Abstract
Purpose of the Review
An increased prevalence of cardiovascular risk factors in rheumatoid arthritis (RA) is reported. The absolute cardiovascular risk in RA patients is higher than in the general population, and although the RA prognosis has gradually improved, premature cardiovascular (CV) mortality remains a matter of fact. The purpose of this review is to shed light on CV and metabolic involvement in RA, with the aim of defining its best management.
Recent Findings
Multiple lines of evidence have revealed common mechanisms behind inflammatory and CV diseases and clarified the metabolic and CV pathways involved in RA and the effects of different pharmacological treatments.
Summary
CV risk assessment should be mandatory in all RA patients, taking into account the impact of both diseases on patient’s prognosis. Therefore, a multidisciplinary approach is the best management, and rheumatologists, cardiologists, and general practitioners must work together to significantly improve outcome and quality of life in RA patients. Future research could investigate the potential beneficial effects of a more aggressive pharmacological treatment of CV and metabolic risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidemiological data clearly show that rheumatic diseases are burdened by a considerable cardiovascular risk, in terms of ischemic heart disease, heart failure, cerebrovascular disease, and peripheral arterial disease. This has led, over the years, to sensitize both rheumatologists and cardiologists to the involvement of the cardiovascular system in these pathologies, bringing to official recommendations by the respective scientific societies, as happened for the first time in 2009 by EULAR (European League Against Rheumatism) [1] and later with the 2015–2016 EULAR update [2] and 2016 ESC (European Society of Cardiology) guidelines on heart failure [3], where the impact of rheumatoid arthritis (RA) is briefly discussed as a comorbidity that can worsen prognosis in heart failure and, on the other hand, have a possible causative role, together its specific treatment, in the onset of heart failure.
Although the cardiovascular repercussions are known for the majority of inflammatory joint diseases (IJD), most of the evidence comes from RA patients.
In RA, an increased prevalence of cardiovascular (CV) risk factors—such as hypertension, diabetes, smoking, obesity, dyslipidemia—is frequently reported [4].
There’s evidence of a growing awareness of both rheumatologists and cardiologists about the need for better CV risk stratification in patients with RA, taking into account the reciprocal effects of these two pathological entities on patient’s quality of life and prognosis.
The purpose of this review is to describe the state of the art about the knowledge on cardiovascular and metabolic implications in RA, examining the most recent evidence, with the aim of defining the best approach, management, and treatment of cardiovascular risk factors for these patients.
Epidemiology
Epidemiological data show that life expectancy in patients with RA is lower than in the general population.
Firstly, in 1953, data on mortality in RA were published, reporting a 29% excess among RA patients compared to unaffected people [5]. Further evidences of a decreased life expectancy in RA patients emerged in the following years, gradually outlining which were the causes of this increased mortality, and two main determinants were identified: cardiovascular system involvement and infections [6].
Although the RA prognosis has improved compared to previous decades, thanks to pharmacological advances, premature mortality still remains a matter of fact, as shown by local registries [7, 8, 9], systematic reviews and meta-analysis [10, 11], with an increased risk of 48% for the incidence of CV disease, a 60% increase in risk of CV death compared to the general population [12], and a reduced life expectancy of 3 to 10 years or more [13].
Among cardiovascular affections in RA patients, the major impact on mortality lies in myocardial infarction rather than cerebrovascular events, with an increased risk of 68 and 41%, respectively [14, 15, 16], while it has been reported that the risk of heart failure is greater than 87% compared to unaffected adult patients (> 46 years) [17].
Several data show that cardiovascular manifestations often precede the diagnosis of RA [18, 19].
However, different observations are reported by some authors, showing that in subjects with recent onset of the disease, on effective pharmacological therapy, there’s not an increased CV risk compared to general population [20].
Mechanisms Involved in Cardiovascular and Metabolic Comorbidities in RA
In all IJD, cardiovascular comorbidities depend on several pathogenic mechanisms, even if atherosclerosis is the most frequently involved. Further mechanisms include microvascular dysfunction, arrhythmias, cardiac autonomic deregulation, inflammation and immunologic abnormalities, as well as the effects of pharmacological treatments.
