Abstract
Purpose of Review
Kidney transplantation is the best treatment for end-stage renal disease. However, due to organ shortage, suboptimal grafts are increasingly being used.
Recent Findings
We carried out a review on the methods and techniques of organ optimization in the cadaveric setting.
Summary
Donor care is the first link in a chain of care. Right after brain death, there is a set of changes, of which hormonal and hemodynamic changes are the most relevant. Several studies have been conducted to determine which drugs to administer, although in most cases, the results are not definitive. The main goal seems rather achieve a set of biochemical and hemodynamic objectives. The ischemia–reperfusion injury is a critical factor for kidney damage in transplantation. One of the ways found to deal with this type of injury is preconditioning. Local and remote ischemic preconditioning has been studied for various organs, but studies on the kidney are scarce. A new promising area is pharmacological preconditioning, which is taking its first steps. Main surgical techniques were established in the late twentieth century. Some minor new features have been introduced to deal with anatomical variations or the emergence of donation after circulatory death. Finally, after harvesting, it is necessary to ensure the best conditions for the kidneys until the time of transplantation. Much has evolved since static cold preservation, but the best preservation conditions are yet to be determined. Conservation in the cold has come to be questioned, and great results have appeared at temperatures closer to physiological.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney transplant is the best treatment for end-stage renal disease [1]. However, due to organ shortage, the majority of patients do not get a transplant. To increase organ pool available, we resort to new forms of donation, like expanded criteria donors (ECD) or donation after circulatory death (DCD) [2]. These new donors are suboptimal comparing with living donors or standard donors after brain dead (DBD) [3].
To get the best results from these organs and to harvest the maximum number of organs from each donor, a keychain of medical and technical care is established since death (DBD or DCD) is declared, until the organ is implanted on the recipient.
We performed a review of current and possible future care in renal procurement on the cadaveric setting, making a summary of the evidence on the various stages of renal procurement: donor care, preconditioning, and surgical.
Donor Optimization Prior Harvest
One of the critical points for graft quality optimization is donor care [4]. Immediately after brain death, changes start to occur in different organ systems (cardiac, pulmonary, endocrine, hematological, and musculoskeletal), which must be optimized before harvest surgery to increase the number of organs transplanted per donor (OTPD) and to improve graft function [5, 6]. Some transplant coordinating entities have established hemodynamic and biochemical goals for the brain-dead donor maintenance until harvest surgery [7]. However, the way to achieve these goals and donor pharmacological management is still a subject of study.
One of the most prominent areas is catecholamine usage. Animal studies [8,9,10] show that among catecholamines, dopamine has the highest therapeutic potential. It reduces kidney graft inflammation by decreasing the expression of major histocompatibility class (MHC) II, P-selectin and tumor necrosis factor-α (TNF-α), and by inducing antioxidant defenses. These effects resulted in less leukocyte infiltration and reduced endothelial injury associated with reperfusion–ischemia and cold storage. However, clinical studies show different results. Two retrospective studies [11, 12] have shown an advantage in donor treatment with dopamine or norepinephrine, by reducing acute rejection and improving long-term graft survival. However, two randomized control trials (RCTs) showed opposite results, as one failed to achieve kidney graft survival improvement [13] and the other one succeeded on that goal [14]. Hence, there is no definite evidence to support systematic catecholamine use in donor management to improve kidney transplant results.
Fluid management is another relevant factor. Various authors raised questions about how to manage donor fluid replenishment and which fluids to use. When addressing fluid resuscitation, retrospective studies [15] showed that an aggressive approach increased the number of OTPD. This observation led to an RCT [16] that aimed to establish a fluid therapy protocol in organ donors, but this showed no advantage over a standard and clinically guided management.
Regarding which fluids to use, different approaches have been studied. A retrospective series [17] that compared crystalloids with colloid resuscitation demonstrated that when only crystalloids were used, there was more delayed graft function (DGF) compared to an approach with crystalloids and colloids. Also, the type of colloid to use is a matter of debate. Hydroxyethyl starch has been the focus of several studies [18,19,20], showing an increased risk of DGF when this colloid was used in donor resuscitation.
As mentioned before, one of the physiological changes that happen on the donor immediately after brain death is serum hormonal level variations [21]. Among these, one of the most relevant pertains to thyroid hormones, as T3 and T4 decrease to a half hour after brain death and become undetectable between 9 and 16 h, while TSH remains stable [21, 22]. Retrospective studies and non-randomized studies [15, 23, 24] showed an advantage in donor thyroid hormone treatment, by facilitating their hemodynamic stability, reducing catecholamines use, and by improving the number of OTPD, especially the kidneys. However, RCT and literature reviews fail to show this advantage [22, 25, 26].
Other hormones whose replacement has been studied are insulin and antidiuretic hormone (ADH). After brain death, insulin decreases to 20% at 13 h, and ADH becomes undetectable at 6 h [21]. Administration of desmopressin to the brain-dead donor showed an advantage in both renal graft survival and the number of kidneys harvested by donor in two retrospective studies [23, 27], but one RCT failed to show any advantage [28]. Regarding insulin administration, it seems that more important than its administration to the donor, which in itself is not beneficial [23], the focus should be on keeping blood glucose below 180 mg/dL, which allows to improve graft function and increase the number OTPD [29].
The other crucial hormonal group studied are the corticosteroids. The rationale for their application lies in the observation that most brain-dead donors have adrenal insufficiency [30] and the presumption that using them would reverse the systemic inflammation affecting the donor [31]. However, retrospective studies [23] and RCT [32, 33] showed no advantage in terms of the number of kidneys harvested by donor, DGF, or graft survival. Furthermore, two systematic reviews [34, 35] showed no beneficial effect.
Controlled hypothermia is a promising donor management care option. An RCT [14] showed that spontaneous donor hypothermia 4–20 h before the harvest was associated with lower kidney DGF. The authors associated this improvement with less systemic inflammation, which resulted in less kidney damage. Another RCT [36] showed the same benefit, which was more significant in expanded criteria donors (ECDs). However, this may not be beneficial for all types of donors, namely for heart transplant donors, since hypothermia has been shown to lead to worse heart graft function [37].
