Abstract
There has been increased awareness of Spontaneous Coronary Artery Dissection (SCAD) as a type of myocardial infarction over the last decade. However, as its underlying pathology and mechanisms are still being understood, and the best management principles for SCAD still being realized, there are little robust guidelines for those not subspecializing in SCAD patient management to help guide management. This book chapter targets general medical practitioners who are faced with taking care of the small but increasing SCAD population in the community, in partnership with SCAD specialists. It provides an updated understanding of SCAD including the “inside out” versus “outside in” hypothesis of pathophysiology, and the management principles to be mindful of in SCAD in comparison to traditional acute coronary syndrome (ACS) management. Uniquely, the chapter provides colloquial answers to frequently asked questions by patients about SCAD, and an in-depth review of the benefits of cardiac rehabilitation for SCAD patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Spontaneous Coronary Artery Dissection (SCAD) is a type of myocardial infarction (MI) that presents acutely with similar characteristics as the more common atherosclerotic MI. SCAD is the cause of up to 4% of acute coronary syndrome (ACS) presentations [1]. However, in females under the age of 50, it can represent up to 35% of ACS presentations [1,2,3].
There is an increasing awareness of SCAD as a unique type of heart attack, and an increasing need for medical practitioners to be equipped to manage SCAD patients in the community. Thus, the aim of this chapter is to review the current definition, classification and understanding of SCAD, and highlight key similarities and differences in diagnosis (Table 4.1), management (Table 4.2) and prognosis between SCAD ACS and atherosclerotic ACS.
In addition, a schematic (Fig. 4.1) and suggested outpatient partnered management (Table 4.3) of SCAD between SCAD specialists, rehabilitation specialists, general cardiologists, and primary care providers is provided. Commonly asked questions by patients are addressed (Table 4.5), including recommendations regarding pregnancy after SCAD. Furthermore, the role of cardiac rehabilitation in this population is highlighted [4].
Definition and Classification of SCAD
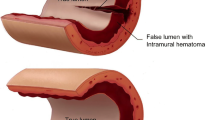
The definition of SCAD is a ‘spontaneous separation of the coronary artery wall, which is not iatrogenic and not related to atherosclerosis or trauma’ [5]. It leads to myocardial infarction as the hematoma within the separated coronary artery wall compresses the lumen of the artery and prevents blood flow, causing ischemia.
Traditionally, SCAD was thought to result from a tear in the intima of the coronary artery, which allowed blood to flow into the intimal layer to create this false lumen that expanded and compressed the true lumen [6]. This is referred to as the “inside out” hypothesis but is now superseded largely by a modified hypothesis, comparatively named the “outside in” hypothesis which suggests that the primary incident is an intramural hematoma, rather than an intimal tear [2, 6]. The intramural hematoma is thought to be caused by spontaneous medial dissection or rupture of the vasa vasorum [2]. From there, it is believed that the underlying hemorrhage expands in either a focal, linear, or spiral fashion to compress into the lumen of the coronary artery [2, 6].
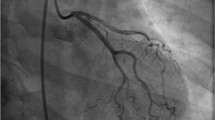
The way the coronary artery lumen is compressed is how SCAD is classified into its three types, an angiographic classification identified and labelled by the Canadian SCAD group (Fig. 4.2). Type 1 is an intimal tear with contrast staining of the false lumen, Type 2 represents a long diffuse and smooth narrowing angiographically, and Type 3 is a focal or tubular angiographic stenosis with both Type 2 and 3 resulting from an intra-mural hematoma [5].

Figure from Saw, 2016, contemporary review of spontaneous coronary dissection [5]
Classification of SCAD.
Pathophysiology of SCAD
Recent literature has suggested conceptualizing SCAD as “SCAB” (spontaneous coronary artery “bleed”) rather than “dissection”, as the presence of an intimal tear is not necessary for diagnosis [7]. Increasing understanding of SCAD suggests that as the “bleed” expands between the coronary artery layers, an intimal tear can occur to decompress the intramural pressure [6]. This newer pathophysiological theory is supported by the evidence that not all SCAD cases have an intimal tear, with recent cohort studies identifying an intimal tear in only 30% of cases [2]. Further, intracoronary imaging studies in more severe SCAD cases have shown to be associated with an intimal tear [8], which supports the “outside in” hypothesis as a unifying mechanism with the tear occurring secondarily for decompression [8, 9].
