Opinion statement
As advancements are made in cancer treatment, there is an increasing recognition of the cardiotoxic potential of chemotherapies and the need to monitor for the development of cardiac dysfunction in survivors. Echocardiography is the cornerstone of cardiac imaging and provides a feasible and non-invasive method to assess cardiac dysfunction in patients with cancer. In recent years, there has been increasing research in echocardiographic techniques to improve diagnosis of cardiotoxicity, including a more accurate assessment of the left ventricular function and the detection of subclinical disease. These specialized techniques include stress and contrast echocardiography, three-dimensional echocardiography, diastolic dysfunction, tissue Doppler imaging, and strain parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer and heart disease are the two major causes of mortality and morbidity in the USA and worldwide. Advances in therapy and technology have improved survival in both, and in recent years, the increasing number of cancer survivors has necessitated treatment of cardiovascular disease in this subset of patients. Accordingly, the field of cardio-oncology is rapidly developing in order to assess and treat these two important diseases together. The spectrum of cardiovascular disease in oncologic patients is wide, ranging from development of cardiomyopathy, valve disease, ischemia, and pericardial disease. Particularly, the use of anthracyclines, trastuzumab, and radiotherapy treatment pose increased risk for developing cardiovascular sequelae.
At the forefront of treatment is the use of cardiac imaging for the detection, prevention, and guidance of treatment in cardiovascular disease. Echocardiography is the mainstay of these imaging modalities and in cancer patients has proven to be the most feasible and readily available. Echocardiography is used in every phase of treatment of cancer patients—prior to, during, and following cancer-related therapies—and remains the ideal imaging modality because it is widely available, non-invasive, and lacks radiation exposure [1••]. The usefulness of echocardiography is in its ability to comprehensively assess myocardial structure and function with no risk to the patient. Echocardiographic techniques such as three-dimensional echocardiogram (3dE) and contrast echocardiogram have improved the ability to accurately measure left ventricular (LV) function. Stress echocardiography, diastolic dysfunction parameters, tissue Doppler imaging (TDI), and strain are able to detect subtle changes in myocardial dysfunction and may aid in early detection of cardiotoxicity. Emerging methods such as 3d strain have promise in increasing sensitivity for detection of chemotherapy induced cardiotoxicity.
Cardiotoxicity related to cancer therapy
The most widely recognized causes of cardiotoxicity are anthracycline-based and trastuzumab chemotherapies used commonly in treatment of breast cancer. With anthracyclines, the decline in LV function is a dose-related phenomenon and is characterized by irreversible myocardial damage. Trastuzumab-related cardiac dysfunction however is not dose related and has a high likelihood of recovery. The use of both therapies is associated with the highest risk of development of cardiotoxicity with up to 42 % incidence of cardiomyopathy or heart failure (HF) [2]. The definition of cardiac dysfunction related to cancer therapeutics has varied in the past [1••, 3–6] and several of the most widely used criteria are listed in Table 1.
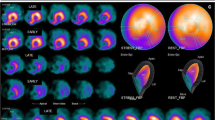
Multiple modalities have been shown to be useful in assessment of left ventricular function in patients with cancer. Figure 1 shows examples of various echocardiographic methods in evaluation of the left ventricle. Table 2 summarizes the advantages, limitations, and clinical implications of the echocardiographic modalities that will be further discussed in this paper.
Two-dimensional echocardiography
Assessment of LV function by two-dimensional echocardiography (2dE) is the most widely used parameter in detection of cardiac dysfunction in patients with cancer. The American Society of Echocardiography (ASE) recommends the use of the modified biplane Simpson’s technique when assessing LV volume and function [7••, 8]. Parameters such as M-mode and linear measurements take into consideration only two walls of the myocardium (anteroseptal and inferolateral) and can be misleading [1••, 7••, 8]. Normal left ventricular ejection fraction (LVEF) is defined as ≥55 %, established by the ASE; however, multiple databases suggest the normal LVEF is approximately 63 % and better reflected by a range of 53–73 % [9–13].
