Abstract
Purpose of Review
In many countries, the average age of paternity is rising. The negative effect of older age on fertility in women is well documented; however, less is known about the impact of paternal age on fecundity. In this review, we summarize the current knowledge of how paternal age affects semen parameters, reproductive success, and offspring health.
Recent Findings
Contemporary evidence confirms that aged men have worse semen parameters, including overall negative changes in sperm genetics. Reproductive outcomes with unassisted pregnancy tend to be worse with older fathers. While most current studies of assisted pregnancy do show a negative effect of paternal age, there are some conflicting results. Studies continue to show an overall increased risk of health problems, particularly neuropsychiatric conditions, in the offspring of older men.
Summary
While men can often maintain fertility potential throughout a lifetime, increasing evidence indicates worsening of semen parameters, including sperm genetics, and potentially worse reproductive success. Older men should also be counseled on their offspring’s possible increased risk of certain medical conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
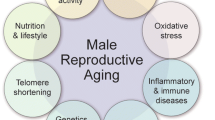
The negative effects of advanced maternal age on reproductive outcomes, maternal health, and fetal health are well-known [1]. Menopause is a biologic event which directly limits the time frame within which a woman can conceive naturally, the colloquially named “biologic clock.” However, it has long been questioned whether such a “biologic clock” exists for males. It is acknowledged that some men develop decreased libido or erectile function with age; however, until more recently, it was not as clear how age may otherwise impact a man’s fecundity. It has been known since antiquity that men can sire children well into old age. The oldest known man to have documented paternity in the medical literature is of a North Carolina man who sired a pregnancy at the age of 94 to a 27-year-old woman [2]. One major problem with assessing the impact of age on paternal fertility is the lack of a consensus on what should be defined as advanced paternal age (APA). The most commonly used criterion to define APA is age > 40 years at time of conception [3].
This issue of APA is becoming increasingly important given that over the past several decades, the average age of paternity has been on the rise in Western countries in parallel to the rising age of maternity. In the USA, the mean paternal age has increased by 3.5 years from 27.4 to 30.9 years in the past four decades. The number of newborns born to fathers > 40 years increased from 4.1 to 8.9% and from 0.5 to 0.9% for fathers > 50 years between 1972 and 2015 [4••]. A similar increase was noted for men in England and Wales with the mean age of fatherhood increasing from 29.2 to 32.1 years between 1980 and 2002 [5]. While the reasons for this increase are likely multifactorial, socioeconomic factors such as changes in parental education level, career and educational goals, acceptance and use of contraception, life expectancy, and financial considerations are all possibly involved. Additionally, the increasing use and acceptance of assisted reproductive technologies (ARTs) have granted more couples the ability to achieve pregnancy despite certain barriers to natural conception, such as increasing age. Either way, this demographic change is important to both clinicians and patients given the increasing data to support the negative impact of APA on paternal fecundity and offspring health.
Urologic Diseases of the Aging Male and Their Impact on Fertility
As men age, the increasing prevalence of some comorbid conditions, particularly urological, can negatively impact a man’s fertility potential. The association between advancing age and erectile dysfunction (ED) is well-known [6]. The barrier of ED to conception is obvious, and the frequency of marital coitus has been shown to decrease in correlation to the husband’s age and the duration of the marriage [7]. While there is a lack of data on the impact of ED on semen parameters, men with ED may suffer from increased abstinence intervals. Longer abstinence periods have been shown to increase the level of sperm DNA fragmentation; however, it also increases the sperm concentration and total sperm count [8].
Hypogonadism is a potential contributing risk factor to both ED and decreased sexual desire as men age. It is well-known that both total and free testosterone levels decrease with age [9,10,11]. Testosterone replacement therapy improves both libido and erectile function in middle and older aged hypogonadal men [12, 13, 14••]. The prevalence of testosterone therapy is on the rise; the use of testosterone in the USA increased in men > 40 years of age by over threefold between 2001 and 2011 from 0.81 to 2.91% [15].
While testosterone therapy may be beneficial for ED and libido in the hypogonadal man, exogenous testosterone use has well-known detrimental side effects on sperm production through suppression of the hypothalamic-pituitary axis. Unfortunately, in a survey conducted in 2010, 25% of urologists who responded reported they would still treat an infertile male with testosterone [16]. Given the rising prevalence of testosterone therapy amongst men, it is important that the clinician be aware of this when evaluating men for fertility and when counseling men on the risks of testosterone therapy. A majority of men will recover some degree of sperm production after cessation of testosterone, but this is not guaranteed and some may not return to their prior baseline [17,18,19]. The use of human chorionic gonadotropin (HCG) has been shown to preserve spermatogenesis while on testosterone therapy and has proven useful for “rebooting” spermatogenesis after testosterone therapy [20, 21••, 22••].
Other urological issues associated with older age may negatively contribute to a man’s fecundity. Ejaculatory dysfunction increases from 3% in men aged 50–54 years to 35% in those aged 70–78 years [23]. Treatments for other conditions associated with the aging male, such as benign prostatic hyperplasia, prostate cancer, and bladder cancer, can all negatively impact a man’s fertility potential as well [24].
These studies serve to remind clinicians that other comorbidities, particularly urological, associated with aging and their respective treatments may contribute to a decreased fertility potential in men.
Effect of Age on Semen Parameters
The cornerstone study to assess a man’s fertility potential remains the semen analysis. While the most commonly used definition of “normal” semen parameters is not without controversy, studies have negatively correlated decreases in semen parameters with reproductive success, some of which may be overcome with ARTs [25]. While individual studies comparing the semen parameters of younger and older men have demonstrated inconsistent findings, possibly due to the inclusion in some studies of men presenting for infertility evaluations, meta-analyses have shown an overall trend towards a decline in semen quality with age [26, 27••].
