antiplatelet therapy
Most experts recommend using aspirin for acute and chronic management of SCAD based on evidence from ACS literature [32]. In SCAD, antiplatelet therapy theoretically prevents thrombus formation in the true lumen and keeps luminal patency to prevent ischemia of the affected vessel. The role of dual antiplatelet therapy (DAPT) in SCAD patients not treated with stents remains uncertain. However, DAPT may further reduce the thrombotic burden in the false lumen and subsequently alleviate true luminal compression. There are anecdotal reports showing the safety of DAPT in SCAD: Rogowski et al. demonstrated effective use of DAPT in 92% of SCAD patients (clopidogrel in 69%, prasugrel in 14%, ticagrelor in 9%). Healing of dissection was confirmed on 6-month follow-up angiogram in the majority of patients [33]. In another large observational study of 134 patients with SCAD, 82% of medically treated patients (n = 78) empirically received DAPT for a mean duration of 11 months and experienced low recurrence rate of 4.7%, suggesting safety of DAPT [34]. Therefore, individualized use of DAPT based on careful assessment of risks and benefits would be prudent in SCAD. In general, DAPT with clopidogrel is often empirically used until the resolution of SCAD is confirmed on follow-up evaluation or for 1 year after the onset of SCAD in the setting of ACS.
Angiotensin converting enzyme inhibitor/angiotensin receptor blocker
Based on the evidence and recommendation for AMI patients with left ventricular dysfunction, angiotensin converting enzyme inhibitor (ACEI)/angiotensin receptor blockers (ARBs) are used in patients with SCAD who have significantly reduced left ventricular ejection fraction (LVEF) [32, 35]. LVEF is frequently reduced in patients with pregnancy-associated SCAD. Tweet et al. reported the rate of LVEF ≤ 35% was significantly higher in pregnancy-associated than non-pregnancy-associated SCAD (26 vs. 10%, p = 0.007) [9]. Although ACEI and ARB should not be used during pregnancy, patients with pregnancy-associated SCAD and reduced LVEF who are not lactating may benefit from ACEI/ARB during the postpartum period.
Anticoagulation, thrombolytic therapy, and statins
The role of anticoagulation in SCAD is not well studied. The potential benefit of preventing thrombus formation is likely to be offset by risks of increasing intramural bleeding and extending the dissection. Therefore, anticoagulation should be discontinued once the diagnosis of SCAD has been made in the absence of another indication for anticoagulation.
There are contradicting reports regarding thrombolytic therapy in SCAD. Leclercq et al. described a patient with SCAD involving left main coronary artery with proximal LAD occlusion, who was successfully treated with thrombolytic therapy. A proposed mechanism is thrombolysis of false lumen thrombus with resultant decompression of true lumen and reperfusion of the vessel [36]. However, a larger body of reports raises safety concerns with regard to thrombolytic therapy extending dissection and IMH in SCAD [37, 38]. In a review of SCAD cases, Shamloo et al. reported 87 SCAD patients who were treated with thrombolysis before the diagnosis of SCAD was made and subsequently whose condition deteriorated requiring rescue PCI or coronary artery bypass graft (CABG). Accordingly, the experts’ consensus is that the use of thrombolytic therapy should be avoided when SCAD is suspected/diagnosed; however, its use should not be discouraged in STEMI when appropriate prior to angiography.
There is a paucity of data on the clinical utility of statins in SCAD. Unlike patients with coronary artery disease, SCAD patients generally do not have significant atherosclerosis and hence, the role of statins in SCAD is less clear. Of note, a retrospective cohort study of 87 SCAD patients showed a potential association between statin use and recurrence of SCAD, whereas another cohort study of 64 SCAD patients (89% received statins) demonstrated three recurrent SCAD cases among 56 medically treated patients during the follow-up of ~ 8 years without observing such association [6, 33]. Because of the uncertainty of statins’ benefit in SCAD, we would recommend statin therapy to suspected SCAD patients with concomitant dyslipidemia or coronary artery disease.