Atherosclerosis and RA share common risk factors and very likely some common pathogenic mechanisms, such as obesity, smoking, and dyslipidemia. Anyway, in RA, the relation between patient’s profile and his CV risk does not correspond to that of the general population, as for example being male, smoker, and already with a CV history do not have the same impact on CV risk compared to a subject without RA [21]: the absolute CV risk for RA patients is higher than that of the general population, even when adjusted for traditional risk factors, meaning that RA represents an independent risk factor for CV diseases [21].
Therefore, a description of the peculiarities of the main CV risk factors in RA patients could be useful.
Cholesterol
It is well-known that cholesterol is an important CV risk factor for general population [22]. However, there’s evidence that this general concept is not equally applicable to RA patients, and that cholesterol impact on the risk stratification in RA patients could be in contrast with the common population trend [23]. Indeed, a large cohort study, based on a population of RA patients, showed a higher cardiovascular risk in subjects with lower levels of total cholesterol (TC) compared to subjects with higher levels [24], and another study reported that, in patients with RA compared to general population, CV risk still remains high despite lower TC levels, as well as low-density lipoprotein cholesterol (LDL-C) (− 7 and − 11%, respectively) [25]. These observations were confirmed in further studies [26, 27], underlying that—for RA patients—low LDL-C levels do not necessarily mean low cardiovascular risk, and—vice versa—high LDL-C levels do not increase patient’s risk profile, leading to the so called “lipid paradox” phenomenon [24].
The natural history of RA explains this paradox showing that LDL-C levels are indirectly correlated with inflammation levels, with lower values at pre-treatment time and during the active phase and relapses, while higher levels are observed during treatment and remission phase [28]. The responsible for the decline of TC, LDL-C, and high-density cholesterol (HDL-C) compared with individuals without RA could be the active inflammation [29].
In fact, in the active phase, a higher catabolism of cholesterol esters could be present, as emerged from studies performed by labeling lipids with stable isotopes, administered to subjects, and then traced in their metabolic pathway; in a recent study [30••], 36 patients with RA in active phase have been compared to healthy volunteers, measuring cholesterol levels at baseline and after 6 weeks of treatment with tofacitinib (TOFA): baseline levels were lower in RA patients compared to controls, suggesting a higher catabolism of cholesterol esters, while after 6 weeks of treatment with TOFA, cholesterol levels increased. Another similar study was conducted with tocilizumab (TOCI) and led to comparable results [31••].
Going through more advanced phases of the disease, the increase in lipid levels could be ascribed to a greater synthesis and to a concomitant reduced lipids removal from blood stream.
Furthermore, the inflammatory process seems to act as well by altering the structure and function of lipid components, changing the known anti-atherogenic effect of HDL-C, traditionally thought as protective against cardiovascular diseases, into pro-atherogenic effect [32]; HDL-C is the main responsible for the removal of excess cholesterol in the arterial wall, through the so called “cholesterol efflux” from cells. The ability of HDL to guarantee the cholesterol efflux depends on their quality, composition, and possible modifications induced by several pathologic processes [33]. To confirm the inflammation role on lipid profile in RA patients, there’s the evidence that anti-inflammatory therapy can modify quantity, composition, and function of lipid components: disease-modifying anti-rheumatic drugs (DMARDs) determined a reductions in high sensitivity protein C (CRP), an increase in LDL-C levels, and concomitant improvements in HDL-C efflux capacity in a longitudinal RA cohort study [34•]; furthermore, another study reported a > 30% increase LDL-C after treatment with methotrexate (MTX) plus etanercept, triple therapy (MTX plus sulfasalazine plus hydroxychloroquine), or aggressively titrated MTX monotherapy [35].