The most consistent finding amongst studies is the decline in semen volume in the aged male. Kidd et al. performed a meta-analysis in 2001 looking at the impact of aging on semen parameters and demonstrated that when comparing the semen of men in their fourth to their sixth decade of life, semen volume declines 3–22% [26]. There was also a 3–37% decline in sperm motility and a 4–18% decline in normal morphology when comparing these cohorts. A more recent meta-analysis by Johnson et al. showed similar findings, with aging males demonstrating a decrease in semen volume, sperm count, motility, and morphology [27••]. Both studies did not show a decline in sperm concentration, but this is likely based upon the parallel decline in both semen volume and sperm count.
In a study of ICSI outcomes using donor oocytes, Beguería et al. demonstrated a decrease in all semen parameters with increasing paternal age [28]. For every 5 years of age, semen volume decreased by 0.22 mL, concentration increased by 3.1 million sperm/mL, and motility decreased by 1.2%. A similar, but much smaller, study demonstrated only a decline in sperm morphology in men > 50 years compared to younger men undergoing ICSI with donor oocytes [29]. These studies must be interpreted with caution, as they provide limited to no information on these men’s comorbidities, prior pregnancies, or other possible issues related to male factor infertility.
Despite some differences, these studies indicate a general decline in traditional semen parameters with age.
Effect of Age on Sperm Genetics
More recently, studies have started to examine the possibility of age-related changes in sperm genetics, such as sperm DNA damage/fragmentation, sperm aneuploidy, telomere length, and epigenetic changes. Many studies have found that sperm DNA damage increases as males age [27••, 29,30,31]. However, Brahem et al. reported that DNA fragmentation was increased with age only in an infertile cohort and not in a cohort of proven fertility [32]. In a study using donor oocytes, García-Ferreyra et al. demonstrated that men ≥ 50 years of age had significantly higher DNA fragmentation (33.6 ± 18.19%) compared to men aged 40–49 years (24.1 ± 14.49%) and ≤ 39 years (25.6 ± 15.63%) [33•]. While the rates of DNA fragmentation are generally higher in this study than in a similar prior study of donor oocyte cycles from the same author, the overall trend of significantly increased DNA fragmentation in men ≥ 50 years of age is maintained [29].
While the exact mechanisms of this increase in sperm DNA damage with age are not known, reactive oxygen species (ROS) are a known cause of DNA damage and older men have demonstrated increased levels of ROS in their seminal ejaculates [34]. Increased ROS in older men may be related to an increase with age in cumulative environmental pollutant exposure, comorbidities, or varicoceles [35].
Similar to their finding with DNA damage, Brahmen et al. demonstrated that sperm diploidy increased with age only in the infertile patients and not in the fertile patients [32]. Kaarouch et al. showed higher rates of DNA fragmentation and sperm aneuploidy rates with age in men undergoing IVF for male factor infertility [36•]. Other studies have shown no difference in sperm numerical chromosomal abnormalities between older and younger men [37, 38]. Many studies of aneuploidy in APA assess aneuploidy based on embryo biopsy, without analysis of sperm [29, 33•].
Telomeres are repeating sequences of DNA found at the end of chromosomes which serve a protective function over the genetic material during cellular division [39]. It is known that telomeres shorten with age, and people with shorter telomeres have reduced survival [40]. Interestingly, contrary to most tissues in the body and oocytes, it has been demonstrated that telomere length increases in sperm as men age [41]. Indeed, offspring of older men have longer telomeres, and this has been shown to be maintained for at least two generations [42].
More recent studies have focused on the role of possible epigenetic changes in the sperm of older men. It has been demonstrated that there is an increase in global sperm methylation with age [43]. Jenkins et al. examined the impact of aging on sperm DNA methylation and found 139 regions of hypomethylation and 8 regions of hypermethylation in aged sperm associated with 117 genes [44]. APA has also been associated with epigenetic changes in the genome of oocytes. Kawai et al. demonstrated that expression of genes important for autophagy and embryonic growth were negatively associated with paternal age, and they hypothesized that this may be due to epigenetic modifications [45].
These studies all indicate that there are age-related changes in sperm genetics, and most of these are likely detrimental to reproductive success.
Paternal Age and Reproductive Success with Unassisted Conception
Most studies have demonstrated a negative association with paternal age and the reproductive success of unassisted conception. One of the most commonly reported associations is that of increased time to pregnancy with APA. In questionnaires completed by couples in the Avon Longitudinal Study of Pregnancy and Childhood, when controlling for female partner age, the odds ratio for conception in ≤ 12 months was 0.51 in men aged ≥ 40 years compared to men aged < 25 years [46]. A similar, but higher, odds of increased time to pregnancy was noted in a study out of the UK [47].
A study by de la Rochebrochard et al. demonstrated that when the female partner was aged ≤ 34 years, paternal age ≥ 40 years did not have an effect on the odds of conception within 1 year [48]. However, when the female partner was aged 35–39 years, there was a higher odds ratio for failure to conceive within 12 months (adjusted odds ratio of 2.21) when paternal age was ≥ 40 years compared to younger men.
Taken together, these studies demonstrate an overall decline in the reproductive success of aged males with unassisted conception.
Paternal Age and Reproductive Success with Assisted Conception
Assisted conception includes both intrauterine insemination (IUI) and in vitro fertilization (IVF) with or without intracytoplasmic sperm injection (ICSI). In a study of 901 cycles of IUI, paternal age was the most significant predicative factor on multivariate analysis contributing to decreased success of IUI [49]. A more recent study by Belloc et al. examined 17,000 IUI cycles and found that there was a negative effect of paternal age on pregnancy rate in parallel to the effect of maternal age [50].