Conservative medical management versus revascularization
Conservative medical management is the initial therapy in SCAD due to high rates of spontaneous healing of dissections and excellent long-term clinical outcomes. In a retrospective study of 189 patients with 2.3 years of median follow-up, spontaneous angiographic healing of dissection was observed in 73% of conservatively managed patients who had repeat angiography [8]. In a cohort of 168 patients and very early follow-up angiography in conservatively managed patients (< 20 days after SCAD) angiography demonstrated no evidence of dissection healing, suggesting that follow-up angiography should be scheduled at least ~ 30 days after the onset of SCAD [4].
Generally, conservative management is thought to lead to excellent clinical outcomes. In a recently updated series of 327 SCAD patients from Vancouver, only 9 out of 272 conservatively managed patients (3.3%) had evidence of extension of dissection requiring subsequent in-hospital revascularization (2.2% PCI and 1.1% CABG) with no in-hospital mortality [13••]. In a series of 189 patients from Mayo Clinic, 9 out of 94 (10%) conservatively managed patients experienced early SCAD progression, and 8 of these patients required subsequent revascularizations (6 PCI and 2 CABG) [8].
In contrast, revascularization therapy was associated with relatively high failure rate [8, 13••]. In the Vancouver cohort, 31% of PCIs were deemed unsuccessful as a result of residual dissection, stenosis > 50% of luminal diameter, worse TIMI (Thrombolysis in Myocardial Infarction trial) flow than baseline, or extension of dissection requiring bail-out CABG [13••]. Mayo Clinic series also demonstrated PCI failure rate of 53% [8]. Therefore, it is recommended that the revascularization therapy be limited to patients with certain high-risk features: ongoing or recurrent ischemia, left main dissection, cardiogenic shock, or ventricular arrhythmias [13••]. A failure of conservative management of SCAD was also observed in the presence of predominantly false lumen [39]; this anatomic feature may warrant preemptive revascularization. When revascularization is necessary, PCI rather than CABG should be performed if the anatomy is feasible.
Revascularization
PCI
PCI in the setting of SCAD is challenging for several reasons. First of all, fragile arterial walls with underlying arteriopathy are vulnerable to iatrogenic dissection during the procedure [4, 23]. It may also be difficult to wire the true lumen, particularly in type 1 SCAD with the presence of intimal rupture [33]. In addition, the dissection can extend during the procedure, particularly when stenting does not adequately cover the distal edge of IMH. The use of long stents or multiple stents may also increase the risk of stent thrombosis and restenosis. Furthermore, involvement of distal small coronary segments may be challenging for angioplasty and stent placement.
To address some of these challenges during PCI, several strategies have been utilized. Intracoronary imaging can guide the access to true lumen and confirm stent expansion/apposition. When the extent of IMH is relatively confined, longer stents are suggested to adequately cover and compress both proximal and distal edges of IMH in order to prevent propagation of the IMH. If a longer lesion is determined to require multiple stents, it may be helpful to stent the distal edge first followed by stenting of the proximal edge in order to prevent the propagation of dissection. Bioabsorbable stents have been shown to be a potential option in SCAD to avoid permanent implants and potential late stent malapposition following resorption of IMH, which may be associated with stent thrombosis [40]. Successful treatment of SCAD using bioabsorbable stents has been reported in a series of 18 patients with no adverse events at a median follow-up of 18 months [41,42,43]. However, an increase in MI and scaffold thrombosis for Absorb stent has been observed in ABSORB II [44] and ABSORB III trials [45], which led to removal of Absorb stent from the US market [46]. SCAD patients have been successfully treated with other bioabsorbable stents as well, including magnesium-based bioabsorbable stents [47, 48]. In addition, investigators have reported successful cutting balloon angioplasty in SCAD, which creates micro communications between the true and false lumens and achieves decompression of IMH with or without subsequent stenting [49,50,51,52]. This novel strategy to fenestrate IMH can be considered when the dissection and IMH extend to the distal small segments where stenting would be challenging. After successful PCI, a subsequent extension of dissection can still occur; thus, close inpatient monitoring and follow-up of SCAD patients is recommended [8]. Intracoronary imaging should be considered for patients who undergo stent placement in order to assess the completeness of the dissection coverage.