Diabetes
There is evidence of a strong association between RA and diabetes, even in this case probably mediated by inflammation [36]. Several inflammatory mediators involved in RA, like interleukin-1 beta (IL-1 β), interleukin 6 (IL-6), and tumor necrosis factor (TNF), have an important role in determining metabolic deregulation and atherosclerosis [37]: these pro-inflammatory cytokines can determine beta-cells dysfunction and disruption [37] and progressively lead to insulin resistance [38]. A double relation between diabetes and RA has been reported:
1) a high prevalence of type-2 diabetes in patients with RA.
2) RA as risk factor for development of type-2 diabetes.
In a study of Ruscitti and colleagues, the prevalence of type-2 diabetes and impaired fasting glucose (IFG) was investigated in an Italian cohort of RA patients and compared with age- and gender-matched controls, showing that RA was significantly associated with glucose metabolism abnormalities both in relation to traditional CV risk factors (i.e., metabolic syndrome) and to specific RA characteristics like CRP levels, duration of the disease, and extension of the articular damage [39•]. The same authors reported a significant risk of type-2 diabetes in RA patients in a longitudinal study after 1-year follow-up, as close as the low control of the disease activity [40]. Recent reports show that some pharmacological treatment used in RA can produce a positive effect in preventing diabetes, as emerged from a study in patients with RA or psoriasis, where TNF-inhibitors and hydroxychloroquine reduced the risk of diabetes [41]. Besides diabetes, insulin resistance is common among patients with RA, too. Interestingly, many data suggest that in RA patients an important contribution to insulin resistance comes from both obesity and inflammation [42].
Hypertension
Systemic hypertension is a very underdiagnosed and undertreated condition in patients with RA [43]. In a recent prospective study [44], the occurrence of new onset of cardiovascular events and subclinical atherosclerosis was investigated; and after 1-year follow-up, a significantly increased number of patients affected by systemic hypertension were observed (from 42.36 to 55.62%) and the onset of systemic hypertension during the follow-up was associated with a significantly increased risk of cardiovascular events. Furthermore, a study conducted on RA patients by 24-h ambulatory blood pressure monitoring (ABPM) revealed that the night-time blood-pressure profile, which represents a strong predictor of future cardiovascular outcomes [45], is characterized by non-dipping-pattern (a decline in BP < 10% of daytime values), associated with subclinical vascular damage, including arterial stiffness [46] and carotid atherosclerosis [47].
Furthermore, the effects of systemic inflammation on peripheral vascular resistance [48] and specific anti-rheumatic drugs are other mechanisms responsible for the development of hypertension in RA patients, as well as the presence of specific genetic polymorphism [49].
Obesity and Physical Inactivity
Obesity is a well-recognized cardiovascular risk factor, since adipose tissue is metabolically active, able to secrete pro-inflammatory cytokines that act on endothelium and vascular function, glucose homeostasis, oxidative-stress, and hemostasis, only to cite some of their effects [50].
Even if there is evidence of the negative impact of obesity in RA patient’s risk profile, as expressed—for example—from the direct correlation between body mass index (BMI) and carotid artery intima-media thickness (cIMT) [51], there’s on the other hand the paradoxical effect of a low BMI (< 20 kg/m2) on RA patient prognosis, since the deleterious effects of the inflammatory process lead to a muscular wasting and body cell consumption, known as rheumatoid cachexia [52]; the role of inflammation in this concern is confirmed from studies that show an improvement in sarcopenia after IL-6 blocking [53].
Caloric intake and the lack of physical activity in RA patients have a role in developing obesity, as well. Because of chronic pain, swelling, and stiffness of the joints, physical inactivity is common in RA patients and it’s correlated with an adverse cardiovascular risk profile. In a cross-sectional study, RA patients were grouped into active, moderately active, and inactive; this latter had a significantly worse cardiovascular risk profile when compared to active patients, and results remained significant even after adjusting for disease severity and activity [54]. Interestingly, it was shown that RA patients who were physically active during the 5-year period before the disease diagnosis developed a milder disease, and that physical activity significantly reduced the risk of having a disease activity score (DAS)-28 above the median, independently from anticyclic citrullinated peptide antibodies (anti-CCP) status, sex, BMI, socioeconomic status, or physically demanding work [55]. These data confirm that body weight control and physical activity are important parameters to include in the global evaluation of patients with RA and to reach as a goal in their overall management.