Epidemiology of SCAD
The mean age of SCAD patients is between 44 and 52 years, and it is uncommon in the extremes of age (under 25 years or over 80 years) [5, 10]. The female predominance is now well established in the literature, with a large meta-analysis of 2172 patients revealing 84% of SCAD patients as female [6].
There has been a temporal increase in the incidence of SCAD, with a rise in awareness paralleling a rise in diagnosis, particularly since 2012 [2]. Although it is likely still under-recognized at present, SCAD is believed to be the cause of up to 4% of ACS presentations [3]. Further, in administrative databases, SCAD represents 15–20% of myocardial infarctions during pregnancy or peripartum, a particularly high-risk period for known SCAD patients [1, 10, 11].
Recent data from the Canadian SCAD registry demonstrated that recurrence rates for SCAD are surprisingly low, 2.4% at 3 years [12]. Further, the 30-day mortality rate is 0.1% [13] and 3-year mortality rate is 1% [14] for SCAD patients.
Risk Factors for SCAD
The most notable risk factor for poor outcome in SCAD is pregnancy and the post-partum state in a patient with prior SCAD. Hypertension also is a risk factor for SCAD-related myocardial infarction during pregnancy [15]. Amongst the SCAD patient population, 15–20% of patients are pregnant or post-partum at the time of diagnosis [1, 16]. However, the exact mechanism of association between hormones and SCAD is not yet understood, and the data is conflicting, as there does not appear to be a clear association between oral contraceptives or hormone replacement therapy and SCAD [16].
Secondly, hypertension has a high prevalence (45%) in SCAD patients [15]. There is a plausible explanation mechanistically, as hypertension can lead to chronic pathological changes in the arterial wall via increasing arterial wall stress, endothelial damage, triggering smooth muscle cell proliferation and breakdown of elastin fibers, that ultimately make the vessel more vulnerable to SCAD [15]. Hypertension is also independently associated with a higher risk of in-hospital mortality (OR 2.19, CI 1.86, 2.58) [17]. Furthermore, in a prospective Spanish registry, hypertensive SCAD patients had higher risk of increased severity of SCAD lesion (15 versus 7%, p > 0.05), higher risk of procedural complications (65 versus 41%, p < 0.05) and lower likelihood of procedural success (65 versus 88%, p < 0.05) [15, 18].
In addition, there should be clinical suspicion for SCAD in the presence of risk factors such as fibromuscular dysplasia (FMD). Between 15 and 70% of SCAD patients are found to have FMD with the wide range explained by variable screening protocol [1, 3, 6, 19]. Further risk factors may include connective tissue disorders, autoimmune and inflammatory disorders [1, 6], as well as microvascular dysfunction [15, 20], however there is not enough data to establish a clear association between these entities and SCAD at present. The role of genetics in the mechanism of SCAD is also not yet fully understood. A recent study noted a higher prevalence of fibrillin 1 (FBN1) in the plasma of SCAD patients compared with non-SCAD ACS patients [21]. This medium sized (n = 70 in each arm) study generated interest due to the mechanistic plausibility as fibrillin 1 is a component of the elastic tissue found in the media of coronary arteries [21]. Presently, there are no specific genetic screening tests for SCAD or fibromuscular dysplasia, but this is an active area of research [22].
Clinical Presentation of SCAD
As a type of ACS, SCAD presents acutely with similar characteristics as atherosclerotic myocardial infarction. Although ACS presentations in young and middle-aged females with no atherosclerosis risk factors should lead to a high suspicion for SCAD, it must be emphasized that ACS by atherosclerotic plaque rupture is significantly more common than SCAD (50 versus 4%) [23]. Thus, atherosclerotic ACS must be ruled out by coronary angiography before the diagnosis of SCAD can be considered in any clinical presentation [10].
Similar to atherosclerotic ACS, most patients with SCAD (85–96%) experience chest pain or discomfort as their primary presenting complaint [10]. However, a small proportion of SCAD patients may have atypical symptoms including dyspnea (20%), back pain (14%), diaphoresis (21%), nausea and vomiting (24%) and presyncope (9%). Clinically, approximately 20–50% of SCAD patients present as STEMI, and up to 5% present with ventricular arrhythmia, and 2% with cardiogenic shock [10].
In addition, SCAD should be suspected in patients with associated extreme physical or emotional trigger, which is notable in 40% and 24% of SCAD presentations, respectively [9]. The postulated mechanism is a hyper-catecholaminergic state, which along with Valsalva-like maneuvers in physical or emotional states can damage the vasculature and arterial wall with shear force [6]. In the recently published study by the Canadian SCAD group, an isometric physical trigger was more commonly observed in men (40.2 versus 24.0%, p = 0.007), whilst emotional stress triggers have been found to be more likely in women (35 versus 60%, p = 0.001) [9, 24].