McPherson et al. examined 4,057 first IVF cycles with and without ICSI and found a combined negative effect of APA with advanced maternal age on live birth rates [51•]. There was an approximately 10% decrease in pregnancy and live birth rates in 35-year-old women when the male partner was > 40 years of age compared to male partner aged < 30 years. However, starting at age 35 years for the female partner, this additive effect was no longer seen, suggesting a much stronger female factor for infertility as women age. It should be noted that the study did not provide information on the semen parameters of these men and that infertility in the couple was attributed more often to male factor when the male partner was older.
Wu et al. did not demonstrate any association of paternal age with fertilization rate at IVF. However, when the maternal age was 31–34 years, increasing paternal age was associated with decreased implantation and pregnancy rate. No information, other than age, was given on the males in this study including comorbidities, semen parameters, or reason for seeking IVF.
In a study by Kaarouch et al., it was shown that couples undergoing IVF for male factor infertility had worse outcomes with APA [36•]. Compared to younger men, IVF cycles performed with men with APA had lower fertilization rates (56 vs 65%), lower cleavage rates (94 vs 96%), lower blastulation rates (24 vs 33%), higher rates of cancelled embryo transfers (29 vs 10%), lower clinical pregnancy rates (17 vs 32%), and higher miscarriage rates (60 vs 42%). Men with APA did demonstrate higher rates of sperm DNA fragmentation and aneuploidy in this study.
Since it is often difficult to interpret the effect of paternal age on IVF outcomes due to maternal factors or abnormal semen parameters in many studies, the oocyte donation model has been used to help isolate the possible effect of APA on IVF success. de la Rochebrochard et al. examined the French National IVF Registry looking at cases of IVF performed in couples due to either bilateral fallopian tube obstruction or absence [52]. This study showed that for men aged ≥ 40 years, the odds ratio was 2.0 for failure to conceive when the female partner was aged 35–37 years and this increased to 5.74 with female partners aged ≥ 41 years compared to couples where both partners were < 30 years of age. Another study using donor oocytes demonstrated a 26% decreased odds of a live birth rate for each 5-year increase in paternal age [53].
However, other studies using the oocyte donation model have failed to show an effect of paternal age on IVF outcomes. In a study of IVF with ICSI outcomes using donor oocytes from women < 36 years of age, Beguería et al. showed there were no differences in any reproductive outcomes (biochemical pregnancy, clinical pregnancy, miscarriage, and live birth) amongst males of different ages [28]. Other studies have also not shown a significant impact of male age on IVF outcomes using donor oocytes [54, 55].
While many studies do show a possible effect of APA on reproductive outcomes with assisted conception, some studies do not show a correlation. Thus, it is difficult to definitively determine if APA is detrimental in the setting of ART at this time.
Potential Health Problems in Offspring of Older Men
The increased risk of certain health issues in the offspring of aged men has been well documented, and more studies are continually being published on this topic. While the exact mechanisms of many of these associations are difficult to prove, some are likely related to the sperm genetic changes described earlier. Additionally, in a study of 78 parent-offspring trios from Iceland, Kong et al. demonstrated that there was an increased rate of de novo mutations in the offspring of older fathers [56]. It should also be noted that when examining studies of neuropsychiatric and behavioral issues, it is often difficult to control for possible differences in environmental and social factors faced by the offspring of fathers of different ages or for reasons of delayed paternity in the first place (i.e., paternal psychiatric illness, etc.).
Compared to infants born to fathers aged 25–29 years, those born to fathers > 45 years have increased rates of late stillbirth, low birth weight, preterm birth, and very preterm birth [57]. In a study of 944,031 pregnancies from a Danish nationwide cohort from 1994 to 2010, the highest hazard ratios for stillbirth were noted when the father was > 40 years of age, with the highest ratio in those > 50 years (1.58 compared to fathers aged 30–34) [58••].
However, using a population-based cohort of > 800,000 live births from Ohio between 2006 and 2012 and controlling for maternal age, Hurley et al. did not find an association of paternal age on perinatal outcomes such as preterm birth or fetal growth restriction with or without the use of ART. When controlling for maternal factors, APA has been associated with lower infant mortality. In a study using the Linked Birth and Infant Death data file from the National Center for Vital Statistics, adolescent fathers (age < 20 years) siring children with older women (aged 21–45 years) demonstrated the highest risk for infant mortality with a hazard ratio of 2.7 [59].
An increased risk of congenital abnormalities in the offspring of men with APA was one of the first associations reported. In 1955, Penrose hypothesized about the increased risk of achondroplasia in the children of older fathers [60]. Since then, other autosomal dominant disorders have been associated with APA such as Apert syndrome, osteogenesis imperfecta, and neurofibromatosis type I [61,62,63].
There is also a reported increased risk of other conditions, such as syndactyly and cleft palate, in the offspring of older fathers [64, 65]. An analysis using the Danish national register data demonstrated that offspring of fathers aged > 45 years had a 69% higher rate of patent duct arteriosus compared to that of younger fathers [66]. There have also been studies suggesting increased risk of disorders of aneupoloidy, such as Down syndrome (trisomy 21) [67]. However, these studies of often poorly account for advanced maternal age and thus these associations are considered weak [68, 69]. More recently, in a study using donor oocytes (with an average age of 24 years), García-Ferreyra et al. demonstrated that men ≥ 50 years of age had significantly higher rates of global aneuploidy, trisomy 21, trisomy 18, and trisomy 13 (65.1, 15.1, 14.9, and 14.2%) on embryo biopsy with preimplantation genetic diagnosis (PGD) compared to men aged 40–49 years (53.5, 5.7, 3.8, and 2.5%) and ≤ 39 years (55.6, 6.1, 4.3, and 5.2%) [33•].