Smoking
Despite the increased prevalence of smoking habit in RA patients is well-recognized, its impact on atherosclerosis has not been fully clarified [56, 57]. In fact, although the well-known pathogenic role of smoking on CV risk, a weak association between smoking and CV events, in RA patients, has been previously shown [58]. The so-called “RA smoking paradox” may be related with an “index event” bias, a frequent bias of observational, retrospective, and epidemiological studies, in which causal factors appear not to apply to disease complications [59].
Inflammation
Several data suggest that inflammatory burden strongly determines the propensity to develop cardiovascular events in RA patients, so that disease activity and severity could directly correlate to cardiovascular risk. Systemic inflammation acts on multiple biological pathways producing effects in glucose metabolism, lipid profile, endothelial function, and oxidative stress. Elevated levels of CRP promote the atherosclerotic process [60], as well as levels of other systemic inflammatory biomarkers predict cardiovascular disease probability and severity [61]. The specific rheumatoid chronic inflammatory state is generally considered to increase CV risk, via different mechanisms, such as pro-thrombotic state and pro-atherogenic metabolic effects [60]. In fact, it has been shown that the failure to fully control the inflammatory process may enhance the risk of developing both CV disease and subclinical atherosclerosis, thus suggesting the need of a good control of RA activity, to prevent the occurrence of such comorbidities [62•, 44]. Concerning RA disease duration, conflicting results are available in literature about its association with CV risk [63, 64]. In fact, it has been proposed that the burden of the inflammatory process over time, more than RA duration, may increase the CV risk in those RA patients with a persistent poorly controlled inflammatory process and active disease [63, 65].
On these bases, there is overall agreement on the inflammatory nature of the atherosclerotic disease, and the inflammatory response in atherosclerosis is significantly similar to that in RA [66]. Vascular inflammatory process other than atherosclerosis, such as vasculitis, may also have an important role in autoimmune rheumatic disease, especially in RA and spondyloarthritis, where coronary arteries and aorta involvement is common, leading to an acceleration of the atherosclerotic process in these vessels [67].
Others
The psychological and behavioral repercussions of RA, determined by its significant impact in patient’s quality of life, seem to negatively influence the immunological pathway, in terms of promoting an increased release of pro-inflammatory cytokines as long as the depressive mood progresses [68]. Conversely, a pro-inflammatory state may promote depression through its negative effects on neurotransmitter metabolism and hypothalamo-hypophisis axis function [69].
Many other factors are described to contribute to cardiovascular risk in RA, like hyperuricemia, hypothyroidism, vitamin D deficiency, and reduction or dysfunction of endothelial progenitor cells [60].
Cardiovascular Risk Stratification
Development and standardization of cardiovascular risk assessment models in patients with RA (or IJD in general) are advisable, aiming to ensure a complete evaluation of the patient. In fact, cardiovascular disease risk in patients with RA is elevated compared with the general population, as stated above [4, 6]. Unfortunately, a RA-specific CV disease risk prediction model is lacking, and the commonly used risk models, based on epidemiological data from general population, underestimate the CV risk in RA patients [70••]. For example, the Framingham and Reynolds risk scores, as well, significantly underestimate CV risk in patients with RA, especially in more advanced ages and in case of positive rheumatoid factor: the observed CVD risk was twofold higher than the Framingham risk score predicted in women and 65% higher in men, and in patients aged ≥ 75 years, it was > 3 times the Framingham predicted risk; the Reynolds risk score was not better in estimation [71]. Furthermore, also the SCORE (the most used CV risk prediction model in Europe) was found to fail in classifying at high risk (carotid ultrasonography evaluation) even the 88% of observed patients in a Spanish cohort of RA patients [72].