What to Do if You Suspect Your Patient Has SCAD
A detailed history and physical examination are key to timely and accurate diagnosis of SCAD (Table 4.1). When there is a suspicion of SCAD, the assessment needs to include questions regarding previous SCAD, pregnancy, menstrual cycle, migraines, physical (particularly isometric) or emotional triggers, known FMD and vascular disorders. Similarly, the physical examination should extend beyond the cardiovascular exam to include auscultating for renal bruits and assessing for signs of connective tissue disorders.
Investigation and management are initially similar for suspected SCAD patients and atherosclerotic ACS patients (Table 4.2). This includes serial electrocardiograms (ECG) and troponin levels. Following that, coronary angiography is vital as it can visualize plaque rupture to identify traditional atherosclerotic ACS, or rule out plaque rupture to confirm the suspected diagnosis of SCAD [10].
We know that there are also ACS presentations that are neither plaque-rupture atherosclerotic ACS nor SCAD, and can be caused by myocardial infarction with no obstructive coronary arteries (MINOCA), coronary vasospasm or microvascular dysfunction. Presentations can also be ACS-mimics such as myocarditis, myopericarditis, takotsubo cardiomyopathy, or other non-cardiac diagnoses including pulmonary emboli or aortic dissection.
Having considered the wider differential for acute chest pain, the general principle is to treat the patient as atherosclerotic ACS until SCAD or an alternate differential is diagnosed on coronary angiography [15]. If SCAD is the true diagnosis, the course of management significantly changes, including avoiding invasive percutaneous coronary intervention (PCI) if possible [18]. However, PCI may still be considered in the presence of clinical high-risk features, including ongoing ischemia/chest pain, cardiogenic shock, sustained ventricular arrhythmia and left main dissection [25].
Further, coronary angiography assists in determining the type of SCAD and the amount of myocardium affected, which can also influence management and follow up frequency and strategy [26]. It is important to explain to the patient the risks of coronary angiography for informed consent, including vascular damage, stroke, myocardial infarction, arrhythmia, contrast risk and focusing specifically on radiation risk for young female patients. Also, the possibility of pregnancy must be assessed, and risks must be discussed.
Moreover, SCAD patients are expected to be admitted in hospital for a longer duration of time (5–7 days) than atherosclerotic ACS patients, as studies have shown that SCAD patients who deteriorate do so usually within 5 days of presentation, compared to atherosclerotic ACS where the highest risk period is the first 48 h [27].
Conservative Versus Invasive Management
Management for revascularization differs between SCAD and atherosclerotic ACS patients. It is well established that if the clinical situation allows, conservative management without PCI is the preferred treatment strategy for SCAD patients, whereas for an atherosclerotic plaque rupture, PCI is recommended. This is explained by the natural history of SCAD, whereby 95% of SCAD are healed spontaneously by 30 days [25]. However, 5–7% of patients who were initially treated conservatively will require PCI in the same presentation, most often due to cardiogenic shock, ongoing ischemic chest pain, or proximal coronary artery involvement [8, 25].
Intervention is considered only for select clinical situations in SCAD because the presence of dissection and intramural hematoma adds to the complexity of the procedure, resulting in higher complication rates [2]. The success rate of PCI for SCAD is 50–70% in large cohort studies, which is much lower than that for the atherosclerotic ACS PCI population [1, 28, 29]. Further, although this may be impacted by a selection bias of the more severe SCAD patients receiving intervention, PCI for SCAD has not been independently associated with reduced infarct size, nor with reduced in-hospital MACE (Major adverse cardiovascular events) compared to conservatively managed SCAD groups [27, 30].
Medical Management
The main debate in the acute medical management of SCAD ACS has been regarding single or dual antiplatelet therapy use. Currently, there are no randomized clinical data or established protocol [31], but there is expert consensus opinion to offer guidance. The most recent literature favors single antiplatelet therapy (SAPT) for SCAD ACS over dual antiplatelet therapy (DAPT) with the multicenter DIssezioni Spontanee COronariche (DISCO) registry from Italy and Spain (n = 199) showing patients on DAPT (n = 132, 66%) experienced significantly higher MACE (defined as all-cause death, non-fatal MI or unplanned PCI; 18.9 versus 6.0%. HR 2.62, 95% CI 1.22–5.61, p = 0.013) than those treated with SAPT (n = 67, 34%) [32]. This result was driven mainly by non-fatal MI (15.2 versus 3.0%, p = 0.009) and unplanned PCI (12.1 versus 1.5%, p = 0.001) [32]. The pathophysiological rationale behind this is increased bleeding and intramural hemorrhage due to DAPT, with no added benefit as there is no acute plaque rupture or disturbance. Similarly, the consensus is that anticoagulation is not necessary for SCAD ACS [1, 2, 33].