Maintaining different hemodynamic and biochemical objectives until the time of harvest seems to be more critical than focusing on a single one drug or parameter [7]. The so-called donor management goals (DMGs), set by the US Department of Health and Human Services and the Health Resources and Services Administration, is a set of nine donor hemodynamic and biochemical parameters proposed to be achieved in brain-dead donor care. Different studies have shown that meeting at least seven of these nine goals increases the number of OTPD and decreases the rate of DGF in kidney transplant recipients [7, 38,39,40].
The use of other drugs such as statins [41, 42], cyclosporin [43], N-acetylcysteine [44], or therapeutics such as blood transfusions [45] is under investigation.
Preconditioning and Ischemia–Reperfusion Injury Modulation
Optimizing organ preservation starting even before harvest until implantation is another critical aspect to achieve a quality graft for transplantation. One of the main, if not the primary, mechanisms of organ damage until transplantation is the so-called ischemia and reperfusion injury (IRI) [46, 47]. After the circulation stops, the organ becomes anaerobic, leading to a depletion in cellular stored ATP and to dysfunction of ATP synthetase. There is an intracellular accumulation of anaerobic products, causing acidosis and hyperosmolarity. An ionic movement from extracellular to intracellular space aggravates this state of hyperosmolarity [46,47,48]. Finally, dysfunction of endoplasmic reticulum protein production and mitochondrial damage activate apoptotic pathways [47, 49, 50]. There are several mechanisms and cellular pathways involved in IRI, including the mitogen-activated protein kinase (MAPK) family pathways [51, 52], antioxidant defenses, reactive oxygen species [53], and IL-8 [54]. To prevent graft IRI, one line of study has focused on modulating the mechanisms of IRI by organ preconditioning [55, 56].
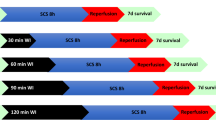
The preconditioning concept was first described by Murry in 1986 when he presented the first studies in myocardial preconditioning [57]. Since his work, the preconditioning study extended to different organs, including the kidney. In its purest form, ischemic preconditioning (IP) consists of exposing an organ to a short period of ischemia, followed by reperfusion and subsequent new ischemia. This was the principle first applied in the field of kidney transplantation by Torras [58], when he presented the first animal studies, concluding that the best IP time scheme consisted of 15 min of warm ischemia followed by 10 min of reperfusion since these were the intervals with the best histological and functional protection of renal function. Torras also demonstrated that NO was involved in the IP process [58]. As the works progressed, it was possible to realize that IP is a two-phase process, with an early and late window [59]. The initial phase is rapid, occurring within minutes and without protein synthesis. The late phase requires hours to begin and involves protein synthesis. Between them, there is a period in which there is no protection for ischemia [59]. More recently, new forms of preconditioning have emerged, such as remote IP [60] (in which organ preconditioning is achieved through limb ischemia) and pharmacological preconditioning.
IP was initially tested in animal models [61, 62] without advantage, like reducing renal dysfunction or morphological injury. It was later retested on rodent kidney transplant models [63,64,65,66], and at this time with success, showing biological improvement. These studies have shown that pathways involved in protection against IRI by IP are related to endothelial NO production, induced NO synthetase, and activation of cellular pathways such as NF-κB or hypoxia-induced factor (HIF) 1α/HIF-2. Another mechanism related to IP is heme oxygenase (HO), a key enzyme in redox homeostasis processes [67]. Overall, IP studies on renal transplantation have shown beneficial effects in inflammation inhibition, coagulation inhibition, oxidative attenuation, induction of antiapoptotic state, and modulation of HIF pathways [55]. However, despite the absence of clinical studies in renal transplantation, a meta-analysis of animal studies showed a reduction in serum creatinine, blood urea nitrogen, and histological damage in IP kidneys, and the beneficial effect was attained both with local and remote IP [68].
The field that has concentrated most of the research is pharmacological preconditioning. One extensively studied drug is erythropoietin [53, 69,70,71,72,73]. Its administration before ischemia has shown beneficial effects in animal models. It increases creatinine clearance and sodium excretion fraction, and reduces visible tubulointerstitial lesions on biopsy [53, 73] and lipid peroxidation of renal tissue [71]. Another study that aimed to evaluate IRI by urinary excretion of neutrophil gelatinase-associated lipocalin (NGAL) concluded that administration of erythropoietin significantly reduced urinary NGAL [73]. An additional beneficial effect of erythropoietin administration is the reduction of proinflammatory cytokines such as IL-6 [72], IL-2, and TNF-α [71]. The positive impact of erythropoietin appears to result from activation of tyrosine kinases, named Janus kinase 2 (JAK2) [70, 71], mediated by heat shock protein-70 (HSP70) [70], and also increased expression of the anti-apoptotic gene Bcl-2 [70].
Other composites tested showed varying results. One is carbon monoxide (CO) [74,75,76]. CO is produced at low doses by mammalian cells through HO catalysis and helps to maintain cellular protection, vascular tone, and neuromessenger [76]. Experimental studies in animal models have shown advantages in CO application on preservation fluid, namely better functional outcomes [75], better histology by reducing fibrosis, inflammatory infiltrate, and lipid peroxidation [75]. One of the mechanisms involved in CO action is cytochrome P450 levels maintenance [74].
Several other compounds are in the early stages of study such as cardiotropin-1 [77], which have been shown to reduce oxidative stress markers, inflammation, and vascular injury. Another example is melgatran, a thrombin inhibitor, which when applied to the kidney storage fluid has been shown to reduce the immune cells’ proinflammatory state after transplantation, improving graft function, reducing inflammation and kidney damage. Hydrogen sulfide (H2S) is an endogenously produced gas with anti-inflammatory, antioxidant, and antiapoptotic functions [78]. Applying H2S to the kidney preservation fluid improved early graft function and survival, decreasing necrosis and apoptosis [78]. The manipulation of IRI-related pathways may also be beneficial. For example, in a study with a HIF hydroxylase inhibitor administration, it reduced the effects of IRI [66]. Other compounds also studied with beneficial effects were cyclosporine [51], 1–25 dihydroxy vitamin D3 [79], tin-protoporphyrin IX (an HO inhibitor) [80], bosentan [81], ozone [82], or sildenafil [83].