Studies examining the association of APA with increased risk of malignancy in offspring have showed generally inconsistent results, except for acute lymphoblastic leukemia (ALL). In a study examining the Danish health registries from 1978 to 2010, there was a 13% increased hazard ratio for ALL for every 5-year increase in paternal age [70••]. An increased risk of 4% per 5-year increase in paternal age for ALL was also demonstrated in a meta-analysis [71•]. There is also some evidence of an association of APA with an increased risk of retinoblastoma, but this data is not as strong [72].
Interestingly, there is some data to suggest that actually a younger paternal age at birth may be associated with an increased risk of some malignancies. Levine et al. examined a cohort of over one million men linked to the Israel National Cancer Registry and showed that an increasing paternal age at birth was linearly associated with a lower risk of testicular germ cell tumors, especially seminomas [73••]. The risk of seminoma decreased by 3.2% for each increase in year of paternal age at birth and the risk of seminoma was 40% higher in sons of fathers aged 15–24 vs > 30 years.
One of the areas with the most literature associating the risk of health problems in offspring of men with APA is in neuropsychiatric disease. A study of the Danish population demonstrated an increased risk of schizophrenia, mental retardation, and autism spectrum disorders (ASDs) in children born to fathers ≥ 45 years of age [74]. A study of the Swedish population, which compared siblings and cousins to possibly account for familial related confounding factors, demonstrated an increased risk of many conditions such as ASDs, psychosis, bipolar disorders, suicide attempts, substance abuse problems, and attention deficit/hyperactivity disorder (ADHD) [75]. Many other newer studies have corroborated the association of neuropsychiatric diseases, particularly schizophrenia and ASDs, with APA [76, 77]. In a study of 389 patients with schizophrenia, Fond et al. found a significant difference in the age of onset of the disease (20.7 vs 22.3 years) in offspring of fathers ≥ 35 years at birth [78]. While most studies show a positive correlation between APA and neuropsychiatric diseases, a more recent study of the Danish population did not find an association of APA with ADHD [79•].
Data is conflicting on the effect of APA on the intelligence and academic achievements of these men’s offspring. The study by D’Onofrio et al. of the Swedish population demonstrated an increased risk of failing a grade and a lower educational attainment in offspring of older men [75]. Saha et al. examined a sample of 33,437 children from the US Collaborative Perinatal Project and showed that the offspring of older fathers demonstrated decreased neurocognitive ability [80].
However, Gajos et al. showed a significant, albeit marginal, nonlinear relationship between paternal age at birth and male children’s verbal IQ scores at age 9 [81]. It has been shown in a population-based study of British twins that male offspring of older fathers demonstrated higher scores on measures assessing IQ and the ability to focus strongly on a subject of interest, and this was also associated with future academic achievement [82].
There are also potential social issues children of older fathers may have to face which may impact their mental health and behavior. Examples include the possible effect on the child of caring for an elderly parent or handling the death of the parent at a potentially earlier age in the child’s life.
Interestingly, it has been hypothesized, but far from proven, that the children of older men may derive some benefit from possibly increased longevity due to the increasing telomere length in the sperm of older men. It has been suggested that this may serve as a mechanism of “adaptive intergenerational plasticity” allowing for longer lifespans as generations reproduce at older ages [83].
Despite the evidence supporting an increased risk of various health conditions in the children of older men, it needs to be noted that the overall incidence of many of these genetic diseases is quite low, and the percentage of children born to fathers of APA, while increasing, remains low [69]. This means that significantly increased incidences, even several fold, for many of these conditions often translate into only small increases in the actual number of children born that are affected. That being said, many of these diseases often have significant morbidity and mortality associated with them.
Conclusions
There has been significant development in our knowledge of the effects of APA on fertility in the past two decades. This information is becoming increasing important to both clinicians and patients given the increasing paternal age in many countries. This article reviews the urologic comorbidities associated with the aged male that can impact fertility potential. Additionally, the impact of APA on decreased semen parameters, increased alterations in sperm genetics, possibly decreased reproductive outcomes, and increased risk of health conditions in offspring of men with APA was reviewed.
Given these findings, clinicians should be obligated to counsel older potential fathers on these possible risks. Because of this, some older men may want to seek earlier work-up with semen testing or pursue IVF with PGD, particularly given the potential for worsening outcomes with the continued ticking of the “biologic clock.” Since it has been shown that men with abnormal semen parameters or with a diagnosis of infertility have an increased risk of mortality and medical comorbidities themselves, it may be also prudent to counsel men on these potential health risks pending the findings of their work-up [84, 85••]. Some men may even consider cryopreserving sperm when younger; however, there is no guarantee of improved reproductive outcomes by doing this and there are additional ethical and financial concerns. Because of all the possible concerns raised in this article about APA, some have proposed placing a paternal age limit for the use of ARTs, but this is highly controversial for numerous reasons [86]. Overall, no guidelines exist to support these considerations at this time [87].
In conclusion, numerous recent studies have highlighted the potential decline in factors associated with productive success and health of the offspring in fathers of advanced paternal age. However, a lot remains unknown about fertility in the aging male, and the choice of siring offspring in older age remains up to the individual father and his partner. Clinicians should be aware of the current data and appropriately counsel patients on the possible risks of advanced paternal age.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sauer MV. Reproduction at an advanced maternal age and maternal health. Fertil Steril. 2015;103(5):1136–43. https://doi.org/10.1016/j.fertnstert.2015.03.004.
Seymour FI, Duffy C, Koerner A. A case of authenticated fertility in a man, aged 94. JAMA. 1935;105(18):1423–4.
Toriello HV, Meck JM, Professional P, Guidelines C. Statement on guidance for genetic counseling in advanced paternal age. Genet Med. 2008;10(6):457–60. https://doi.org/10.1097/GIM.0b013e318176fabb.