The key medication for the management of SCAD long term are beta blockers. A single center observational study has shown a significant decrease of SCAD recurrence with the use of beta blockers, by over 50%, over 3 years [33]. The second step of optimal medical management of SCAD is good blood pressure control, aiming for SBP < 130 mmHg. Angiotensin Converting Enzyme-Inhibitors (ACE-I) or Angiotensin Receptor Blockers (ARBs) are used to treat hypertension, which is a clear risk factor for both SCAD ACS and its recurrence, and are also indicated for the management of left ventricular dysfunction after SCAD-related myocardial infarction. If more blood pressure control is necessary, calcium channel blockers or diuretics may also be used as secondary agents, in line with treatment guidelines for essential hypertension [34].
Unlike atherosclerotic ACS, there is no evidence for the routine use of statins in SCAD [31]. Cholesterol-laden plaque is not implicated in the pathophysiology of SCAD ACS, while it is central to the pathophysiology of traditional atherosclerotic-myocardial infarction. This is a challenging concept for most cardiologists and family physicians to accept, as the use of statin therapy after ACS is firmly entrenched in the practice patterns of most physicians. Similarly, anti-anginal medications (nitrates, and calcium channel blockers) may be very useful for symptom control for chest pain syndromes after SCAD, but are not prescribed routinely [2].
Outpatient Management of SCAD Patients
The optimal outpatient management of SCAD patients is continually being developed as SCAD becomes more understood. Currently, as a lesser-known form of ACS with minimal public awareness or education, many SCAD patients experience social isolation, anxiety and depression during this vulnerable period in their recovery [4].
Indeed, good quality clinical research on outpatient management of SCAD patients are lacking, with many current recommendations being derived from the larger vessel (aortic) dissection literature whilst direct experience with SCAD patients is still building. Thus, the partnered management of patients between SCAD specialists, general cardiologists, cardiac rehabilitation specialists and primary care providers is important for the communication of accurate information (Table 4.5), education of effective management strategies, and screening of associated pathologies [35]. Such management partnerships should be identified and linked in with the SCAD patient prior to hospital discharge.
This schematic (Fig. 4.1) is a timeline from the point of SCAD ACS presentation to diagnosis and management in the hospital, followed by discharge home and the subsequent outpatient avenues of follow up (Table 4.3) with both the primary care provider (Time points: 1, 3, 4), and the planned SCAD multi-disciplinary team (MDT).
The first follow up with the family physician (Point 1* in Fig. 4.1 and Table 4.3) after discharge from a SCAD admission to hospital is recommended to occur within 4–6 weeks. One of the key message that needs to be reiterated to young females of child-bearing age is that after SCAD ACS, pregnancy is not recommended due to the high risk of severe and potentially life-threatening recurrence of SCAD during pregnancy or post-partum [16, 36]. Thus, in the event of pregnancy despite such warning, patients must seek involvement of SCAD expert and the high-risk obstetrics team as early as possible to consider their options. Seeking expert opinion prior to conception is most ideal if pregnancy is desired despite awareness of its high risk.
Regarding exertion, SCAD patients are often advised to avoid high intensity physical exertion and Valsalva maneuvers, which involves not lifting anything heavier than 30lbs for women, or 50lbs for men. However, this should not preclude patients from regaining an active lifestyle with regular moderate activity post SCAD. Participation in cardiac rehabilitation as early as possible is vital in facilitating this.
Between 8 and 12 weeks after hospital discharge, a SCAD MDT follow up (Point 2* in Fig. 4.1) is ideal for further assessment and investigations for associated disorders. The most important and well documented association is FMD, and all patients who have experienced SCAD must have an initial screening test for FMD via a head-to-pelvis computed tomographic (CT) angiography looking for aneurysm, tortuosity, dissection, irregularity, or stenosis of arterial beds. This may have already been done during the inpatient stay if a CT angiography was available.