On the basis of these considerations, in 2009, the EULAR evidence-based recommendations for cardiovascular risk management in patients with RA and other forms of inflammatory arthritis [1] suggested an annual CV risk assessment and advised that common risk score models had to be adapted by introducing a 1.5 multiplication factor in patients with at least 2 criteria between disease duration > 10 years, RF, or ACCP positivity, extra-articular manifestation.
EULAR recommendations were later updated in 2015/2016 [2], and some modifications were made: the CV risk assessment schedule was changed from annual to once every 5 years, or following major changes in anti-rheumatic therapy (i.e., the initiation of biologic DMARDs or other drugs that may alter LDL-C or other CV risk factors); the 1.5 multiplication factor was applied for all RA patients, independently from disease-specific criteria, because new data evidenced the increased CV risk even in the early stages of RA and in patients without extra-articular manifestations [73, 74]. This change was due to the consideration that 2009 recommendation did not reclassify as many patients as was expected into a more appropriate risk category.
Nevertheless, another risk calculator (QRISK 2), including a 1.4 multiplication factor for RA patients, tended to overestimate CV risk in AR patients [75].
Ultimately, the EULAR taskforce recommends that CV risk assessment should be performed according to national guidelines and the SCORE CV disease risk prediction model should be used if no national guidelines are available [2]; however, a better strategy to define CV risk profile should also include the evaluation the activity of the disease, since there is a great deal of evidence on a good outcome in patients with optimal control of disease activity [62•]. On the other hand, there are conflicting data concerning the association between disease duration and CV risk [63].
Another critical point in cardiovascular risk stratification is which lipid parameters to use and when, taking into account the “lipid paradox” phenomenon described above. In practice, both TC and HDL-C are used in risk calculators, and—since disease activity and anti-inflammatory therapy significantly modify concentrations of lipid components—the latter should be measured during a stable phase of the disease [76]. TC/HDL-C ratio, rather than individual lipid components, has been found to be a better CV risk predictor in RA patients, especially in those with high inflammatory activity [32]. Furthermore, RA treatments generally increase individual lipids components, without changes in TC/HDL-C ratio.
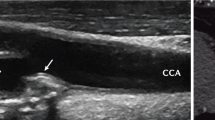
Finally, cardiovascular risk stratification may be improved by screening for asymptomatic atherosclerotic plaque by carotid ultrasound. In fact, RA specific factors contribute to carotid atherosclerosis [77, 78], and the presence of carotid plaques is associated with vascular events in AR patients [78, 62, 79]. As a screening for asymptomatic atherosclerosis, EULAR taskforce recommends the use of carotid ultrasound, in agreement with the ESC guidelines on CV prevention [80]: detection of carotid arteries plaques has been shown to reclassify an important percentage of patients in a more appropriate risk group [75].
Cardiovascular Risk Management
The EULAR taskforce states that the responsibility for CV risk management in RA patients should be defined according to local healthcare system and economy, relying on primary-care physicians, cardiologists, rheumatologists, and many other healthcare professionals who treat patients, even if the rheumatologist should firsthand ensure that CV risk evaluation and management is regularly being performed.
As already seen, the abovementioned recommendations suggest that RA patients categorized as having a low-moderate CV risk (SCORE < 5%) can be reassessed every 5 years, as there is no evidence that an annual screening reduces the CV risk compare to a more deferred interval [81]; patients at high risk (SCORE ≥ 5 and < 10%) or at very high risk (≥ 10%) can be re-evaluated sooner, in particular in case of rapid disease progression.
Cardiovascular risk management of RA patients is based on general recommendations and more specific measures and targets.
At the base of the CV risk management, there is the treatment of the traditional risk factors.