The ASE released an update to the recommendations on chamber quantification in adults in 2015 [7••]. Notably, there was a move from compartmentalizing severity of disease from mild, moderate, and severe and recognition that these values are different based on body mass, gender, and age. Left atrial biplane volumes were recommended over two-dimensional measurements. New measurement techniques were also discussed, including focus on right ventricular measurements, strain, and 3dE. These emerging techniques have been studied in the cardio-oncology field and, with the new ASE updates, provide standardization in acquisition and reporting.
Right ventricular (RV) abnormalities can occur in the setting of primary or metastatic disease, pre-existing structural (valve or ventricular) dysfunction, or secondary to chemotherapy. It is difficult to quantify the frequency of RV disorders due to lack of focus in prior studies. The thin structure of the RV free wall may make it susceptible to damage by cardiotoxic therapy [14]. Evaluation of the RV per ASE recommendations should include RV basal and mid chamber diameter [8], longitudinal M-mode-derived tricuspid annular plane systolic excursion and pulsed Doppler tissue imaging-derived systolic peak velocity of the tricuspid annulus [1••, 8]. There is no sufficient evidence to warrant routine RV 3dE or strain assessment. When sufficient tricuspid regurgitant jet is present, the RV systolic pressure estimate should be reported.
Calleja et al. recently conducted a study of 30 women with HER2+ breast cancer who developed LV cardiotoxicity after treatment with trastuzumab to determine the incidence of concomitant RV dysfunction. The echocardiographic measurements included were RV fractional area change (RVFAC) and global longitudinal strain (GLS). The results showed that RV function was decreased in women with LV cardiotoxicity by abnormal RVFAC and strain. The proportion of women with abnormal strain (40 %) was higher than RVFAC (10 %) demonstrating the increased sensitivity of strain to identify subtle dysfunction. Recovery of LV function was also lower in patients with associated RV dysfunction. This study further expands on the knowledge that exists regarding the importance of RV cardiotoxicity.
Pericardial disease is common in oncologic patients and can occur in the setting of chemotherapy, radiation, or due to primary or metastatic disease. 2dE is the most common modality in initial evaluation of pericardial disease or accompanying effusion. Assessment of pericardial effusion size, presence of tamponade, and constriction should be performed based on ASE guidelines including M-mode and 2d techniques measurements [15].The presence of constriction in oncologic patients can occur after high-dose chemotherapy or as a result of radiotherapy. The use of cardiac magnetic resonance (CMR) imaging and computed tomography (CT) can aid in confirming diagnosis of pericarditis. In the age of modern radiotherapy (RT), the incidence of pericardial disease has decreased significantly.
Cardiac tumors are rare and if present, are often benign. 2dE is the primary diagnostic modality for assessment of cardiac masses. Tumors, metastatic lesions, thrombus, and other cardiac masses can be appropriately evaluated by transthoracic echo (TTE) and if warranted, transesophageal echocardiogram (TEE). Location of a cardiac mass can often narrow the diagnosis and contrast echocardiography can also aid in further tissue characterization [16]. CMR and CT can also aid in diagnosis of cardiac tumors.
Valvular heart disease (VHD) present in oncologic patients may be from a primary or metastatic tumor, pre-existing valvulopathy, secondary to RT, or result of infection in the setting of immunocompromised state [17–19]. In evaluation of valves, TTE may be sufficient, but increased resolution of TEE may be beneficial, especially in diagnosis of endocarditis. RT given for mediastinal or breast disease may result in incidental cardiac exposure and increase risk of ischemic and VHD [20]. Recent data showed that in survivors of Hodgkin’s lymphoma (HL) receiving mediastinal irradiation, 32 % developed asymptomatic valve defects at 6 years and up to 42 % at 20 years with increasing risk above radiation doses of 30 Gy [21, 22]. Recognition of these risks and decrease in use of sole radiotherapy have led to reduction in doses of RT in the treatment for HL [23].
In 2015, a retrospective review [24] of 1852 5-year survivors of HL was conducted to further establish the relationship between radiation dose to the heart and risk of clinically significant VHD defined by presence of at least moderate severity lesion. There was little increase in clinical VHD in those receiving less than 30 Gy. However, for doses above 30 Gy, the percentage of clinical VHD increased progressively with increasing dose. When compared to controls, for doses to the affected valves of less than or equal to 30, 31–35, 36–40, and more than 40 Gy, VHD rates increased by factors of 1.4, 3.1, 5.4, and 11.8, respectively. This study supports findings of prior studies and establishes a dose-response relationship for development of clinical VHD.