Optimization of Surgical Harvesting Technique
Establishment of organ harvest main surgical techniques was done at the end of twentieth century [84,85,86]. Despite having similar surgical procedures, DBD and DCD surgical techniques are different.
In DBD, there are two different approaches. The oldest is the “warm dissection technique” [87] in which the anatomical structures are dissected before perfusing the corpse. This technique is associated with more vasospasm and vascular and parenchymal lesions [88], requiring a 30- to 60-min recovery period before ischemia to reverse some of the damages. However, this technique facilitates anatomical dissection. The most commonly used method is “dissection in the cold” [88, 89]. With this technique, there is minimal dissection until ischemia and perfusion are established. Dissection and organ separation only takes place after exsanguination and when the organs are cold. Studies indicate that functional results are similar between these two techniques [90].
With DCD, we have two main techniques: “super-rapid” and “premortem cannulation.” With “super-rapid” method, the main principle is to perform the laparotomy and cannulate the distal aorta in less than 4 min [91]. Following cannulation, the supraceliac aorta should be cross-clamped and the intrapericardial inferior vena cava should be incised. With “premortem cannulation,” the cannulas are inserted on the femoral vessels (artery and vein) before withdrawn of support on the donor. Immediately after death is declared, perfusion is started, and the explantation surgery starts. This approach decreases warm ischemia time [89].
The main surgical procedure for kidney harvest is the same in DBD and DCD and was already described by our group [92]. It starts with a midline incision from the xiphoid process to the symphysis pubis. In high BMI donors, a cruciform prolongation or chest incision might be needed [93]. The round and the falciform hepatic ligaments are sectioned up to the diaphragm. To get access to the retroperitoneum, we have to perform a Cattell Braasch maneuver. For that, an incision is made in the white line of Toldt, starting in the right iliac artery, laterally to the ascending colon, up to the hepatoduodenal ligament. An incision is performed on the peritoneum at the right side of the duodenum, as well as in the inferior border of the foramen ovale. The head of the pancreas and the duodenum are mobilized. An incision is performed on the mesenteric root to free the duodenum. The left white line of Toldt is cut, and the freed bowel is covered with gauze and is held outside of the upper part of the abdomen.
After this exposition, the aorta and the inferior vena cava (IVC) are dissected immediately above the bifurcation. It is essential to identify an accessorial lower pole renal artery originating from the iliac artery, which happens in 1–3% of individuals. In that case, cannulating the ipsilateral iliac has to be considered, instead of the aorta. The inferior mesenteric artery is ligated and cut, and a thick silk thread is passed behind the aorta (two threads) and the IVC (one thread).
If only the kidneys are being harvested, the superior mesenteric artery (SMA) must be ligated and cut, and the aorta above the superior mesenteric artery is encircled. Another thread is passed behind the IVC above the renal veins. If an abdominal multiorgan harvest is performed, the supraceliac aorta must be controlled instead. To access this aortic segment, the left liver lobe must be freed. After that, the lesser omentum is inspected to check the presence of a left accessory hepatic artery (occurs in approximately 15% of individuals) and is cut next to gastric smaller curve. The diaphragm crus is exposed and should be divided, and the supraceliac aorta is encircled.
In order to get the best results, it is of paramount importance to administer 25,000–30,000 U (or 300–500 U/kg) of non-fractioned heparin at least 3 min before cannulation. To cannulate, the two inferior threads (on the aorta and IVC) are tied, the perfusion cannula is placed on the aorta, and the second inferior aortic thread is tied to fix it in place. If the portal perfusion is considered necessary, another cannula can be placed on the inferior mesenteric vein.
To start perfusion on a kidney-only harvest, all the threads are tied, and an incision on the IVC next to the inferior ligature is made. In an abdominal multiorgan harvest, the diaphragm and the pericardium are opened, and the intrathoracic IVC is identified, the thread above the supraceliac aorta is tied, and the intrathoracic IVC is cut. Quickly after starting perfusion, the abdomen is filled with ice. Perfusion is most frequently made with Celsior (40–60 mL/kg) or UW (75–100 mL/kg).
After perfusion, to remove the kidneys, the inferior ligatures are cut. The left renal vein is sectioned at its entrance on the IVC. On the right side, the IVC must be divided above and below the right renal vein to perform elongation plasty on the bench. The anterior wall of the aorta is opened longitudinally until the SMA origin. After inspection of the aorta and identification of the renal arteries’ origin, the Carrel patch is cut. Each kidney is mobilized with perirenal and pararenal fat, and the ureter is sectioned near the bladder. In the end, the organs are inspected and perfused on the bench.
Organ Preservation Methods Optimization
After surgical harvest, a crucial stage starts in transplantation: the organ preservation. The first and still most used strategy used for organ preservation is cold storage (CS) after perfusion with preservative liquid. Different perfusion solutions available were designed to maintain cellular integrity during CS [94]. The most commonly used solution is the University of Wisconsin (UW) because it is compatible with different organs preservation, has buffers to keep the pH close to neutrality, and presents a high concentration of impermeable molecules that prevent cellular edema [94]. Alongside the UW, the other commonly used solutions are histidine–tryptophan–ketoglutarate (HTK), Eurocollins, and Celsior. Two retrospective studies comparing UW and HTK showed that HTK increases the risk of primary non-functioning (PNF) kidneys [95] and decreases graft survival after the first 12 months [96]. Regarding DGF, one of the previous retrospective studies [96] and a meta-analysis [97] (citing two RCTs) showed no difference between these two fluids. However, another retrospective study comparing UW and HTK showed a higher DGF in deceased donors’ kidneys preserved with HTK, but, on the other hand, the DGF risk was higher with UW-treated grafts in living donor kidneys [98]. Comparing UW with the Eurocollins solution, the same meta-analysis [97] cites two RCTs where the Eurocollins solution had a higher risk of DGF. Comparing UW with Celsior, there are two retrospective studies [99, 100] and one review [101] showing similar results in transplanted kidneys preserved with either solution. However, small details such as a UW higher fluid viscosity have been pointed out as an essential property to attend to at the time of choosing the preservation solution to use, as organ perfusion time increases [102].