The importance of a behavioral education toward a healthy lifestyle, which includes smoking cessation, regular exercise, and healthy diet, is underlined in EULAR recommendations and is as valid as for the general population. As stated above, physical inactivity is common in AR patients, and exercise therapy has positive effects on CV risk factors and inflammation [82, 83, 84]. Furthermore, a Mediterranean diet (high consumption of fruit, vegetable, cereals and legumes, and less red meat and more fish) is clearly associated with a reduced CV risk in general population [85], probably also due to its effects on disease activity in AR patients [86].
As regards hyperlipidemia and hypertension, the same thresholds for general population should be used in decision-making for pharmacologic therapies. In patients at low or moderate risk (SCORE < 5%, i.e., a 10-year risk of cardiovascular death less than 5%), drug treatment is considered for LDL-C values 100–155 mg/dl if lifestyle intervention is insufficient; for patients at high risk (SCORE 5–10%) or at very high risk (SCORE > 10%), drug intervention is suggested for LDL-C values < 100 and < 70 mg/dl, respectively [87].
For systemic hypertension, drug treatment should be considered in patients with grade 1 (BP ≥ 140/90 mmHg) or 2 (BP ≥ 160/100 mmHg) hypertension who are at high CV risk. In patients at low to moderate total CV risk and with grade 1 or 2 hypertension, lifestyle measures are recommended, and drug treatment may be considered if lifestyle measures fail to reduce BP [77].
Preference for an anti-hypertensive treatment, like angiotensin-converting enzyme inhibitor (ACE-I) and angiotensin-receptor blockers (ARBs), is no longer suggested in 2015/2016 EULAR recommendations, in contrast to the previous ones.
Influence of RA Therapies on Cardiovascular and Metabolic Comorbidities
Therapies used to treat RA includes non-steroidal anti-inflammatory drugs (NSAIDs), cyclo-oxygenase-2 inhibitors (COXIB), corticosteroids, disease-modifying anti-rheumatic drugs both conventional synthetic, in particular methotrexate (MTX), and biological ones (anti-TNF, anti-IL 1, and 6). In RA, TNF-inhibitors and MTX are associated with a decreased risk of all CV events, while corticosteroids and NSAIDs are associated with an increased risk [88].
Corticosteroids, despite being very effective at treating inflammation, can increase insulin resistance, induce hypertension, and lead to metabolic syndrome, thus increasing cardiovascular risk. For patients on corticosteroid therapy, the minimum effective dosage is advisable, considering their association with increased CV risk, in a dose- and duration-dependent way [89], even if no conclusive evidence about corticosteroids long-term effects on CV safety is available [90], due to the fact that their efficacy in reducing the inflammatory state, which translates into an overall reduction of the CV risk, may be counteracted by the long-term adverse CV effects that they intrinsically possess.
NSAIDs and COXIB are associated with several potential adverse effects, including cardiovascular, such as ischemic coronary disease, heart failure, increased blood pressure, and cardiac arrhythmias. The risk of different events varies depending upon the clinical context, medication, and dose [91].
The 2009 EULAR recommendations suggested to be very cautious in their prescription in patients with documented CV disease or with CV risk factors. However, more recent data [92] show that NSAIDs increase CV risk in RA patients in comparison with general population to a lesser extent than previously reported. Therefore, in the EULAR update, the attitude in prescription and use of NSAIDs is recommended to be the same for patients without RA; they show beneficial effects in many patients with RA, by reducing inflammation and improving physical activity.
MTX seems to be effective in lowering cardiovascular morbidity and mortality mainly by the suppression of inflammatory burden [88]. Furthermore, methotrexate showed direct in vivo anti-atherosclerotic action in rabbits with atherosclerosis induced by cholesterol feeding [93] .
Finally, as seen in previous sections, lipid components undergo significant fluctuations in relation to disease activity and treatment. The most evident effect in increasing lipid components belongs to biologic-DMARDs [94].
Conclusions
The relation between inflammatory process at the base of RA and cardiovascular risk and comorbidities is clinically relevant, and the management of CV aspects should become a challenge in the approach to RA patients.