Three-dimensional echocardiography and contrast echocardiography
Although 2dE-derived LVEF is a strong predictor of cardiovascular outcomes, it may miss incremental changes in LV function. The 2dE techniques for quantification of LV are limited by image quality, loading conditions, geometric assumptions, and presence of regional wall motion abnormalities [1••, 25, 26] as noted in Table 2. Given these limitations, 3dE has emerged as a more accurate measurement of LV volume and function [27, 28]. Particularly in patients receiving anthracyclines and trastuzumab, 3dE appears to be more reproducible when compared to 2dE methods for serial examination [29]. Limitations to use of 3dE include decreased availability as specialized software is required.
Contrast echocardiography is useful in those patients with difficult windows to enhance the endocardial border and provide accurate assessment of LV function and wall motion abnormalities. The use of contrast agent is indicated when two contiguous LV segments are not visualized on non-contrast images. There is limited literature on the use of contrast echocardiography in monitoring and diagnosis of cardiotoxicity, with one study favoring the use of 3dE-derived LVEF over 2dE with and without contrast due to lower temporal variability [29]. In one small study, 3dE and contrast echocardiography were found to have similar accuracy for LVEF compared with CMR [30]. In addition to delineation of endocardial borders, contrast can be used to delineate structures in the LV cavity including masses and thrombi. This can be especially useful at the apex, an area of the myocardium prone to significant artifact on noncontract images.
The St. Jude Lifetime Cohort Study [31•] evaluated 3dE, Global longitudinal strain, and diastolic dysfunction in 1820 adult survivors of childhood cancer exposed to anthracycline therapy to identify prevalence of cardiac dysfunction. Published recently, this is the largest study to date evaluating advanced echocardiographic techniques. Only 5.8 % had abnormal 3d LVEF (<50 %), with 32 % having normal 3dE LVEF with evidence of abnormal GLS and diastolic dysfunction. The findings suggest that diastolic dysfunction and decreased strain are more sensitive markers of cardiac dysfunction than 3dE and the subset with abnormal diastology or GLS are higher risk and hence should be monitored more closely for development of LV dysfunction long term.
Evaluation of subclinical disease
Decrease in LVEF during cancer treatment may be a late finding of cardiotoxicity, and prior studies suggest that a more sensitive marker may be helpful in prevention of subsequent heart failure [32].
Stress echocardiography is routinely used to evaluate patients for coronary artery disease by assessment of wall motion abnormalities and augmentation of LV function at peak stress. In the oncologic population, stress echocardiography can be used to evaluate subclinical LV dysfunction [33–42] and evaluate contractile reserve in those patients with decreased LV function [43–49].
Tissue Doppler imaging (TDI) uses Doppler to measure the excursion and velocity of the myocardium and allow for assessment of cardiac function. Commonly measured TDI indices include peak myocardial velocity (Sm), peak systolic mitral annular velocity (Sa), peak early diastolic myocardial velocity (Em), peak early mitral annular diastolic velocity (Ea), and isovolumetric contraction time (IVCT) and isovolumetric relaxation time (ISRT). TDI in several studies has been shown to detect subclinical LV dysfunction prior to decrease of LVEF [50, 51] in patients undergoing anthracycline or trastuzumab therapy. Changes in peak early diastolic velocity were detected within 1–3 months whereas changes in LVEF were not detected until 3 years after therapy was completed. Fallah-Rad et al. followed a higher risk group of patients who received anthracyclines and trastuzumab, and decrease in peak systolic annular velocity was noted 3 months after chemotherapy. All patients with decreased TDI developed heart failure at later stages, with changes in LVEF not detected until 6 months [51]. TDI has several limitations, the most significant being the angle-dependent nature of Doppler parameters as only vectors parallel to the ultrasound beam are measured. Any slight change in angle can lead to varying results. TDI cannot distinguish passive (tethering) from active motion. These shortcomings lead to decreased reproducibility. The Tei index is a Doppler-derived parameter that assesses the global index of cardiac function [52]. It is the sum of the isovolumetric contraction and relaxation time divided by ejection time [52]. Several studies [53, 54] support the early subclinical detection of LV cardiac dysfunction with the Tei index, although it is unsure the long-term clinical outcomes related to its use.