•• Khandwala YS, Zhang CA, Lu Y, Eisenberg ML. The age of fathers in the USA is rising: an analysis of 168 867 480 births from 1972 to 2015. Hum Reprod. 2017;32(10):2110–6. https://doi.org/10.1093/humrep/dex267. A retrospective study demonstrating a mean increase in paternal age of 3.5 years (from 27.4 to 30.9 years) within the USA between 1972 and 2015.
Bray I, Gunnell D, Davey SG. Advanced paternal age: how old is too old? J Epidemiol Community Health. 2006;60(10):851–3. https://doi.org/10.1136/jech.2005.045179.
Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120(2):151–7. https://doi.org/10.1016/j.amjmed.2006.06.010.
Brewis A, Meyer M. Marital coitus across the life course. J Biosoc Sci. 2005;37(4):499–518.
Agarwal A, Gupta S, Du Plessis S, Sharma R, Esteves SC, Cirenza C, et al. Abstinence time and its impact on basic and advanced semen parameters. Urology. 2016;94:102–10. https://doi.org/10.1016/j.urology.2016.03.059.
Travison TG, Vesper HW, Orwoll E, Wu F, Kaufman JM, Wang Y, et al. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102(4):1161–73. https://doi.org/10.1210/jc.2016-2935.
Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–98. https://doi.org/10.1210/jcem.87.2.8201.
Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96(8):2430–9. https://doi.org/10.1210/jc.2010-3012.
Corona G, Isidori AM, Buvat J, Aversa A, Rastrelli G, Hackett G, et al. Testosterone supplementation and sexual function: a meta-analysis study. J Sex Med. 2014;11(6):1577–92. https://doi.org/10.1111/jsm.12536.
Cunningham GR, Stephens-Shields AJ, Rosen RC, Wang C, Bhasin S, Matsumoto AM, et al. Testosterone treatment and sexual function in older men with low testosterone levels. J Clin Endocrinol Metab. 2016;101(8):3096–104. https://doi.org/10.1210/jc.2016-1645.
•• Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611–24. https://doi.org/10.1056/NEJMoa1506119. Part of the Testosterone Trials, this prospective study showed that treatment of symptomatic hypogonadism in older men showed benefit with respect to sexual function, mood, and depressive symptoms.
Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465–6. https://doi.org/10.1001/jamainternmed.2013.6895.
Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES Jr. Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol. 2012;187(3):973–8. https://doi.org/10.1016/j.juro.2011.10.137.
World Health Organization Task Force on methods for the regulation of male fertility. Contraceptive efficacy of testosteroneinduced azoospermia in normal men. Lancet. 1990;336(8721):955–9.
Turek PJ, Williams RH, Gilbaugh JH 3rd, Lipshultz LI. The reversibility of anabolic steroid-induced azoospermia. J Urol. 1995;153(5):1628–30.
Samplaski MK, Loai Y, Wong K, Lo KC, Grober ED, Jarvi KA. Testosterone use in the male infertility population: prescribing patterns and effects on semen and hormonal parameters. Fertil Steril. 2014;101(1):64–9. https://doi.org/10.1016/j.fertnstert.2013.09.003.
Hsieh TC, Pastuszak AW, Hwang K, Lipshultz LI. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol. 2013;189(2):647–50. https://doi.org/10.1016/j.juro.2012.09.043.
•• Wenker EP, Dupree JM, Langille GM, Kovac J, Ramasamy R, Lamb D, et al. The use of HCG-based combination therapy for recovery of spermatogenesis after testosterone use. J Sex Med. 2015;12(6):1334–7. https://doi.org/10.1111/jsm.12890. This was a restrospective study of 49 men who had azoospermia or severe oligospermia while taking testosterone. They were given human chorionic gonadotropin (HCG) supplementated with clomiphene, tamoxifen, anastrazole, or recombinent follicle-stimulating hormone (or combination) with improvements in sperms counts seen in 47 (95.9%) of the men.
•• Kohn TP, Louis MR, Pickett SM, Lindgren MC, Kohn JR, Pastuszak AW, et al. Age and duration of testosterone therapy predict time to return of sperm count after human chorionic gonadotropin therapy. Fertil Steril. 2017;107(2):351–7 e1. https://doi.org/10.1016/j.fertnstert.2016.10.004. This study retrospectively examined 66 men who presented for an infertility evaluation after testosterone use and were placed on a human chorionic gonadotropin (HCG) + SERM regimen. The authors demonstrated that increasing age and duration of testosterone use reduced the likelihood of recovery of sperm in the ejaculate at both 6 and 12 months. Only 64.8% of azoospermic men achieved a total motile sperm count > 5 million sperm by 12 months.
Blanker MH, Bosch JL, Groeneveld FP, Bohnen AM, Prins A, Thomas S, et al. Erectile and ejaculatory dysfunction in a community-based sample of men 50 to 78 years old: prevalence, concern, and relation to sexual activity. Urology. 2001;57(4):763–8.
Avellino G, Theva D, Oates RD. Common urologic diseases in older men and their treatment: how they impact fertility. Fertil Steril. 2017;107(2):305–11. https://doi.org/10.1016/j.fertnstert.2016.12.008.
Wang C, Swerdloff RS. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102(6):1502–7. https://doi.org/10.1016/j.fertnstert.2014.10.021.
Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75(2):237–48.
•• Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22–33. https://doi.org/10.1016/j.arr.2014.10.007. A meta-analysis demonstrating a decline in semen parameters (volume, motility, morphology) and an increase in DNA fragmenation as men age.