The evaluation of CV risk should be mandatory in all RA patients, measuring blood pressure and BMI in each ambulatory visit and dosing metabolic parameter (glycaemia and lipid components) every 1–5 years, according to patient’s risk category [2]. The use of RA specific risk multiplicator is advisable, although further efforts are needed to improve accuracy of prediction models.
Lifestyle recommendation (i.e., smoking cessation, physical activity, Mediterranean diet) should be given to all patients with moderate risk, and drug treatment for hypertension or dyslipidemia should be proposed to those at higher CV risk, according to current international guidelines [80, 87]. Further studies are needed to evaluate the beneficial effects of a more aggressive approach considering RA a high risk condition per se, as current guidelines do for diabetes.
In conclusion, RA should be considered not only a rheumatologic disease but also a cardiac and metabolic pathology. Therefore, a multidisciplinary approach is the better management, and rheumatologists, cardiologists, and general physicians must work together to significantly improve outcome and quality of life in RA patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Peters MJL, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–31.
Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders 2015/2016 update. Vol. 76, pp. 17–28.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–200.
Nurmohamed MT, Heslinga M, Kitas GD. Cardiovascular comorbidity in rheumatic diseases. Nat Rev Rheumatol. 2015;11:693–704.
Cobbs S, Anderson F, Bauer W. Length of life and cause of death in rheumatoid arthritis. N Engl J Med. 1953;249:553–6.
Gabriel SE, Crowson CS, Kremers HM, Doran MF, Turesson C, O'Fallon WM. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum. 2003;48:54–8.
Widdifield J, Bernatsky S, Paterson JM, Tomlinson G, Tu K, Kuriya B, et al. Trends in excess mortality among patients with rheumatoid arthritis in Ontario, Canada. Arthritis Care Res. 2015;67(8):1047–53.
Avouac J, Amrouche F, Meune C, Rey G, Kahan A, Allanore Y. Mortality profile of patients with rheumatoid arthritis in France and its change in 10 years. Semin Arthritis Rheum. 2017;46(5):537–43.
Humphreys JH, Warner A, Chipping J, Marshall T, Lunt M, Symmons DP, et al. Mortality trends in patients with early rheumatoid arthritis over 20 years: results from the Norfolk Arthritis Register. Arthritis Care Res. 2014;66(9):1296–301.
Dadoun S, Zeboulon-Ktorza N, Combescure C, Elhai M, Rozenberg S, Gossec L. Mortality in rheumatoid arthritis over the last fifty years: systematic review and meta-analysis. Joint Bone Spine. 2013;80(1):29–33.
Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524–9.
Meune C, Touzé E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology. 2009;48:1309–13.
Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379–85.
Solomon DH, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–7.
Levy L, et al. Incidence and risk of fatal myocardial infarction and stroke events in rheumatoid arthritis patients. A systematic review of the literature. Clin Exp Rheumatol. 2008;26:673–9.
Turesson C, et al. (2004) Increased incidence of cardiovascular disease in patients with rheumatoid arthritis: results from a community based study. Ann Rheum Dis, pp. 952–955.
Nicola PJ, et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005;52:412–20.
Maradit-Kremers H, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52(2):402–11.
Bartoloni E, et al. How early is the atherosclerotic risk in rheumatoid arthritis? Autoimmun Rev. 2010;9(10):701–7.
Kerola AM, et al. No increased cardiovascular mortality among early rheumatoid arthritis patients: a nationwide register study in 2000–2008. ClinExp Rheumatol. 2015;33:391–8.
Gonzalez A, et al. 2008Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis. 67:64–9.
Stampfer MJ, et al. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1991;325:373–81.
Choy E, Sattar N. Interpreting lipid levels in the context of high grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis. 2009;68:460–9.
Myasoedova E, et al. Lipid paradox in rheumatoid arthritis:the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70(3):482–7.
Liao KP, et al. Lipid and lipoprotein levels and trends in rheumatoid arthritis compared to the general population. Arthritis Care Res. 2013;65(12):2046–50.
Liao KP, et al. The association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-RA. Arthritis Rheum. 2015;67(8):2004–10.