Diastolic dysfunction is an important aspect of development of heart failure and has been evaluated in the patients receiving cancer-related therapies for early detection of cardiotoxicity. Several studies [50] have shown abnormal diastolic parameters precede a drop in LVEF; however, it is unclear how these findings translate to long-term development of heart failure.
In a small study of 85 women with breast cancer receiving anthracyclines, trastuzumab, or both, echocardiographic parameters including LVEF, Doppler measurements, and TDI parameters were measured before induction of chemotherapy and for a follow-up of 2 years after to assess changes in cardiac function. The study also evaluated changes after initiation of HF medications including ace inhibitor (ACEi), angiotensin receptor blockers (ARB), and beta-blockers. There was significant reduction of TDI as early as 6 months after the initiation of chemotherapy and at 2 years. LVEF measured by a modified biplane Simpson’s technique was significantly reduced at 2 years. TDI was found to be a more sensitive in the early diagnosis of LV dysfunction. Those treated with ACEi, ARBs, and beta-blockers showed less changes in TDI markers (Sm and IVCT) on follow-up, confirming the cardioprotective role of HF therapy initiation.
The limitations of 2dE, including dependence on loading conditions and tethering of myocardial segments can hinder recognition of subtle regional variations. Markers of deformation (strain, strain rate) have been studied and proven to be a reliable way to detect cardiac dysfunction before LVEF is decreased. Strain is defined as the distance a material deforms relative to its length at baseline. Strain can be measured by TDI and also by speckle tracking. 2d speckle tracking echocardiography (2d-STE) identifies small echo dense structures, or speckles, and tracks these throughout the cardiac cycle. Strain is measured as longitudinal, radial, and circumferential and is reported routinely as global longitudinal strain. Decrease in strain in patients undergoing anthracycline therapy with and without trastuzumab therapy has been previously described [50, 55, 56•, 57, 58]. These changes have been found to occur earlier than detected with LVEF [57] and predictive of development of cardiotoxicity at 6 to 12 months of therapy [56•]. Long-term outcomes have yet to be published so the significance of strain abnormalities over time is unknown.
Although strain echocardiography is promising in detection subclinical LV dysfunction, it has important limitations. Foremost, strain is derived from 2dE images, and its accuracy depends on baseline image quality. Reproducibility is likewise dependent on the experience of the sonographer and although longitudinal strain is noted to be the most reproducible, circumferential and radial strain measurements have high intra and inter-observer variability [59]. The varying ranges of normal values across vendors have also limited the generalizability of studies. The JUSTICE study summarizes GLS values by age, gender for each vendor [60]. As with all LV parameters, strain measurements, especially TDI strain, are affected by loading conditions.
In a recent study, Ryerson et al. combined the use of TDI, strain, and stress echocardiography in 80 asymptomatic childhood survivors that were at least 5 years post treatment. A small high-risk group had significant presence of diastolic dysfunction by TDI at rest in the setting of normal LVEF. However, this difference resolved with stress suggesting that these patients were able to compensate for mild cardiac dysfunction seen in diastole [33]. The authors suggest that in young cancer survivors, mild cardiac dysfunction is well compensated for during exercise, but long-term follow-up is required to determine subsequent development of clinical disease.
One hundred twenty patients with normal LVEF and cancer undergoing chemotherapy were reviewed for LV GLS and all-cause mortality. Abnormal LV GLS was noted in 43 %, and over the course of 21–35 months, 48 % of the cohort passed away with performance status, male sex, and LV GLS significantly associated with all-cause mortality. In the study, there was only one death definitely due to cardiotoxicity, and the authors imply that strain detects changes in cardiac function caused by severe illness in addition to frank cardiotoxicity. Several proposed mechanisms included increase in inflammatory cytokines and autonomic dysfunction associated with advanced cancer as a cause for abnormal LV GLS [61].