Hypothermic machine perfusion (HMP) was the next step in organ conservation. These machines improve the condition of the organs, particularly the kidneys, by various mechanisms. A study in an animal model [103] showed that one of the physiological mechanisms that the HMP helps to preserve is endothelial nitric oxide (NO) production. This improvement translates into better and earlier reperfusion of the kidney. Other physiological mechanisms proposed are ATP production preservation and organ immunogenicity modulation [104].
Regarding the experience of using the pulsed machine, the initial study by Moers [105] showed lower DGF and more prolonged graft survival by the end of the first and the third years, particularly in ECD [106], although other early studies have not seen this advantage [107]. Experimental animal studies [103, 108], clinical human studies in donation after cardiac death (DCD) [109], ECD [110], and meta-analysis [111,112,113] were unanimous in showing lower DGF and less PNF kidneys with HMP preservation, although long-term results of its usage are still unknown [114].
More recently, there have been advances in the composition of the preservation fluids and organ preservation temperature. Conservation in hypothermia has increasingly been questioned, as hypothermia can aggravate IRI [115, 116]. There are several proposed and proved mechanisms for hypothermic preservation–induced organ damage. One of those mechanisms is protein conformational alteration, as hypothermia reduces protein hydrogen bond length, leading to altered conformation and function [115]. Another proven mechanism in an animal model is endothelial injury that leads to the expression of several adhesion molecules, which will lead to increased inflammation within the graft [117]. Reduced ATP production, redox imbalance, and increased intracellular calcium levels were other proven mechanisms in experimental models [118]. Hypoxia has also been questioned as it has harmful effects on cell function, namely in protein folding and in cytoskeleton elements [119, 120]. Different studies are underway in the field of organ preservation based on the physiological mechanisms associated with animal hibernation [121]. During hibernation, the metabolic rate and oxygen consumption drop more than body temperature, hinting that the use of these pathways may in the future offer hope in organ preservation optimization.
Regarding oxygenated preservation techniques of the kidney, there are different methods to achieve oxygenation: retrograde persufflation, hyperbaric oxygenation, hypothermic perfusion, artificial oxygen carriers, and oxygenation at normothermic temperatures [122]. Initial studies in animal models have shown different results [123, 124]. The various human clinical studies carried out to date have not yet led to conclusions about the usefulness of these techniques [125].
Experimental studies in animal models comparing kidneys preserved only in an HMP with preservation in an HMP followed by controlled heating with oxygenated liquid showed better mitochondrial recovery, with less activation of apoptotic pathways and better graft function after transplantation [126]. In other studies, this controlled heating has also been shown to lead to less parenchymal, tubular, and endothelial damage, better mitochondrial function in renal cells, and better kidney graft function [116, 127], and may even promote graft regeneration [128]. Early human studies have shown that preservation with normothermic machine perfusion (NMP) reduces DGF, albeit not improving graft survival at 12 months [129]. More recently, human clinical studies have shown that the use of the NMP has allowed the use of kidney grafts that would otherwise be considered not viable through better evaluation of previously discarded kidney for transplantation [130, 131].
Conclusion
This review summarized the evidence available on organ optimization in the cadaveric setting. Organ shortage and the subsequent increased use of suboptimal organs call upon the need for better strategies in donor management and organ preservation. As previously stated, optimal donor care and meeting DMG increase the OTPD. To do that, it is essential to correct imbalances, mainly endocrine and hemodynamic. Preconditioning is a promising area but is currently making its first steps in the clinical setting. Surgical techniques were established in the late twentieth century, and more innovations are needed. Finally, preservation has evolved since CS, but the best conditions to do so are still to be determined.
References
Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–30.
NHS Blood and Transplant. Annual Report on Kidney Transplantation 2017/18, https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/12256/nhsbt-kidney-transplantation-annual-report-2017-2018.pdf (2018).
Summers DM, Watson CJE, Pettigrew GJ, Johnson RJ, Collett D, Neuberger JM, et al. Kidney donation after circulatory death (DCD): state of the art. Kidney Int. 2015;88:241–9.
Wood KE, Becker BN, McCartney JG, et al. Care of the potential organ donor. N Engl J Med. 2004;351:2730–9.
Anderson TA, Bekker P, Vagefi PA. Anesthetic considerations in organ procurement surgery: a narrative review. Can J Anesth Can d’anesthésie. 2015;62:529–39.
van Der Hoeven JAB, Molema G, Ter Horst GJ, et al. Relationship between duration of brain death and hemodynamic (in)stability on progressive dysfunction and increased immunologic activation of donor kidneys. Kidney Int. 2003;64:1874–82.
Malinoski DJ, Patel MS, Ahmed O, Daly MC, Mooney S, Graybill CO, et al. The impact of meeting donor management goals on the development of delayed graft function in kidney transplant recipients. Am J Transplant. 2013;13:993–1000.
Gottmann U, Brinkkoetter PT, Bechtler M, Hoeger S, Karle C, Schaub M, et al. Effect of pre-treatment with catecholamines on cold preservation and ischemia/reperfusion-injury in rats. Kidney Int. 2006;70:321–8.
Schaub M, Ploetz CJ, Gerbaulet D, Fang L, Kranich P, Stadlbauer TH, et al. Effect of dopamine on inflammatory status in kidneys of brain-dead rats. Transplantation. 2004;77:1333–40.
Liu Z, Hoeger S, Schnuelle P, Feng Y, Goettmann U, Waldherr R, et al. Donor dopamine pretreatment inhibits tubulitis in renal allografts subjected to prolonged cold preservation. Transplantation. 2007;83:297–303.
Schnuelle P, Lorenz D, Mueller A, et al. Donor catecholamine use reduces acute allograft rejection and improves graft survival after cadaveric renal transplantation1. Kidney Int. 1999;56:738–46. 1 by Lu, p. 756.