Begueria R, Garcia D, Obradors A, Poisot F, Vassena R, Vernaeve V. Paternal age and assisted reproductive outcomes in ICSI donor oocytes: is there an effect of older fathers? Hum Reprod. 2014;29(10):2114–22. https://doi.org/10.1093/humrep/deu189.
Garcia-Ferreyra J, Luna D, Villegas L, Romero R, Zavala P, Hilario R, et al. High aneuploidy rates observed in embryos derived from donated oocytes are related to male aging and high percentages of sperm DNA fragmentation. Clin Med Insights Reprod Health. 2015;9:21–7. https://doi.org/10.4137/CMRH.S32769.
Moskovtsev SI, Willis J, Mullen JB. Age-related decline in sperm deoxyribonucleic acid integrity in patients evaluated for male infertility. Fertil Steril. 2006;85(2):496–9. https://doi.org/10.1016/j.fertnstert.2005.05.075.
Das M, Al-Hathal N, San-Gabriel M, Phillips S, Kadoch IJ, Bissonnette F, et al. High prevalence of isolated sperm DNA damage in infertile men with advanced paternal age. J Assist Reprod Genet. 2013;30(6):843–8. https://doi.org/10.1007/s10815-013-0015-0.
Brahem S, Mehdi M, Elghezal H, Saad A. Detection of DNA fragmentation and meiotic segregation in human with isolated teratozoospermia. J Assist Reprod Genet. 2011;28(1):41–8. https://doi.org/10.1007/s10815-010-9482-8.
• Garcia-Ferreyra J, Hilario R, Duenas J. High percentages of embryos with 21, 18 or 13 trisomy are related to advanced paternal age in donor egg cycles. JBRA Assist Reprod. 2018; https://doi.org/10.5935/1518-0557.20180004. This was a restropective review of IVF/ICSI cycles with PGD which demonstrated that the sperm of aged men had higher rates of DNA fragmentation and aneuploidy. Additonally, there were significantly elevated rates of global aneuploidy and trisomy 21, 18, and 12 in embryos of fathers of advanced paternal age.
Cocuzza M, Athayde KS, Agarwal A, Sharma R, Pagani R, Lucon AM, et al. Age-related increase of reactive oxygen species in neat semen in healthy fertile men. Urology. 2008;71(3):490–4. https://doi.org/10.1016/j.urology.2007.11.041.
Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. Etiologies of sperm oxidative stress. Int J Reprod Biomed (Yazd). 2016;14(4):231–40.
• Kaarouch I, Bouamoud N, Madkour A, Louanjli N, Saadani B, Assou S, et al. Paternal age: negative impact on sperm genome decays and IVF outcomes after 40 years. Mol Reprod Dev, https://doi.org/10.1002/mrd.22963. 2018; In this study, 83 couples undergoing IVF for male factor infertility were evaluated. The sperm of older men demonstrated increased DNA fragmentation and aneuploidy rates compared to younger men. IVF outcomes were also worse in the older men.
Donate A, Estop AM, Giraldo J, Templado C. Paternal age and numerical chromosome abnormalities in human spermatozoa. Cytogenet Genome Res. 2016;148(4):241–8. https://doi.org/10.1159/000446724.
Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A. 2006;103(25):9601–6. https://doi.org/10.1073/pnas.0506468103.
Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978;120(1):33–53.
Ehrlenbach S, Willeit P, Kiechl S, Willeit J, Reindl M, Schanda K, et al. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int J Epidemiol. 2009;38(6):1725–34. https://doi.org/10.1093/ije/dyp273.
Kimura M, Cherkas LF, Kato BS, Demissie S, Hjelmborg JB, Brimacombe M, et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4(2):e37. https://doi.org/10.1371/journal.pgen.0040037.
Eisenberg DT, Hayes MG, Kuzawa CW. Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc Natl Acad Sci U S A. 2012;109(26):10251–6. https://doi.org/10.1073/pnas.1202092109.
Jenkins TG, Aston KI, Cairns BR, Carrell DT. Paternal aging and associated intraindividual alterations of global sperm 5-methylcytosine and 5-hydroxymethylcytosine levels. Fertil Steril. 2013;100(4):945–51. https://doi.org/10.1016/j.fertnstert.2013.05.039.
Jenkins TG, Aston KI, Pflueger C, Cairns BR, Carrell DT. Age-associated sperm DNA methylation alterations: possible implications in offspring disease susceptibility. PLoS Genet. 2014;10(7):e1004458. https://doi.org/10.1371/journal.pgen.1004458.
Kawai K, Harada T, Ishikawa T, Sugiyama R, Kawamura T, Yoshida A, et al. Parental age and gene expression profiles in individual human blastocysts. Sci Rep. 2018;8(1):2380. https://doi.org/10.1038/s41598-018-20614-8.
Ford WC, North K, Taylor H, Farrow A, Hull MG, Golding J. Increasing paternal age is associated with delayed conception in a large population of fertile couples: evidence for declining fecundity in older men. The ALSPAC Study Team (Avon Longitudinal Study of Pregnancy and Childhood). Hum Reprod. 2000;15(8):1703–8.
Hassan MA, Killick SR. Effect of male age on fertility: evidence for the decline in male fertility with increasing age. Fertil Steril. 2003;79(Suppl 3):1520–7.
de La Rochebrochard E, Thonneau P. Paternal age >or=40 years: an important risk factor for infertility. Am J Obstet Gynecol. 2003;189(4):901–5.
Mathieu C, Ecochard R, Bied V, Lornage J, Czyba JC. Cumulative conception rate following intrauterine artificial insemination with husband's spermatozoa: influence of husband’s age. Hum Reprod. 1995;10(5):1090–7.