During the late follow up period, an unplanned chest pain presentation (Point 4* in Fig. 4.1) may occur. These need to be approached in a similar way to a new chest pain presentation, without presumption that it is related to SCAD as recurrence rates of SCAD are very low (2.4% over 3 years) [37, 38]. Thereby, hemodynamic stability, acute onset of symptoms and dynamic ECG changes and troponin levels are used to determine if the patient is best for in-hospital or community management. Notably, women are significantly more likely than men to be re-admitted to hospital for chest pain following prior SCAD (8 versus 1%, p = 0.001) [24].
Further, these outpatient scheduled reviews or unscheduled chest pain presentations are opportune to review medications and ensure patients are taking beta blockers long term following their SCAD ACS episode, assess their psychosocial state, and ensure referral to outpatient cardiac rehabilitation [26].
Longer term, after two to three years of consistent follow up by the SCAD MDT and a stable clinical state, shared decision making is advised to individualize the best method for ongoing patient access to SCAD resources and healthcare. Anecdotally, most patients and family physicians have felt comfortable at this stage to take ownership of the follow up long term, given the established relationship with the SCAD MDT and ability to re-refer if concerns arise.
Cardiac Rehabilitation
Cardiovascular rehabilitation (CR) is a comprehensive secondary prevention program that includes patient education, nutritional counseling, exercise training and psychosocial interventions [39, 40]. Participation has been associated with up to a 50% reduction in morbidity and mortality, as well as improved quality of life following a cardiac event such as MI or cardiac procedure [41].
Unfortunately, less than 30% of eligible patients participate in CR [42] and women in particular, are less likely to be referred and participate in programming [43]. Younger age at diagnosis, lack of referral, caregiving/family responsibilities, transportation, scheduling and logistical difficulties with attending an onsite program have been identified as predominant barriers for enrollment in CR [26, 44, 45]. Despite the significant benefits associated with CR program completion, women diagnosed with SCAD are significantly under-represented in CR programs across Canada [12, 46]. Fear, anxiety and hesitancy regarding physical activity after SCAD are common and often lead patients to curtail or avoid physical activity altogether [11]. CR programs are recommended to safely support the resumption of moderate intensity physical activity after SCAD-related events [11, 47, 48]. Recommendations for physical activity should be tailored to the individual and may be best determined and guided during participation in CR [47, 48].
Cardiac Rehabilitation and SCAD Patients
Referral to CR is considered the standard of care for all patients who have experienced a cardiac event or procedure, including those with a SCAD diagnosis [40].
Recent North American studies have shown improvements in aerobic capacity, body composition and mental health with no reported adverse events in SCAD patients completing CR [46, 48]. Structured rehabilitation (Table 4.4), particularly multi-dimensional interventions that offer comprehensive components (e.g. exercise, psycho-educational support, mindful living sessions, nutrition counselling, peer support networks) have produced both physical and emotional benefits [49].
Krittanawong et al. [49] examined the usefulness of CR in a large cohort of SCAD patients (48/50 US states, Canada, Europe, Australia and New Zealand) enrolled in the Mayo Clinic SCAD registry (n = 354). In this cohort, 66% of patients participated in > 10 CR sessions while 32% chose not to participate. A lack of recommendation to CR by a health care provider was cited as the primary reason for non-participation. An inherent reluctance for clinician referral may be attributed to the relative unfamiliarity with SCAD, a lack of perceived benefit in this population due to the younger age without traditional risk factors, and concerns that exercise training may prompt a recurrent SCAD event.
Wagers et al. [50] further reinforced the positive experiences and safety outcomes for SCAD survivors (n = 409) participating in a CR program. However, a focus on the ‘typical’ or traditional CR program was deemed by patients as “not a good fit” considering the patient's younger age, gender and/or prior activity levels. Some participants reported feelings of isolation during CR due to lack of younger counterparts to whom they could relate, while others felt their program was geared to the “standard heart attack” patient. Baechler et al. [26] interviewed 38 patients diagnosed with SCAD and observed that frustration with the lack of mental health resources was mentioned among the most common reported themes. Patients described that an ideal CR program should be specific for patients with SCAD and would include mental health support [26]. The importance of psychosocial support for SCAD survivors has been highlighted in several studies [51, 52] and reinforces that it should be accessible to every patient attending CR [39, 40], notably to women [53].
Neubeck et al. [52] performed an extensive systematic review of 28 studies and analyzed the physical and psychosocial recovery following discharge from hospital in 4167 patients (93.5% female) with SCAD. They observed lack of specific guidance about physical activity and high levels of psychosocial distress. These findings suggest ideal programs with tailored exercise and psycho-educational components including peer support networks require further examination to test feasibility and effectiveness specific to the unique needs of these patients. Moreover, it is imperative that care providers are aware of the safety and associated benefits of CR participation to improve the recovery trajectory for this understudied population.