Schnuelle P, Berger S, de Boer J, et al. Effects of catecholamine application to brain-dead donors on graft survival in solid organ transplantation. Transplantation. 2001;72:455–63.
Schnuelle P, Schmitt WH, Weiss C, Habicht A, Renders L, Zeier M, et al. Effects of dopamine donor pretreatment on graft survival after kidney transplantation: a randomized trial. Clin J Am Soc Nephrol. 2017;12:493–501.
Schnuelle P, Mundt HM, Drüschler F, Schmitt WH, Yard BA, Krämer BK, et al. Impact of spontaneous donor hypothermia on graft outcomes after kidney transplantation. Am J Transplant. 2018;18:704–14.
Salim A, Velmahos GC, Brown C, Belzberg H, Demetriades D. Aggressive organ donor management significantly increases the number of organs available for transplantation. J Trauma. 2005;58:991–4.
Al-Khafaji A, Elder M, Lebovitz DJ, et al. Protocolized fluid therapy in brain-dead donors: the multicenter randomized MOnIToR trial. Intensive Care Med. 2015;41:418–26.
Limnell N, Schramko AA. Is brain-dead donor fluid therapy with colloids associated with better kidney grafts? Exp Clin Transplant. 2018;16:55–60.
Cittanova ML, Leblanc I, Legendre C, Mouquet C, Riou B, Coriat P. Effect of hydroxyethylstarch in brain-dead kidney donors on renal function in kidney-transplant recipients. Lancet. 1996;348:1620–2.
Blasco V, Colavolpe JC, Antonini F, et al. Long-term outcome in kidney recipients from donors treated with hydroxyethylstarch 130/0.4 and hydroxyethylstarch 200/0.6. Br J Anaesth. 2015;115:798.
Patel MS, Niemann CU, Sally MB, de la Cruz S, Zatarain J, Ewing T, et al. The impact of hydroxyethyl starch use in deceased organ donors on the development of delayed graft function in kidney transplant recipients: a propensity-adjusted analysis. Am J Transplant. 2015;15:2152–8.
Novitzky D, Cooper DKC, Rosendale JD, Kauffman HM. Hormonal therapy of the brain-dead organ donor: experimental and clinical studies. Transplantation. 2006;82:1396–401.
Buchanan IA, Mehta VA. Thyroid hormone resuscitation after brain death in potential organ donors: a primer for neurocritical care providers and narrative review of the literature. Clin Neurol Neurosurg. 2018;165:96–102.
Novitzky D, Mi Z, Sun Q, Collins JF, Cooper DK. Thyroid hormone therapy in the management of 63,593 brain-dead organ donors. Transplantation. 2014;98:1119–27.
Novitzky D, Cooper DK, Reichart B. The value of hormonal therapy in improving organ viability in the transplant donor. Transplant Proc. 1987;19:2037–8.
Venkateswaran RV, Steeds RP, Quinn DW, Nightingale P, Wilson IC, Mascaro JG, et al. The haemodynamic effects of adjunctive hormone therapy in potential heart donors: a prospective randomized double-blind factorially designed controlled trial. Eur Heart J. 2009;30:1771–80.
Macdonald PS, Aneman A, Bhonagiri D, Jones D, O'Callaghan G, Silvester W, et al. A systematic review and meta-analysis of clinical trials of thyroid hormone administration to brain dead potential organ donors. Crit Care Med. 2012;40:1635–44.
Benck U, Gottmann U, Hoeger S, et al. Donor desmopressin is associated with superior graft survival after kidney transplantation. Transplantation. 2011;92:1252–8.
Guesde R, Barrou B, Leblanc I, Ourahma S, Goarin JP, Coriat P, et al. Administration of desmopressin in brain-dead donors and renal function in kidney recipients. Lancet. 1998;352:1178–81.
Sally MB, Ewing T, Crutchfield M, et al. Determining optimal threshold for glucose control in organ donors after neurologic determination of death. J Trauma Acute Care Surg. 2014;76:62–9.
Nicolas-Robin A, Barouk JD, Darnal E, Riou B, Langeron O. Free cortisol and accuracy of total cortisol measurements in the diagnosis of adrenal insufficiency in brain-dead patients. Anesthesiology. 2011;115:568–74.
Barklin A. Systemic inflammation in the brain-dead organ donor. Acta Anaesthesiol Scand. 2009;53:425–35.
Kainz A, Wilflingseder J, Mitterbauer C, et al. Steroid pretreatment of organ donors to prevent Postischemic renal allograft failure. Ann Intern Med. 2010;153:222.
Reindl-Schwaighofer R, Kainz A, Jelencsics K, et al. Steroid pretreatment of organ donors does not impact on early rejection and long-term kidney allograft survival: results from a multicenter randomized, controlled trial. Am J Transplant. 2019;19:1770–6.
Dupuis S, Amiel J-A, Desgroseilliers M, et al. Corticosteroids in the management of brain-dead potential organ donors: a systematic review. Br J Anaesth. 2014;113:346–59.
D’Aragon F, Belley-Cote E, Agarwal A, et al. Effect of corticosteroid administration on neurologically deceased organ donors and transplant recipients: a systematic review and meta-analysis. BMJ Open. 2017;7:e014436.
Niemann CU, Feiner J, Swain S, Bunting S, Friedman M, Crutchfield M, et al. Therapeutic hypothermia in deceased organ donors and kidney-graft function. N Engl J Med. 2015;373:405–14.
Schnuelle P, Benck U, Krämer BK, et al. Impact of donor core body temperature on graft survival after heart transplantation. Transplantation. 2018;102:1891–900.
Cardinal H, Lamarche F, Grondin S, Marsolais P, Lagacé AM, Duca A, et al. Organ donor management and delayed graft function in kidney transplant recipients: a multicenter retrospective cohort study. Am J Transplant. 2019;19:277–84.
Patel MS, Zatarain J, De La Cruz S, et al. The impact of meeting donor management goals on the number of organs transplanted per expanded criteria donor. JAMA Surg. 2014;149:969.
Patel MS, De La Cruz S, Sally MB, et al. Active donor management during the hospital phase of care is associated with more organs transplanted per donor. J Am Coll Surg. 2017;225:525–31.