Emerging echocardiography techniques
Three-dimensional speckle tracking echocardiography (3d-STE) is a novel technique in assessing full-volume deformation of the LV cavity. This technique avoids the pitfalls of 2dE and allows the operator to acquire images efficiently, eliminating the time consuming acquisition associated with 2d-STE. Xu et al. compared the ability of 3d-STE and 2d-STE and found that GLS evaluation was similar to 2d-STE >84.9 % of the time and acquisition of 3d-STE was five times faster than 2d-STE or by modified biplane method [62]. Using 3d-STE, Yu evaluated the use of 3d-STE in 53 cancer survivors treated with anthracycline therapy. Compared to controls, survivors had significantly reduced LV global strain [63]. Another study by Mornos demonstrated decrease in 3d-STE prior to decline in 2dE LVEF in patients undergoing anthracycline therapy. Larger studies are needed to validate these findings, but the few studies performed highlight the potential of 3d-STE.
Conclusion
Detection of cardiac dysfunction in patients with cancer is important due to the cardiotoxic potential of chemotherapies and increase in number of survivors requiring long-term care. Early identification of cardiotoxicity has important clinical implications in reducing overall cardiovascular morbidity. Echocardiography remains the most widely used imaging technique in diagnosis of cardiotoxicity. It offers a spectrum of parameters to assess cardiac structure and function; however, further studies are needed to define long term benefits of novel echocardiographic techniques.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–39. Detailed resource on the role of imaging in evaluation of cardiac disease in cancer therapy.
Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60:2504–12.
Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–21.
Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the herceptin adjuvant (HERA) trial. J Clin Oncol. 2010;28:3422–8.
Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25:3859–65.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70. Extensive guide for assessment and measurement of cardiac chambers.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs Jr DR, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16.
Kuznetsova T, Herbots L, Lopez B, Jin Y, Richart T, Thijs L, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–12.
Lancellotti P, Badano LP, Lang RM, Akhaladze N, Athanassopoulos GD, Barone D, et al. Normal reference ranges for echocardiography: rationale, study design, and methodology (NORRE Study). Eur Heart J Cardiovasc Imaging. 2013;14:303–8.
Muraru D, Badano LP, Peluso D, Dal Bianco L, Casablanca S, Kocabay G, et al. Comprehensive analysis of left ventricular geometry and function by three-dimensional echocardiography in healthy adults. J Am Soc Echocardiogr. 2013;26:618–28.
Rietzschel ER, De Buyzere ML, Bekaert S, Segers P, De Bacquer D, Cooman L, et al. Rationale, design, methods and baseline characteristics of the Asklepios study. Eur J Cardiovasc Prev Rehabil. 2007;14:179–91.
Calleja A, Poulin F, Khorolsky C, Shariat M, Bedard PL, Amir E, et al. Right ventricular dysfunction in patients experiencing cardiotoxicity during breast cancer therapy. J Oncol. 2015;2015:609194.
Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2013;26:965–1012 e15.
Kirkpatrick JN, Wong T, Bednarz JE, Spencer KT, Sugeng L, Ward RP, et al. Differential diagnosis of cardiac masses using contrast echocardiographic perfusion imaging. J Am Coll Cardiol. 2004;43:1412–9.
Bansal RC. Infective endocarditis. Med Clin North Am. 1995;79:1205–40.
Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7.
Lancellotti P, Nkomo VT, Badano LP, Bergler-Klein J, Bogaert J, Davin L, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:721–40.
Schellong G, Riepenhausen M, Bruch C, Kotthoff S, Vogt J, Bolling T, et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr Blood Cancer. 2010;55:1145–52.
Machann W, Beer M, Breunig M, Stork S, Angermann C, Seufert I, et al. Cardiac magnetic resonance imaging findings in 20-year survivors of mediastinal radiotherapy for Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 2011;79:1117–23.
Cella L, Liuzzi R, Conson M, Torre G, Caterino M, De Rosa N, et al. Dosimetric predictors of asymptomatic heart valvular dysfunction following mediastinal irradiation for Hodgkin’s lymphoma. Radiother Oncol. 2011;101:316–21.
Specht L, Yahalom J, Illidge T, Berthelsen AK, Constine LS, Eich HT, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the international lymphoma radiation oncology group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89:854–62.
Cutter DJ, Schaapveld M, Darby SC, Hauptmann M, van Nimwegen FA, Krol AD, Janus CP, van Leeuwen FE, Aleman BM. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Nat Cancer Inst. 2015;107.