Physical Activity After SCAD
Fear and hesitancy regarding physical activity after SCAD are common among SCAD survivors and clinicians despite a lack of evidence for protection from recurrent SCAD [11]. Additionally, imposing physical restrictions to young individuals could lead to lifelong impact possibly promoting sedentarism, weight gain, and psychosocial issues. In this realm, CR programs can contribute substantially to overall physical and mental health [11].
As outlined previously, CR has demonstrated overall benefit and safety [46, 48,49,50]; therefore, pursuing regular, moderate exercise likely outweighs the theoretical risks of recurrent SCAD [11, 47, 48]. It is important to note that SCAD survivors, especially those who experienced recurrent SCAD or who have noncoronary aneurysms or dissections should avoid extreme endurance training, exercising to exhaustion, elite competitive sports, or vigorous exertion in extremes of ambient temperature [11, 47, 48].
The Vancouver General Hospital (VGH) SCAD-CR protocol has been adopted worldwide and provides safe parameters for patients initiating CR [48]. Each patient should have an individualized exercise prescription, considering clinical parameters, physical exercise prior to SCAD and personal goals. These considerations do not substantially differ from non-SCAD patients however, it is suggested to start physical activity at a lower level, as recommended by Chou et al. [48], then gradual progression should be targeted (Table 4.4). Further, SCAD survivors should avoid lifting or carrying heavy objects that require straining or prolonged Valsalva [11, 47, 48]. Nevertheless, there is no evidence that heavier loads with proper technique (no straining or Valsalva) are harmful [47].
The length of time between a SCAD event and CR initiation should be considered, and empirically exercise prescription could be more conservative in the first months post SCAD as suggested in Table 4.4, and less restrictive after 6–12 months. The presence of FMD can also represent an additional challenge. Patients with carotid or vertebral artery dissections should avoid resistance training including body weight exercises such as push-ups and sit-ups during the first 8–12 weeks after the acute dissection after which the recommendations would be similar as for SCAD [47].
Future Directions and Opportunities
The evolving understanding of SCAD over the last ten years is promising to translate into partnered care and better outcomes for SCAD ACS patients. More robust data have clarified prior assumptions, and management strategies have become more unified globally. The concern for pregnancy following a SCAD ACS episode continues, particularly as recurrence in this scenario is more likely to be severe.
There are numerous future opportunities that need to be addressed in order to better understand the presentation, diagnosis, early recognition and treatment as well as long term management of patients diagnosed with SCAD.
Cardiac rehabilitation is safe and feasible, early studies have shown the beneficial effects on clinical cardiovascular parameters and mental health in SCAD survivors [46, 49, 50]. Future studies need to specifically examine the clinical experiences and preferences of SCAD patients in order to develop a better understanding of alternative, flexible program models, the integration of peer support networks and how best to tailor CR programming to meet the patient’s psychosocial, physiological and educational needs.
It is crucial that care providers are educated and understand the differences between SCAD and atherosclerotic diseases, and how best to mitigate barriers to SCAD care. Consideration of family systems and roles as well as involvement of family members in SCAD education may help alleviate patient and family anxiety which may subsequently optimize recovery transitions.