Hoeger S, Benck U, Petrov K, et al. Atorvastatin donor pre-treatment in a model of brain death and allogeneic kidney transplantation in rat. Ann Transplant. 17:79–85.
Tuuminen R, Nykänen AI, Saharinen P, Gautam P, Keränen MA, Arnaudova R, et al. Donor simvastatin treatment prevents ischemia–reperfusion and acute kidney injury by preserving microvascular barrier function. Am J Transplant. 2013;13:2019–34.
Orban J-C, Fontaine E, Cassuto E, et al. Effects of cyclosporine A pretreatment of deceased organ donors on kidney graft function (Cis-A-rein): study protocol for a randomized controlled trial. Trials. 2018;19:231.
Orban J-C, Quintard H, Cassuto E, et al. Effect of N-acetylcysteine pretreatment of deceased organ donors on renal allograft function. Transplantation. 2015;99:746–53.
de la Cruz JS, Sally MB, Zatarain JR, et al. The impact of blood transfusions in deceased organ donors on the outcomes of 1,884 renal grafts from United Network for Organ Sharing Region 5. J Trauma Acute Care Surg. 2015;79:S164–70.
Chen C-C, Chapman WC, Hanto DW. Ischemia–reperfusion injury in kidney transplantation. Front Biosci (Elite Ed). 2015;7:117–34.
Kosieradzki M, Rowiński W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008;40:3279–88.
Huang Y, Shan J, Wang C, Ma J, Li D, Li L, et al. Can ischemic preconditioning alone really protect organs from ischemia reperfusion injury in transplantation. Transpl Immunol. 2009;20:127–31.
Kitamura M. Endoplasmic reticulum stress and unfolded protein response in renal pathophysiology: Janus faces. Am J Physiol Physiol. 2008;295:F323–34.
Mitchell T, Saba H, Laakman J, Parajuli N, MacMillan-Crow L. Role of mitochondrial-derived oxidants in renal tubular cell cold-storage injury. Free Radic Biol Med. 2010;49:1273–82.
Woo Yang C, Jong Ahn H, Young Jung J, et al. Preconditioning with cyclosporine A or fk506 differentially regulates mitogen-activated protein kinase expression in rat kidneys with ischemia/reperfusion injury. Transplantation. 2003;75:20–4.
Yin T, Sandhu G, Wolfgang CD, Burrier A, Webb RL, Rigel DF, et al. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. J Biol Chem. 1997;272:19943–50.
Hussein AE-AM, Shokeir AA, Sarhan ME, et al. Effects of combined erythropoietin and epidermal growth factor on renal ischaemia/reperfusion injury: a randomized experimental controlled study. BJU Int. 2011;107:323–8.
Araki M, Fahmy N, Zhou L, Kumon H, Krishnamurthi V, Goldfarb D, et al. Expression of IL-8 during reperfusion of renal allografts is dependent on ischemic time. Transplantation. 2006;81:783–8.
Chatauret N, Thuillier R, Hauet T. Preservation strategies to reduce ischemic injury in kidney transplantation: pharmacological and genetic approaches. Curr Opin Organ Transplant. 2011;16:180–7.
Ambros JT, Herrero-Fresneda I, Borau OG, Boira JM. Ischemic preconditioning in solid organ transplantation: from experimental to clinics. Transpl Int. 2007;20:219–29.
Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36.
Torras J, Herrero-Fresneda I, Lloberas N, Riera M, Ma Cruzado J, Ma Grinyó J. Promising effects of ischemic preconditioning in renal transplantation. Kidney Int. 2002;61:2218–27.
Tsai BM, Wang M, March KL, Turrentine MW, Brown JW, Meldrum DR. Preconditioning: evolution of basic mechanisms to potential therapeutic strategies. Shock. 2004;21:195–209.
Selzner N, Boehnert M, Selzner M. Preconditioning, postconditioning, and remote conditioning in solid organ transplantation: basic mechanisms and translational applications. Transplant Rev. 2012;26:115–24.
Kosieradzki M, Ametani M, Southard JH, Mangino MJ. Is ischemic preconditioning of the kidney clinically relevant? Surgery. 2003;133:81–90.
Behrends M, Walz MK, Kribben A, et al. No protection of the porcine kidney by ischaemic preconditioning. Exp Physiol. 2000;85:819–27.
Yamasowa H, Shimizu S, Inoue T, Takaoka M, Matsumura Y. Endothelial nitric oxide contributes to the renal protective effects of ischemic preconditioning. J Pharmacol Exp Ther. 2005;312:153–9.
Park KM, Byun J-Y, Kramers C, Kim JI, Huang PL, Bonventre JV. Inducible nitric-oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J Biol Chem. 2003;278:27256–66.
Cao C, Wang S, Fan L, Wan X, Liu X, Chen X. Renal protection by ischemic preconditioning is associated with p50/p50 homodimers. Am J Nephrol. 2010;31:1–8.
Hill P, Shukla D, Tran MGB, Aragones J, Cook HT, Carmeliet P, et al. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia–reperfusion injury. J Am Soc Nephrol. 2008;19:39–46.
van der Woude FJ, Schnuelle P, Yard BA. Preconditioning strategies to limit graft immunogenicity and cold ischemic organ injury. J Investig Med. 2004;52:323–9.
Wever KE, Menting TP, Rovers M, et al. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS One. 2012;7:e32296.
Baker JE. Erythropoietin mimics ischemic preconditioning. Vasc Pharmacol. 2005;42:233–41.
Yang CW, Li C, Jung JY, Shin SJ, Choi BS, Lim SW, et al. Preconditioning with erythropoietin protects against subsequent ischemia–reperfusion injury in rat kidney. FASEB J. 2003;17:1754–5.
Ates E, Yalcin AU, Yilmaz S, et al. Protective effect of erythropoietin on renal ischemia and reperfusion injury. ANZ J Surg. 2005;75:1100–5.
Ishii Y, Sawada T, Murakami T, et al. Renoprotective effect of erythropoietin against ischaemia–reperfusion injury in a non-human primate model. Nephrol Dial Transplant. 2011;26:1157–62.