CABG
CABG should be reserved for patients with left main dissection, extensive multivessel dissections, those with PCI failure, or in those with anatomy not suitable for PCI. In Mayo Clinic series, a total of 20 patients underwent CABG during the initial hospitalization [8]. There was one in-hospital death after bail-out CABG following PCI failure, but no deaths at 5-year follow-up. Among those who underwent CABG, 11 patients underwent repeat angiography at a median follow-up of 3.5 years. Only 5 of 16 grafts were patent, which can be explained by the competitive filling in healed native artery, resulting in graft occlusion. Despite the high rate of late bypass graft failure, excellent short- and long-term clinical outcomes post-CABG indicate that CABG can be an important revascularization option for selected patients with SCAD.
Cardiac rehabilitation after SCAD
A survey study of 354 patients with SCAD showed a participation rate of 76% in cardiac rehabilitation [53]. Among the nonparticipants, 67% did not receive the referral from their health care providers; lack of referral was the primary reason of nonparticipation. Of the participants, 82% perceived physical health benefits and 75% reported emotional health benefits. Another cohort study of 70 SCAD patients demonstrated safety of cardiac rehabilitation in SCAD patients as well as beneficial effects of rehabilitation on patients’ physical and psychosocial well-being [54]. As cardiac rehabilitation for patients with SCAD appears safe and beneficial, we would encourage the utilization of such programs.
Conclusion
Patients with SCAD represent a unique population with a high prevalence of underlying arteriopathy and few atherosclerotic risk factors. Clinicians need to increase the awareness of this disorder in order to properly diagnose and treat patients with SCAD. Familiarity with angiographic characteristics of SCAD along with utilization of complementary intracoronary imaging increases the diagnostic yield of SCAD. While the optimal management for SCAD remains controversial, conservative management should be considered as the first-line strategy for patients without high-risk features or recurrent symptoms.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Elkayam U, Jalnapurkar S, Barakkat MN, Khatri N, Kealey AJ, Mehra A, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation. 2014;129(16):1695–702. https://doi.org/10.1161/circulationaha.113.002054.
Maehara A, Mintz GS, Castagna MT, Pichard AD, Satler LF, Waksman R, et al. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am J Cardiol. 2002;89(4):466–8.
Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv. 2013;6(1):44–52. https://doi.org/10.1016/j.jcin.2012.08.017.
Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7(5):645–55. https://doi.org/10.1161/circinterventions.114.001760.
Prasad M, Tweet MS, Hayes SN, Leng S, Liang JJ, Eleid MF, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol. 2015;115(12):1672–7. https://doi.org/10.1016/j.amjcard.2015.03.011.
Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126(5):579–88. https://doi.org/10.1161/circulationaha.112.105718.
Luong C, Starovoytov A, Heydari M, Sedlak T, Aymong E, Saw J. Clinical presentation of patients with spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2017;89(7):1149–54. https://doi.org/10.1002/ccd.26977.
Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7(6):777–86. https://doi.org/10.1161/circinterventions.114.001659.
• Tweet MS, Hayes SN, Codsi E, Gulati R, Rose CH, Best PJM. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol. 2017;70(4):426–35. https://doi.org/10.1016/j.jacc.2017.05.055. This prospective observational study provides important information about the unique clinical presentation of pregnancy-associated SCAD.
Havakuk O, Goland S, Mehra A, Elkayam U. Pregnancy and the risk of spontaneous coronary artery dissection: an analysis of 120 contemporary cases. Circ Cardiovasc Interv. 2017;10(3). doi: 10.1161/circinterventions.117.004941.
Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2014;84(7):1115–22. https://doi.org/10.1002/ccd.25293.