References
Kim ES (2020) Spontaneous coronary-artery dissection. N Engl J Med 383(24):2358–2370
Lewey J, El Hajj SC, Hayes SN (2022) Spontaneous coronary artery dissection: new insights into this not-so-rare condition. Annu Rev Med 73:339–354
Yang C, Alfadhel M, Saw J (2020) Spontaneous coronary artery dissection: latest developments and new frontiers. Curr Atheroscler Rep 22(9):1–8
Johnson AK et al (2020) Analysis of posttraumatic stress disorder, depression, anxiety, and resiliency within the unique population of spontaneous coronary artery dissection survivors. J Am Heart Assoc 9(9):e014372
Saw J, Mancini GJ, Humphries KH (2016) Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 68(3):297–312
Di Fusco SA, Rossini R, Zilio F, Pollarolo L, di Uccio FS, Iorio A, Lucà F, Gulizia MM, Gabrielli D, Colivicchi F (2022) Spontaneous coronary artery dissection: overview of pathophysiology. Trends Cardiovasc Med 32(2):92–100
Iismaa SE et al (2021) Spontaneous coronary artery dissection and fibromuscular dysplasia: vasculopathies with a predilection for women. Heart Lung Circ 30(1):27–35
Kotecha D et al (2021) Risks and benefits of percutaneous coronary intervention in spontaneous coronary artery dissection. Heart 107(17):1398–1406
Aslam A et al (2021) Spontaneous coronary artery dissection: an underdiagnosed clinical entity—a primer for cardiac imagers. Radiographics 41(7):1897–1915
Adlam D et al (2021) Spontaneous coronary artery dissection: pitfalls of angiographic diagnosis and an approach to ambiguous cases. JACC Cardiovasc Interv 14(16):1743–1756
Hayes SN et al (2020) Spontaneous coronary artery dissection: JACC state-of-the-art review. J Am Coll Cardiol 76(8):961–984
Saw J et al (2022) Canadian spontaneous coronary artery dissection cohort study: 3-year outcomes. J Am Coll Cardiol 80(17):1585–1597
Saw J et al (2019) Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J 40(15):1188–1197
Adams C et al (2021) Mortality in spontaneous coronary artery dissection: a systematic review and meta-analysis. Catheter Cardiovasc Interv 98(7):1211–1220
Alfonso F, García-Guimaraes M, Alvarado T, Sanz-Ruiz R, Roura G, Amat-Santos IJ, Abdul-Jawad Altisent O, Tizón-Marcos H, Flores-Ríos X, Masotti M, Pérez-de Prado A, Ferre GF, Ruiz-Poveda FL, Valero E, Portero-Portaz JJ, Diez-Villanueva P, Salamanca J, Bastante T, Rivero F (2022) Clinical implications of arterial hypertension in patients with spontaneous coronary artery dissection. Coron Artery Dis 33(2):75–80
Tweet MS, Miller VM, Hayes SN (2019) The evidence on estrogen, progesterone, and spontaneous coronary artery dissection. JAMA Cardiology 4(5):403–404
Mahmoud AN et al (2018) Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovas Interv 11(1):80–90
Alfonso F, García-Guimaraes M (2021) Spontaneous coronary artery dissection: Where do we stand? Med Intensiva 45(6):371–374
Kim Y et al (2021) Clinical characteristics of spontaneous coronary artery dissection in young female patients with acute myocardial infarction in Korea. Korean J Intern Med 36(1):106
Sedlak T et al (2020) Coronary flow reserve in patients with prior spontaneous coronary artery dissection and recurrent angina. J Am Heart Assoc 9(16):e015834
Hui P et al (2020) The value of plasma fibrillin-1 level in patients with spontaneous coronary artery dissection. Int J Cardiol 302:150–156
Amrani-Midoun A, Adlam D, Bouatia-Naji N (2021) Recent advances on the genetics of spontaneous coronary artery dissection. Circ Genomic Precis Med 14(6):e003393
Nishiguchi T et al (2016) Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 5(3):263–270
McAlister C et al (2022) Differences in demographics and outcomes between men and women with spontaneous coronary artery dissection. Cardiovasc Interv 15(20):2052–2061
Hassan S et al (2019) Natural history of spontaneous coronary artery dissection with spontaneous angiographic healing. Cardiovasc Interv 12(6):518–527
Baechler CJ et al (2022) Spontaneous coronary artery dissection and evidence-based medicine. Am J Cardiol 171:65–68
Isogai T et al (2022) Factors associated with revascularization in women with spontaneous coronary artery dissection and acute myocardial infarction. Am J Cardiol 166:1–8
Gilhofer TS, Saw J (2019) Spontaneous coronary artery dissection: a review of complications and management strategies. Expert Rev Cardiovasc Ther 17(4):275–291
Hassan S et al (2021) Outcomes of percutaneous coronary intervention in patients with spontaneous coronary artery dissection. J Interv Cardiol 2021
García-Guimarães M et al (2022) Characteristics, acute results, and prognostic impact of percutaneous coronary interventions in spontaneous coronary artery dissection (from the Prospective Spanish Registry on SCAD [SR-SCAD]). Am J Cardiol 171:177–178