Moriyama MT, Tanaka T, Morita N, Ishii T, Chikazawa I, Suga K, et al. Renal protective effects of erythropoietin on ischemic reperfusion injury. Cell Transplant. 2010;19:713–21.
Nakao A, Faleo G, Shimizu H, et al. Ex vivo carbon monoxide prevents cytochrome P450 degradation and ischemia/reperfusion injury of kidney grafts. Kidney Int. 2008;74:1009–16.
Yoshida J, Ozaki KS, Nalesnik MA, Ueki S, Castillo-Rama M, Faleo G, et al. Ex vivo application of carbon monoxide in UW solution prevents transplant-induced renal ischemia/reperfusion injury in pigs. Am J Transplant. 2010;10:763–72.
Ozaki KS, Kimura S, Murase N. Use of carbon monoxide in minimizing ischemia/reperfusion injury in transplantation. Transplant Rev. 2012;26:125–39.
García-Cenador B, Blanco-Gozalo V, López-Montañés D, Sanz Giménez-Rico JR, López-Novoa JM, López-Hernández FJ. Cardiotrophin-1 improves kidney preservation, graft function, and survival in transplanted rats. Transplantation. 2018;102:e404–12.
Lobb I, Davison M, Carter D, et al. Hydrogen sulfide treatment mitigates renal allograft ischemia–reperfusion injury during cold storage and improves early transplant kidney function and survival following allogeneic renal transplantation. J Urol. 2015;194:1806–15.
Kim YO, Li C, Sun BK, Kim JS, Lim SW, Choi BS, et al. Preconditioning with 1,25-Dihydroxyvitamin D3 protects against subsequent ischemia–reperfusion injury in the rat kidney. Nephron Exp Nephrol. 2005;100:e85–94.
Kaizu T, Tamaki T, Tanaka M, Uchida Y, Tsuchihashi S, Kawamura A, et al. Preconditioning with tin-protoporphyrin IX attenuates ischemia/reperfusion injury in the rat kidney. Kidney Int. 2003;63:1393–403.
Herrero I, Torras J, Riera M, et al. Prevention of cold ischaemia–reperfusion injury by an endothelin receptor antagonist in experimental renal transplantation. Nephrol Dial Transplant. 1999;14:872–80.
Barber E, Menéndez S, León OS, Barber MO, Merino N, Calunga JL, et al. Prevention of renal injury after induction of ozone tolerance in rats submitted to warm ischaemia. Mediat Inflamm. 1999;8:37–41.
Lledó-García E, Subirá-Ríos D, Rodríguez–Martínez D, et al. Sildenafil as a protecting drug for warm ischemic kidney transplants: experimental results. J Urol. 2009;182:1222–5.
Starzl TE, Miller C, Broznick B, et al. An improved technique for multiple organ harvesting. Surg Gynecol Obstet. 1987;165:343–8.
Rosenthal JT, Shaw BW Jr, et al. Principles of multiple organ procurement from cadaver donors. Ann Surg. 1983;198:617.
Starzl TE, Hakala TR, Shaw BW, et al. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223–30.
Imagawa DK, Olthoff KM, Yersiz H, et al. Rapid en bloc technique for pancreas–liver procurement: improved early liver function. Transplantation. 1996;61:1605–9.
Wunderlich H, Brockmann JG, Voigt R, Rauchfuss F, Pascher A, Brose S, et al. DTG procurement guidelines in heart beating donors. Transpl Int. 2011;24:733–57.
Reich DJ, Mulligan DC, Abt PL, Pruett TL, Abecassis MM, D'Alessandro A, et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9:2004–11.
Belli LS, De Carlis L, Romani F, et al. Kidney and liver initial graft function under different procurement techniques. Transplant Proc. 1989;21:1209–10.
Sánchez-Hidalgo JM, Rodríguez-Ortiz L, Arjona-Sánchez Á, Ayllón-Terán MD, Gómez-Luque I, Ciria-Bru R, et al. “Super-rapid” technique in donation after circulatory death liver donors: advantages and disadvantages. Transplant Proc. 2019;51:25–7.
Marconi L, Figueiredo A. Cadaver donor organ procurement: technical aspects and surgical options. In: Figueiredo A, Lledó-García E, editors. European textbook on kidney transplantation: ESTU-EAU; 2017. p. 303–3018.
Yilmaz M, Piskin T, Akbulut S, Ersan V, Gonultas F, Yilmaz S. Is routine sternotomy necessary for organ recovery from deceased donors? A comparative retrospective study. Transplant Proc. 2012;44:1644–7.
Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235–47.
Stevens RB, Skorupa JY, Rigley TH, Yannam GR, Nielsen KJ, Schriner ME, et al. Increased primary non-function in transplanted deceased-donor kidneys flushed with histidine–tryptophan–ketoglutarate solution. Am J Transplant. 2009;9:1055–62.
Stewart ZA, Lonze BE, Warren DS, et al. Histidine–tryptophan–ketoglutarate (HTK) is associated with reduced graft survival of deceased donor kidney transplants. Am J Transplant. 2009;9:1048–54.
O’Callaghan JM, Knight SR, Morgan RD, et al. Preservation solutions for static cold storage of kidney allografts: a systematic review and meta-analysis. Am J Transplant. 2012;12:896–906.
Lynch RJ, Kubus J, Chenault RH, Pelletier SJ, Campbell DA, Englesbe MJ. Comparison of histidine–tryptophan–ketoglutarate and University of Wisconsin preservation in renal transplantation. Am J Transplant. 2008;8:567–73.
Nunes P, Mota A, Figueiredo A, Macário F, Rolo F, Dias V, et al. Efficacy of renal preservation: comparative study of Celsior and University of Wisconsin solutions. Transplant Proc. 2007;39:2478–9.
Tillou X, Collon S, Surga N, Jaureguy M, Viart L, Mazouz H, et al. Comparison of UW and Celsior: long-term results in kidney transplantation. Ann Transplant. 2013;18:146–52.
Dikdan GS, Mora-Esteves C, Koneru B. Review of randomized clinical trials of donor management and organ preservation in deceased donors. Transp J. 2012;94:425–41.