Eleid MF, Guddeti RR, Tweet MS, Lerman A, Singh M, Best PJ, et al. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv. 2014;7(5):656–62. https://doi.org/10.1161/circinterventions.114.001676.
•• Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, et al. Spontaneous Coronary Artery Dissection: Clinical Outcomes and Risk of Recurrence. J Am Coll Cardiol. 2017;70(9):1148–58. https://doi.org/10.1016/j.jacc.2017.06.053. This study of the prospectively followed largest cohort with SCAD provides insights on long-term clinical outcomes of SCAD. This series confirms the appropriateness of conservative medical management for clinically stable patients. Also, the finding of beta-blocker associated with reduced risk of recurrent SCAD is novel and directs future research.
Gornik HL. Spontaneous coronary artery dissection: the zebra has been spotted: now let’s study its stripes. J Am Coll Cardiol. 2017;70(4):436–8. https://doi.org/10.1016/j.jacc.2017.06.019.
Saw J, Mancini GB, Humphries K, Fung A, Boone R, Starovoytov A, et al. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv. 2016;87(2):E54–61. https://doi.org/10.1002/ccd.26022.
Paulo M, Sandoval J, Lennie V, Dutary J, Medina M, Gonzalo N, et al. Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC Cardiovasc Imaging. 2013;6(7):830–2. https://doi.org/10.1016/j.jcmg.2013.02.010.
Poon K, Bell B, Raffel OC, Walters DL, Jang IK. Spontaneous coronary artery dissection: utility of intravascular ultrasound and optical coherence tomography during percutaneous coronary intervention. Circ Cardiovasc Interv. 2011;4(2):e5–7. https://doi.org/10.1161/circinterventions.110.959593.
Ong DS, Jang IK. Fundamentals of optical coherence tomography: image acquisition and interpretation. Interv Cardiol Clin. 2015;4(3):225–37. https://doi.org/10.1016/j.iccl.2015.02.001.
Alfonso F, Paulo M, Gonzalo N, Dutary J, Jimenez-Quevedo P, Lennie V, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol. 2012;59(12):1073–9. https://doi.org/10.1016/j.jacc.2011.08.082.
Franco C, Eng L, Saw J. Optical coherence tomography in the diagnosis and management of spontaneous coronary artery dissection. Interv Cardiol Clin. 2015;4(3):309–20. https://doi.org/10.1016/j.iccl.2015.02.007.
Lowe HC, Narula J, Fujimoto JG, Jang IK. Intracoronary optical diagnostics current status, limitations, and potential. JACC Cardiovasc Interv. 2011;4(12):1257–70. https://doi.org/10.1016/j.jcin.2011.08.015.
Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39(4):604–9.
Prakash R, Starovoytov A, Heydari M, Mancini GB, Saw J. Catheter-induced iatrogenic coronary artery dissection in patients with spontaneous coronary artery dissection. JACC Cardiovasc Interv. 2016;9(17):1851–3. https://doi.org/10.1016/j.jcin.2016.06.026.
Russo V, Marrozzini C, Zompatori M. Spontaneous coronary artery dissection: role of coronary CT angiography. Heart. 2013;99(9):672–3. https://doi.org/10.1136/heartjnl-2012-303215.
Torres-Ayala SC, Maldonado J, Bolton JS, Bhalla S. Coronary computed tomography angiography of spontaneous coronary artery dissection: a case report and review of the literature. Am J Case Rep. 2015;16:130–5. https://doi.org/10.12659/ajcr.892805.
Eleid MF, Tweet MS, Young PM, Williamson E, Hayes SN, Gulati R. Spontaneous coronary artery dissection: challenges of coronary computed tomography angiography. Eur Heart J Acute Cardiovasc Care 2017:2048872616687098. doi:https://doi.org/10.1177/2048872616687098.
Roura G, Ariza-Sole A, Rodriguez-Caballero IF, Gomez-Lara J, Ferreiro JL, Romaguera R, et al. Noninvasive follow-up of patients with spontaneous coronary artery dissection with CT angiography. JACC Cardiovasc Imaging. 2016;9(7):896–7. https://doi.org/10.1016/j.jcmg.2015.06.011.
Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Anesth Analg. 2010;111(2):279–315. https://doi.org/10.1213/ANE.0b013e3181dd869b.
Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J. 2012;33(1):26–35b. https://doi.org/10.1093/eurheartj/ehr186.
Ryden L, Ariniego R, Arnman K, Herlitz J, Hjalmarson A, Holmberg S, et al. A double-blind trial of metoprolol in acute myocardial infarction. Effects on ventricular tachyarrhythmias. N Engl J Med. 1983;308(11):614–8. https://doi.org/10.1056/nejm198303173081102.
Roberts R, Rogers WJ, Mueller HS, Lambrew CT, Diver DJ, Smith HC, et al. Immediate versus deferred beta-blockade following thrombolytic therapy in patients with acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) II-B study. Circulation. 1991;83(2):422–37.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):2354–94. https://doi.org/10.1161/cir.0000000000000133.
Rogowski S, Maeder MT, Weilenmann D, Haager PK, Ammann P, Rohner F, et al. Spontaneous coronary artery dissection: angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter Cardiovasc Interv. 2017;89(1):59–68. https://doi.org/10.1002/ccd.26383.
Lettieri C, Zavalloni D, Rossini R, Morici N, Ettori F, Leonzi O, et al. Management and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 2015;116(1):66–73. https://doi.org/10.1016/j.amjcard.2015.03.039.
O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–425. https://doi.org/10.1161/CIR.0b013e3182742cf6.
Leclercq F, Messner-Pellenc P, Carabasse D, Lucke N, Rivalland F, Grolleau R. Successful thrombolysis treatment of a spontaneous left main coronary artery dissection without subsequent surgery. Eur Heart J. 1996;17(2):320–1.
Zupan I, Noc M, Trinkaus D, Popovic M. Double vessel extension of spontaneous left main coronary artery dissection in young women treated with thrombolytics. Catheter Cardiovasc Interv. 2001;52(2):226–30.
Shamloo BK, Chintala RS, Nasur A, Ghazvini M, Shariat P, Diggs JA, et al. Spontaneous coronary artery dissection: aggressive vs. conservative therapy. J Invasive Cardiol. 2010;22(5):222–8.
Diez-Delhoyo F, Sanz-Ruiz R, Sarnago-Cebada F, Gutierrez-Ibanes E, Rivera-Juarez A, Elizaga J, et al. Spontaneous coronary artery dissection: failure of the conservative strategy due to predominance of the false lumen. JACC Cardiovasc Interv. 2017;10(15):e139–e40. https://doi.org/10.1016/j.jcin.2017.04.032.
Lempereur M, Fung A, Saw J. Stent mal-apposition with resorption of intramural hematoma with spontaneous coronary artery dissection. Cardiovasc Diagn Ther. 2015;5(4):323–9. https://doi.org/10.3978/j.issn.2223-3652.2015.04.05.
Macaya F, Peral V, Alameda M, Pascual M, Gomez-Jaume A, Asmarats L, et al. Bioresorbable scaffolds to treat spontaneous coronary artery dissection. Circ Cardiovasc Interv. 2016;9(1):e003133.
Watt J, Egred M, Khurana A, Bagnall AJ, Zaman AG. 1-year follow-up optical frequency domain imaging of multiple bioresorbable vascular scaffolds for the treatment of spontaneous coronary artery dissection. JACC Cardiovasc Interv. 2016;9(4):389–91. https://doi.org/10.1016/j.jcin.2015.11.030.
Cockburn J, Yan W, Bhindi R, Hansen P. Spontaneous coronary artery dissection treated with bioresorbable vascular scaffolds guided by optical coherence tomography. Can J Cardiol. 2014;30(11):1461.e1–3. https://doi.org/10.1016/j.cjca.2014.06.025.