Seidl S, Rickli H, Rogowski S, Weilenmann D, Ammann P, Haager PK, Joerg L, Rohner F, Chronis J, Rigger J, Maeder MT (2021) Long-term follow-up of medically treated patients with spontaneous coronary artery dissection: a prospective, Swiss single-centre cohort study. Swiss Med Wkly 151:w30067
Cerrato E et al (2021) Antiplatelet therapy in patients with conservatively managed spontaneous coronary artery dissection from the multicentre DISCO registry. Eur Heart J 42(33):3161–3171
Kermali M et al (2021) Spontaneous coronary artery dissection: presentation and management options. Coron Artery Dis 32(2):152–163
Ferdinand KC, Nasser SA (2017) Management of essential hypertension. Cardiol Clin 35(2):231–246
Vaca KC, Tremmel JA, Edwards KS (2021) Preliminary support for group cognitive behavioral therapy (CBT) to reduce psychological distress in patients with spontaneous coronary artery dissection (SCAD). J Clin Psychol Med Settings 28(4):826–832
Tweet MS et al (2020) Association of pregnancy with recurrence of spontaneous coronary artery dissection among women with prior coronary artery dissection. JAMA Netw Open 3(9):e2018170–e2018170
Kok SN, Tweet MS (2021) Recurrent spontaneous coronary artery dissection. Expert Rev Cardiovasc Ther 19(3):201–210
Krittanawong C et al (2020) Recurrent spontaneous coronary artery dissection in the United States. Int J Cardiol 301:34–37
Hamm LF et al (2011) Core competencies for cardiac rehabilitation/secondary prevention professionals: 2010 update: position statement of the American Association of Cardiovascular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev 31(1):2–10
Stone JA, Arthur HM (2005) Canadian guidelines for cardiac rehabilitation and cardiovascular disease prevention, 2004: executive summary. Can J Cardiol 21:3D–19D
Alter DA, Oh PI, Chong A (2009) Relationship between cardiac rehabilitation and survival after acute cardiac hospitalization within a universal health care system. Eur J Cardiovasc Prev Rehabil 16(1):102–113
Anderson L, Taylor RS (2014) Cardiac rehabilitation for people with heart disease: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev (12):CD011273
Colella TJ et al (2015) Sex bias in referral of women to outpatient cardiac rehabilitation? A meta-analysis. Eur J Prev Cardiol 22(4):423–441
Galati A et al (2018) Cardiac rehabilitation in women: state of the art and strategies to overcome the current barriers. J Cardiovasc Med 19(12):689–697
Supervía M, Medina-Inojosa JR, Yeung C, Lopez-Jimenez F, Squires RW, Pérez-Terzic CM, Brewer LC, Leth SE, Thomas RJ (2017) Cardiac rehabilitation for women: a systematic review of barriers and solutions. Mayo Clin Proc 13:S0025-6196(17)30026-5
Silber TC et al (2015) Cardiac rehabilitation after spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev 35(5):328–333
Tweet MS et al (2021) Physical activity and exercise in patients with spontaneous coronary artery dissection and fibromuscular dysplasia. Eur Heart J 42(37):3825–3828
Chou AY et al (2016) The first dedicated cardiac rehabilitation program for patients with spontaneous coronary artery dissection: description and initial results. Can J Cardiol 32(4):554–560
Krittanawong C et al (2016) Usefulness of cardiac rehabilitation after spontaneous coronary artery dissection. Am J Cardiol 117(10):1604–1609
Wagers TP et al (2018) Spontaneous coronary artery dissection (SCAD): female survivors’ experiences of stress and support. J Cardiopulm Rehabil Prev 38(6):374
Bouchard K et al (2021) Recovering from spontaneous coronary artery dissection: patient-reported challenges and rehabilitative intervention needs. Health Psychol 40(7):472
Neubeck L, McHale S, Ross M, MacGillivray S, Galbraith M, Hanson C (2022) Spontaneous coronary artery dissection: a systematic review of physical and psychosocial recovery following discharge from hospital. Eur J Cardiovasc Nurs 21(7):665–676
de Melo Ghisi GL et al (2022) Women-focused cardiovascular rehabilitation: an international council of cardiovascular prevention and rehabilitation clinical practice guideline. Can J Cardiol
Petropoulos T, Madan M (2022) Spontaneous coronary artery dissection, patient guide 2022. Sunnybrook Health Sciences Centre
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Namkoong, J.Y., Colella, T.J., Carvalho, C.G., Madan, M., Liu, S. (2023). Spontaneous Coronary Artery Dissection (SCAD): An Overview of the Condition, Diagnostic Work Up and Management. In: Kirshenbaum, L., Rabinovich-Nikitin, I. (eds) Biology of Women’s Heart Health. Advances in Biochemistry in Health and Disease, vol 26. Springer, Cham. https://doi.org/10.1007/978-3-031-39928-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-39928-2_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-39927-5
Online ISBN: 978-3-031-39928-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)