Mühlbacher F, Langer F, Mittermayer C. Preservation solutions for transplantation. Transplant Proc. 1999;31:2069–70.
Chatauret N, Coudroy R, Delpech PO, Vandebrouck C, Hosni S, Scepi M, et al. Mechanistic analysis of nonoxygenated hypothermic machine perfusion’s protection on warm ischemic kidney uncovers greater eNOS phosphorylation and vasodilation. Am J Transplant. 2014;14:2500–14.
Jochmans I, Nicholson ML, Hosgood SA. Kidney perfusion. Curr Opin Organ Transplant. 2017;22:260–6.
Moers C, Smits JM, Maathuis M-HJ, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7–19.
Moers C, Pirenne J, Paul A, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2012;366:770–1.
Watson CJE, Wells AC, Roberts RJ, et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am J Transplant. 2010;10:1991–9.
Hosgood SA, Yang B, Bagul A, et al. A comparison of hypothermic machine perfusion versus static cold storage in an experimental model of renal ischemia reperfusion injury. Transplantation. 2010;89:830–7.
Jochmans I, Moers C, Smits JM, et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death. Ann Surg. 2010;252:756–64.
Gallinat A, Moers C, Treckmann J, et al. Machine perfusion versus cold storage for the preservation of kidneys from donors ≥65 years allocated in the Eurotransplant Senior Programme. Nephrol Dial Transplant. 2012;27:4458–63.
Hameed AM, Pleass HC, Wong G, et al. Maximizing kidneys for transplantation using machine perfusion. Medicine (Baltimore). 2016;95:e5083.
O’Callaghan JM, Morgan RD, Knight SR, et al. Systematic review and meta-analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant outcomes. Br J Surg. 2013;100:991–1001.
Leite RR d A, Schanaider A, Da-Fonseca ER, et al. Máquina de perfusão versus armazenamento estático na preservação renal de doadores com morte encefálica: revisão sistemática e metanálise. Rev Col Bras Cir. 2019;46:e2079.
Martínez Arcos L, Fabuel Alcañiz JJ, Gómez Dos Santos V, Burgos Revilla FJ. Functional results of renal preservation in hypothermic pulsatile machine perfusion versus cold preservation: systematic review and meta-analysis of clinical trials. Transplant Proc. 2018;50:24–32.
Thuillier R, Delpy E, Matillon X, Kaminski J, Kasil A, Soussi D, et al. Preventing acute kidney injury during transplantation: the application of novel oxygen carriers. Expert Opin Investig Drugs. 2019;28:643–57.
Minor T, VonHorn C. Rewarming injury after cold preservation. Int J Mol Sci:20. Epub ahead of print 2019. https://doi.org/10.3390/ijms20092059.
Dragun D, Hoff U, Park J-K, et al. Prolonged cold preservation augments vascular injury independent of renal transplant immunogenicity and function. Kidney Int. 2001;60:1173–81.
Brinkkoetter P-T, Song H, Lösel R, et al. Hypothermic injury: the mitochondrial calcium, ATP and ROS love–hate triangle out of balance. Cell Physiol Biochem. 2008;22:195–204.
Thuillier R, Hauet T. Impact of hypothermia and oxygen deprivation on the cytoskeleton in organ preservation models. Biomed Res Int. 2018;2018:8926724.
Le Pape S, Pasini-Chabot O, Couturier P, et al. Decoding cold ischaemia time impact on kidney graft: the kinetics of the unfolded protein response pathways. Artif Cells, Nanomedicine, Biotechnol. 2018;46:S873–85.
Ratigan ED, McKay DB. Exploring principles of hibernation for organ preservation. Transplant Rev. 2016;30:13–9.
Hosgood SA, Nicholson HFL, Nicholson ML. Oxygenated kidney preservation techniques. Transplantation. 2012;93:455–9.
Treckmann J, Nagelschmidt M, Minor T, Saner F, Saad S, Paul A. Function and quality of kidneys after cold storage, machine perfusion, or retrograde oxygen persufflation: results from a porcine autotransplantation model. Cryobiology. 2009;59:19–23.
Gallinat A, Paul A, Efferz P, et al. Role of oxygenation in hypothermic machine perfusion of kidneys from heart beating donors. Transplantation. 2012;94:809–13.
O’Callaghan JM, Pall KT, Pengel LHM, et al. Supplemental oxygen during hypothermic kidney preservation: a systematic review. Transplant Rev. 2017;31:172–9.
Schopp I, Reissberg E, Lüer B, et al. Controlled rewarming after hypothermia: adding a new principle to renal preservation. Clin Transl Sci. 2015;8:475–8.
Mahboub P, Ottens P, Seelen M, et al. Gradual rewarming with gradual increase in pressure during machine perfusion after cold static preservation reduces kidney ischemia reperfusion injury. PLoS One. 2015;10:e0143859.
Hamar M, Urbanellis P, Kaths MJ, et al. Normothermic ex vivo kidney perfusion reduces warm ischemic injury of porcine kidney grafts retrieved after circulatory death. Transplantation. 102. Epub ahead of print 2018. https://doi.org/10.1097/TP.0000000000002245.
Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant. 2013;13:1246–52.
Hosgood SA, Saeb-Parsy K, Hamed MO, Nicholson ML. Successful transplantation of human kidneys deemed untransplantable but resuscitated by ex vivo normothermic machine perfusion. Am J Transplant. 2016;16:3282–5.
Hosgood SA, Thompson E, Moore T, et al. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br J Surg. 105. Epub ahead of print 2018. https://doi.org/10.1002/bjs.10733.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Edgar Tavares da Silva and Arnaldo Figueiredo each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of Topical Collection on Kidney Diseases
Rights and permissions
About this article
Cite this article
Tavares-da-Silva, E., Figueiredo, A. Renal Procurement: Techniques for Optimizing the Quality of the Graft in the Cadaveric Setting. Curr Urol Rep 21, 12 (2020). https://doi.org/10.1007/s11934-020-0963-8
Published:
DOI: https://doi.org/10.1007/s11934-020-0963-8