Serruys PW, Chevalier B, Sotomi Y, Cequier A, Carrie D, Piek JJ, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet (London, England). 2016;388(10059):2479–91. https://doi.org/10.1016/s0140-6736(16)32050-5.
Kereiakes DJ, Ellis SG, Metzger C, Caputo RP, Rizik DG, Teirstein PS, et al. 3-year clinical outcomes with everolimus-eluting bioresorbable coronary scaffolds: the ABSORB III trial. J Am Coll Cardiol. 2017;70:2852–62. https://doi.org/10.1016/j.jacc.2017.10.010.
Abbott. Absorb GT1 bioresorbable vascular scaffold. Abbott. https://www.vascular.abbott/us/products/coronary-intervention/absorb-bioresorbable-scaffold-dissolving-stent.html. Accessed November 15 2017.
Quadri G, Tomassini F, Cerrato E, Varbella F. First reported case of magnesium-made bioresorbable scaffold to treat spontaneous left anterior descending coronary artery dissection. Catheter Cardiovasc Interv. 2017;90(5):768–72. https://doi.org/10.1002/ccd.27214.
Haude M, Ince H, Kische S, Abizaid A, Tolg R, Alves Lemos P, et al. Sustained safety and clinical performance of a drug-eluting absorbable metal scaffold up to 24 months: pooled outcomes of BIOSOLVE-II and BIOSOLVE-III. EuroInterv: J EuroPCR Collab Working Group Interv Cardiol Eur Soc Cardiol. 2017;13(4):432–9. https://doi.org/10.4244/eij-d-17-00254.
Yumoto K, Sasaki H, Aoki H, Kato K. Successful treatment of spontaneous coronary artery dissection with cutting balloon angioplasty as evaluated with optical coherence tomography. JACC Cardiovasc Interv. 2014;7(7):817–9. https://doi.org/10.1016/j.jcin.2013.10.027.
Motreff P, Barber-Chamoux N, Combaret N, Souteyrand G. Coronary artery fenestration guided by optical coherence tomograhy before stenting: new interventional option in rescue management of compressive spontaneous intramural hematoma. Circ Cardiovasc Interv. 2015;8(4):e002266. https://doi.org/10.1161/circinterventions.114.002266.
Alkhouli M, Cole M, Ling FS. Coronary artery fenestration prior to stenting in spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2016;88(1):E23–7. https://doi.org/10.1002/ccd.26161.
Ito T, Shintani Y, Ichihashi T, Fujita H, Ohte N. Non-atherosclerotic spontaneous coronary artery dissection revascularized by intravascular ultrasonography-guided fenestration with cutting balloon angioplasty. Cardiovasc Interv Ther. 2017;32(3):241–3. https://doi.org/10.1007/s12928-016-0397-x.
Krittanawong C, Tweet MS, Hayes SE, Bowman MJ, Gulati R, Squires RW, et al. Usefulness of cardiac rehabilitation after spontaneous coronary artery dissection. Am J Cardiol. 2016;117(10):1604–9. https://doi.org/10.1016/j.amjcard.2016.02.034.
Chou AY, Prakash R, Rajala J, Birnie T, Isserow S, Taylor CM, et al. The first dedicated cardiac rehabilitation program for patients with spontaneous coronary artery dissection: description and initial results. Can J Cardiol. 2016;32(4):554–60. https://doi.org/10.1016/j.cjca.2016.01.009.
Acknowledgements
We thank Amy Zhong, MA for her medical illustration of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ilhwan Yeo and Luke K. Kim each declare no potential conflicts of interest.
Dmitriy N. Feldman is a section editor for Current Treatment Options in Cardiovascular Medicine.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Coronary Artery Disease
Rights and permissions
About this article
Cite this article
Yeo, I., Feldman, D.N. & Kim, L.K. Spontaneous Coronary Artery Dissection: Diagnosis and Management. Curr Treat Options Cardio Med 20, 27 (2018). https://doi.org/10.1007/s11936-018-0622-2
Published:
DOI: https://doi.org/10.1007/s11936-018-0622-2