Gulati G, Zhang KW, Scherrer-Crosbie M, Ky B. Cancer and cardiovascular disease: the use of novel echocardiography measures to predict subsequent cardiotoxicity in breast cancer treated with anthracyclines and trastuzumab. Curr Heart Fail Rep. 2014;11:366–73.
Jacobs LD, Salgo IS, Goonewardena S, Weinert L, Coon P, Bardo D, et al. Rapid online quantification of left ventricular volume from real-time three-dimensional echocardiographic data. Eur Heart J. 2006;27:460–8.
Jenkins C, Chan J, Hanekom L, Marwick TH. Accuracy and feasibility of online 3-dimensional echocardiography for measurement of left ventricular parameters. J Am Soc Echocardiogr. 2006;19:1119–28.
Jenkins C, Moir S, Chan J, Rakhit D, Haluska B, Marwick TH. Left ventricular volume measurement with echocardiography: a comparison of left ventricular opacification, three-dimensional echocardiography, or both with magnetic resonance imaging. Eur Heart J. 2009;30:98–106.
Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84.
Jenkins C, Marwick TH. Baseline and follow-up assessment of regional left ventricular volume using 3-Dimensional echocardiography: comparison with cardiac magnetic resonance. Cardiovasc Ultrasound. 2009;7:55.
Armstrong GT, Joshi VM, Ness KK, Marwick TH, Zhang N, Srivastava D, et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65:2511–22. Evlauation of strain and diastolic dysfunction in largest to date cohort of long term survivors. Abnormal GLS and diastolic dysfunction were associated with treatment exposure.
Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–20.
Ryerson AB, Border WL, Wasilewski-Masker K, Goodman M, Meacham L, Austin H, et al. Assessing anthracycline-treated childhood cancer survivors with advanced stress echocardiography. Pediatr Blood Cancer. 2015;62:502–8.
Weidemann F, Breunig F, Beer M, Sandstede J, Stork S, Voelker W, et al. The variation of morphological and functional cardiac manifestation in Fabry disease: potential implications for the time course of the disease. Eur Heart J. 2005;26:1221–7.
Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266:1672–7.
Pieroni M, Chimenti C, Ricci R, Sale P, Russo MA, Frustaci A. Early detection of Fabry cardiomyopathy by tissue Doppler imaging. Circulation. 2003;107:1978–84.
Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145–53.
Kilickap S, Barista I, Akgul E, Aytemir K, Aksoyek S, Aksoy S, et al. cTnT can be a useful marker for early detection of anthracycline cardiotoxicity. Ann Oncol. 2005;16:798–804.
Ganame J, Claus P, Eyskens B, Uyttebroeck A, Renard M, D'Hooge J, et al. Acute cardiac functional and morphological changes after anthracycline infusions in children. Am J Cardiol. 2007;99:974–7.
Galderisi M, Marra F, Esposito R, Lomoriello VS, Pardo M, de Divitiis O. Cancer therapy and cardiotoxicity: the need of serial Doppler echocardiography. Cardiovasc Ultrasound. 2007;5:4.
Colan SD, Sanders SP, Borow KM. Physiologic hypertrophy: effects on left ventricular systolic mechanics in athletes. J Am Coll Cardiol. 1987;9:776–83.
Borow KM, Henderson IC, Neuman A, Colan S, Grady S, Papish S, et al. Assessment of left ventricular contractility in patients receiving doxorubicin. Ann Intern Med. 1983;99:750–6.
Bountioukos M, Doorduijn JK, Roelandt JR, Vourvouri EC, Bax JJ, Schinkel AF, et al. Repetitive dobutamine stress echocardiography for the prediction of anthracycline cardiotoxicity. Eur J Echocardiogr. 2003;4:300–5.
Jarfelt M, Kujacic V, Holmgren D, Bjarnason R, Lannering B. Exercise echocardiography reveals subclinical cardiac dysfunction in young adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49:835–40.
De Wolf D, Suys B, Maurus R, Benoit Y, Verhaaren H, Matthijs D, et al. Dobutamine stress echocardiography in the evaluation of late anthracycline cardiotoxicity in childhood cancer survivors. Pediatr Res. 1996;39:504–12.
Lanzarini L, Bossi G, Laudisa ML, Klersy C, Arico M. Lack of clinically significant cardiac dysfunction during intermediate dobutamine doses in long-term childhood cancer survivors exposed to anthracyclines. Am Heart J. 2000;140:315–23.