Belloc S, Cohen-Bacrie P, Benkhalifa M, Cohen-Bacrie M, De Mouzon J, Hazout A, et al. Effect of maternal and paternal age on pregnancy and miscarriage rates after intrauterine insemination. Reprod BioMed Online. 2008;17(3):392–7.
• McPherson NO, Zander-Fox D, Vincent AD, Lane M. Combined advanced parental age has an additive negative effect on live birth rates—data from 4057 first IVF/ICSI cycles. J Assist Reprod Genet. 2017; https://doi.org/10.1007/s10815-017-1054-8. A retrospective review of 4057 first IVF cycles showing an additive negative effect on pregnancy and live birth rates when both parents are of advanced age.
de La Rochebrochard E, de Mouzon J, Thepot F, Thonneau P, French National IVFRA. Fathers over 40 and increased failure to conceive: the lessons of in vitro fertilization in France. Fertil Steril. 2006;85(5):1420–4. https://doi.org/10.1016/j.fertnstert.2005.11.040.
Robertshaw I, Khoury J, Abdallah ME, Warikoo P, Hofmann GE. The effect of paternal age on outcome in assisted reproductive technology using the ovum donation model. Reprod Sci. 2014;21(5):590–3. https://doi.org/10.1177/1933719113506497.
Whitcomb BW, Turzanski-Fortner R, Richter KS, Kipersztok S, Stillman RJ, Levy MJ, et al. Contribution of male age to outcomes in assisted reproductive technologies. Fertil Steril. 2011;95(1):147–51. https://doi.org/10.1016/j.fertnstert.2010.06.039.
Gu L, Zhang H, Yin L, Bu Z, Zhu G. Effect of male age on the outcome of in vitro fertilization: oocyte donation as a model. J Assist Reprod Genet. 2012;29(4):331–4. https://doi.org/10.1007/s10815-012-9719-9.
Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488(7412):471–5. https://doi.org/10.1038/nature11396.
Alio AP, Salihu HM, McIntosh C, August EM, Weldeselasse H, Sanchez E, et al. The effect of paternal age on fetal birth outcomes. Am J Mens Health. 2012;6(5):427–35. https://doi.org/10.1177/1557988312440718.
•• Urhoj SK, Andersen PK, Mortensen LH, Davey Smith G, Nybo Andersen AM. Advanced paternal age and stillbirth rate: a nationwide register-based cohort study of 944,031 pregnancies in Denmark. Eur J Epidemiol. 2017;32(3):227–34. https://doi.org/10.1007/s10654-017-0237-z. A restrospective study based on a large sample from Denmark examing the impact of paternal age on the rate of stillbirth. It showed advanced paternal age, particularly in those > 40 years of age, was associated with an increased risk of still birth.
Doamekpor LA, Amutah NN, Ramos LJ. Fathers matter: the role of paternal age in infant mortality. Am J Mens Health. 2014;8(2):175–82. https://doi.org/10.1177/1557988313511492.
Penrose LS. Parental age and mutation. Lancet. 1955;269(6885):312–3.
Glaser RL, Broman KW, Schulman RL, Eskenazi B, Wyrobek AJ, Jabs EW. The paternal-age effect in Apert syndrome is due, in part, to the increased frequency of mutations in sperm. Am J Hum Genet. 2003;73(4):939–47. https://doi.org/10.1086/378419.
Bunin GR, Needle M, Riccardi VM. Paternal age and sporadic neurofibromatosis 1: a case-control study and consideration of the methodologic issues. Genet Epidemiol. 1997;14(5):507–16. https://doi.org/10.1002/(SICI)1098-2272(1997)14:5<507::AID-GEPI5>3.0.CO;2-Y.
Blumsohn A, McAllion SJ, Paterson CR. Excess paternal age in apparently sporadic osteogenesis imperfecta. Am J Med Genet. 2001;100(4):280–6.
Polednak AP. Paternal age in relation to selected birth defects. Hum Biol. 1976;48(4):727–39.
Herkrath AP, Herkrath FJ, Rebelo MA, Vettore MV. Parental age as a risk factor for non-syndromic oral clefts: a meta-analysis. J Dent. 2012;40(1):3–14. https://doi.org/10.1016/j.jdent.2011.10.002.
Su XJ, Yuan W, Huang GY, Olsen J, Li J. Paternal age and offspring congenital heart defects: a national cohort study. PLoS One. 2015;10(3):e0121030. https://doi.org/10.1371/journal.pone.0121030.
Zhu JL, Madsen KM, Vestergaard M, Olesen AV, Basso O, Olsen J. Paternal age and congenital malformations. Hum Reprod. 2005;20(11):3173–7. https://doi.org/10.1093/humrep/dei186.
Crane E, Morris JK. Paternal age and birth defects: how strong is the association. Hum Reprod. 2007;22(8):2349–50. https://doi.org/10.1093/humrep/dem134.
Nybo Andersen AM, Urhoj SK. Is advanced paternal age a health risk for the offspring? Fertil Steril. 2017;107(2):312–8. https://doi.org/10.1016/j.fertnstert.2016.12.019.
•• Urhoj SK, Raaschou-Nielsen O, Hansen AV, Mortensen LH, Andersen PK, Nybo Andersen AM. Advanced paternal age and childhood cancer in offspring: a nationwide register-based cohort study. Int J Cancer. 2017;140(11):2461–72. https://doi.org/10.1002/ijc.30677. This was a retrospective study examining the Danish health registries from 1978 to 2010. It showed a 13% increased hazard ratio for acute lymphoblastic leukemia (ALL) for every 5-year increase in paternal age.