Elbl L, Hrstkova H, Chaloupka V, Novotny J, Michalek J. The evaluation of left ventricular function in childhood cancer survivors by pharmacological stress echocardiography. Neoplasma. 2003;50:191–7.
Hamada H, Ohkubo T, Maeda M, Ogawa S. Evaluation of cardiac reserved function by high-dose dobutamine-stress echocardiography in asymptomatic anthracycline-treated survivors of childhood cancer. Pediatr Int. 2006;48:313–20.
Civelli M, Cardinale D, Martinoni A, Lamantia G, Colombo N, Colombo A, et al. Early reduction in left ventricular contractile reserve detected by dobutamine stress echo predicts high-dose chemotherapy-induced cardiac toxicity. Int J Cardiol. 2006;111:120–6.
Ho E, Brown A, Barrett P, Morgan RB, King G, Kennedy MJ, et al. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: a speckle tracking echocardiographic study. Heart. 2010;96:701–7.
Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–70.
Tei C. New non-invasive index for combined systolic and diastolic ventricular function. J Cardiol. 1995;26:135–6.
Belham M, Kruger A, Mepham S, Faganello G, Pritchard C. Monitoring left ventricular function in adults receiving anthracycline-containing chemotherapy. Eur J Heart Fail. 2007;9:409–14.
Dodos F, Halbsguth T, Erdmann E, Hoppe UC. Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clin Res Cardiol. 2008;97:318–26.
Mercuro G, Cadeddu C, Piras A, Dessi M, Madeddu C, Deidda M, et al. Early epirubicin-induced myocardial dysfunction revealed by serial tissue Doppler echocardiography: correlation with inflammatory and oxidative stress markers. Oncologist. 2007;12:1124–33.
Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26:493–8. Comparison of various echocardiographic modalities including strain, ejection fraction and diastolic parameters in breast cancer patients.
Neilan TG, Jassal DS, Perez-Sanz TM, Raher MJ, Pradhan AD, Buys ES, et al. Tissue Doppler imaging predicts left ventricular dysfunction and mortality in a murine model of cardiac injury. Eur Heart J. 2006;27:1868–75.
Stoodley PW, Richards DA, Boyd A, Hui R, Harnett PR, Meikle SR, et al. Left ventricular systolic function in HER2/neu negative breast cancer patients treated with anthracycline chemotherapy: a comparative analysis of left ventricular ejection fraction and myocardial strain imaging over 12 months. Eur J Cancer. 2013;49:3396–403.
Koopman LP, Slorach C, Manlhiot C, McCrindle BW, Jaeggi ET, Mertens L, et al. Assessment of myocardial deformation in children using digital imaging and communications in medicine (DICOM) data and vendor independent speckle tracking software. J Am Soc Echocardiogr. 2011;24:37–44.
Takigiku K, Takeuchi M, Izumi C, Yuda S, Sakata K, Ohte N, et al. Normal range of left ventricular 2-dimensional strain: Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) study. Circ J. 2012;76:2623–32.
Rhea IB, Uppuluri S, Sawada S, Schneider BP, Feigenbaum H. Incremental prognostic value of echocardiographic strain and its association with mortality in cancer patients. J Am Soc Echocardiogr. 2015;28:667–73.
Xu TY, Sun JP, Lee AP, Yang XS, Qiao Z, Luo X, et al. Three-dimensional speckle strain echocardiography is more accurate and efficient than 2D strain in the evaluation of left ventricular function. Int J Cardiol. 2014;176:360–6.
Yu HK, Yu W, Cheuk DK, Wong SJ, Chan GC, Cheung YF. New three-dimensional speckle-tracking echocardiography identifies global impairment of left ventricular mechanics with a high sensitivity in childhood cancer survivors. J Am Soc Echocardiogr. 2013;26:846–52.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Both authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Patel, A.A., Labovitz, A.J. Advanced Echocardiographic Techniques in Detection of Cardiotoxicity. Curr Treat Options Cardio Med 18, 28 (2016). https://doi.org/10.1007/s11936-016-0450-1
Published:
DOI: https://doi.org/10.1007/s11936-016-0450-1