• Sergentanis TN, Thomopoulos TP, Gialamas SP, Karalexi MA, Biniaris-Georgallis SI, Kontogeorgi E, et al. Risk for childhood leukemia associated with maternal and paternal age. Eur J Epidemiol. 2015;30(12):1229–61. https://doi.org/10.1007/s10654-015-0089-3. A meta-analysis examing the risk of childhood leukemias associated with advanced parental age. It demonstrated that both older maternal and paternal ages were associated with an increased risk of acute lymphoblastic leukemia (ALL) in their offspring. It also showed that children of mothers at the extremes of both ends of age and younger fathers had an increased risk for acute myeloid leukemia (AML).
Heck JE, Lombardi CA, Meyers TJ, Cockburn M, Wilhelm M, Ritz B. Perinatal characteristics and retinoblastoma. Cancer Causes Control. 2012;23(9):1567–75. https://doi.org/10.1007/s10552-012-0034-7.
•• Levine H, Keinan-Boker L, Leiba A, Derazne E, Rais A, Kark JD. Paternal age and risk of testicular germ cell tumors: a cohort study of 1,000,000 men. Andrology. 2017;5(6):1124–30. https://doi.org/10.1111/andr.12422. A retrospective analysis of a large population-based cohort that demonstrated an increased risk of testicular germ cell tumors, especially seminoma, in the male offspring of younger fathers.
McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, Pedersen CB. A comprehensive assessment of parental age and psychiatric disorders. JAMA Psychiatry. 2014;71(3):301–9. https://doi.org/10.1001/jamapsychiatry.2013.4081.
D'Onofrio BM, Rickert ME, Frans E, Kuja-Halkola R, Almqvist C, Sjolander A, et al. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry. 2014;71(4):432–8. https://doi.org/10.1001/jamapsychiatry.2013.4525.
Fountoulakis KN, Gonda X, Siamouli M, Panagiotidis P, Moutou K, Nimatoudis I, Kasper S Paternal and maternal age as risk factors for schizophrenia: a case-control study. Int J Psychiatry Clin Pract. 2017:1–7. https://doi.org/10.1080/13651501.2017.1391292.
Merikangas AK, Calkins ME, Bilker WB, Moore TM, Gur RC, Gur RE. Parental age and offspring psychopathology in the Philadelphia Neurodevelopmental Cohort. J Am Acad Child Adolesc Psychiatry. 2017;56(5):391–400. https://doi.org/10.1016/j.jaac.2017.02.004.
Fond G, Godin O, Boyer L, Llorca PM, Andrianarisoa M, Brunel L, et al. Advanced paternal age is associated with earlier schizophrenia onset in offspring. Results from the national multicentric FACE-SZ cohort. Psychiatry Res. 2017;254:218–23. https://doi.org/10.1016/j.psychres.2017.04.002.
• Hvolgaard Mikkelsen S, Olsen J, Bech BH, Obel C. Parental age and attention-deficit/hyperactivity disorder (ADHD). Int J Epidemiol. 2017;46(2):409–20. https://doi.org/10.1093/ije/dyw073. Using a large Danish population cohort, the authors retrospectively reviewed the risk of parental age on ADHD. When comparing full siblings, there was increased risk of ADHD with younger mothers, but no association with paternal age.
Saha S, Barnett AG, Foldi C, Burne TH, Eyles DW, Buka SL, et al. Advanced paternal age is associated with impaired neurocognitive outcomes during infancy and childhood. PLoS Med. 2009;6(3):e40. https://doi.org/10.1371/journal.pmed.1000040.
Gajos JM, Beaver KM. The role of paternal age in the prediction of offspring intelligence. J Genet Psychol. 2017;178(6):319–33. https://doi.org/10.1080/00221325.2017.1377678.
Janecka M, Rijsdijk F, Rai D, Modabbernia A, Reichenberg A. Advantageous developmental outcomes of advancing paternal age. Transl Psychiatry. 2017;7(6):e1156. https://doi.org/10.1038/tp.2017.125.
Eisenberg DT, Kuzawa CW. Commentary: The evolutionary biology of the paternal age effect on telomere length. Int J Epidemiol. 2013;42(2):462–5. https://doi.org/10.1093/ije/dyt027.
Eisenberg ML, Li S, Behr B, Cullen MR, Galusha D, Lamb DJ, et al. Semen quality, infertility and mortality in the USA. Hum Reprod. 2014;29(7):1567–74. https://doi.org/10.1093/humrep/deu106.
•• Eisenberg ML, Li S, Cullen MR, Baker LC. Increased risk of incident chronic medical conditions in infertile men: analysis of United States claims data. Fertil Steril. 2016;105(3):629–36. https://doi.org/10.1016/j.fertnstert.2015.11.011. This was a retrospective review using the Truven Health MarketScan claims database from 2001 to 2009 to determine the incidence of chronic medical conditions in infertile men. The authors demonstrated that men diagnosed with male factor infertility had a significantly higher risk of adverse health outcome in later years.
Braverman AM. Old, older and too old: age limits for medically assisted fatherhood? Fertil Steril. 2017;107(2):329–33. https://doi.org/10.1016/j.fertnstert.2016.12.006.
Jennings MO, Owen RC, Keefe D, Kim ED. Management and counseling of the male with advanced paternal age. Fertil Steril. 2017;107(2):324–8. https://doi.org/10.1016/j.fertnstert.2016.11.018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Daniel J. Mazur declares no potential conflicts of interest.
Larry I. Lipshultz is a consultant for AbbVie, Lipocine, Aytu Bioscience, and Endo Pharmaceuticals and a speaker for American Medical Systems and Endo Pharmaceuticals.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Andrology and Infertility
Rights and permissions
About this article
Cite this article
Mazur, D.J., Lipshultz, L.I. Infertility in the Aging Male. Curr Urol Rep 19, 54 (2018). https://doi.org/10.1007/s11934-018-0802-3
Published:
DOI: https://doi.org/10.1007/s11934-018-0802-3