Zhang J, et al. The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis. 2014;73(7):1301–8.
Plutzky J, et al. Lipids in RA: is less not necessarily more? Curr Rheumatol Rep. 2018;20(2):8.
Robertson J, et al. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat Rev Rheumatol. 2013;9:513–23.
•• Charles-Schoeman C, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheum. 2015;67(3):616–25.
•• Robertson J, et al. Interleukin-6 blockade raises LDL via reduced catabolism rather than via increased synthesis: a cytokine-specific mechanism for cholesterol changes in rheumatoid arthritis. Ann Rheum Dis. 2017;76(11):1949–52 These two studies showed that during the active phase of the disease LDL-C catabolism increases, explaining cholesterol levels fluctuations that characterize the natural history of the disease and its treatment.
Toms TE, et al. Are lipid ratios less susceptible to change with systemic inflammation than individual lipid components in patients with rheumatoid arthritis? Angiology. 2011;62:167–75.
Favari E, et al. Cholesterol efflux capacity (CEC): an index of atheroprotective activity of HDL? Giornale Italiano dell’Arteriosclerosi. 2014;5(3):3–8.
• Liao KP, et al. The association between reduction in inflammation and HDL cholesterol efflux capacity in rheumatoid arthritis. J AmHeart Assoc. 2015;4(2):e001588 This study shed light on another mechanism involved in lipids regulation in RA patients, demonstrating that disease-modifying antirheumatic drugs improve HDL cholesterol efflux capacity.
Navarro-Millan I, et al. Changes in lipoproteins associated with methotrexate or combination therapy in early rheumatoid arthritis: results from the treatment of early rheumatoid arthritis trial. Arthritis RheumVolucella. 2013;65(6):1430–8.
Chung CP, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum. 2008;58:2105–12.
Donath MY, et al. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107.
Zuliani G, et al. Insulin resistance and systemic inflammation but not metabolic syndrome phenotype, predict 9 years mortality in older adults. Atherosclerosis. 2014;235:538–45.
• Ruscitti P, et al. Prevalence of type 2 diabetes and impaired fasting glucose in patients affected by rheumatoid arthritis: results from a cross-sectional study. Medicine. 2017;96(34):e7896 This study has pointed out that RA is significantly associated with glucose metabolism abnormalities, possibly leading to a metabolic syndrome, and that they correlate with disease duration and articular damage extension.
Ruscitti P, et al. Poor clinical response in rheumatoid arthritis is the main risk factor for diabetes development in the short term: a 1-year, single center, longitudinal study. PlosOne. 2017;12(7):e0181203.
Solomon DH, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525–31.
Stavropoulos-Kalinoglou A, et al. Anti-tumour necrosis factor α therapy improves insulin sensitivity in normal-weight but not in obese patients with rheumatoid arthritis. Arthritis Res Ther. 2012;14:R160.
Panoulas VF, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477–82.
Ruscitti P, et al. Increased cardiovascular events and subclinical atherosclerosis in rheumatoid arthritis patients: 1 year prospective single centre study. PLoS One. 12(1):e0170108.
O’Brien. Twenty-four-hour ambulatory blood pressure measurement in clinical practice and research: a critical review of a technique in need of implementation. J Intern Med. 2011;269:478–95.
Cicek Y, et al. Non-dipping pattern in untreated hypertensive patients is related to increased pulse wave velocity independent of raised nocturnal blood pressure. Blood Press. 2013;22:34–8.
Cuspidi C, et al. Nondipping pattern and carotid atherosclerosis: a systematic review and meta-analysis. J Hypertens. 2016;34:382–5.
Wong M, et al. Reduced arterial elasticity in rheumatoid arthritis and the relationship to vascular disease risk factors and inflammation. Arthritis Rheum. 2003;48:81–9.
Panoulas VF, et al. Polymorphisms of the endothelin-1 gene associate with hypertension in patients with rheumatoid arthritis. Endothelium. 2008;15:203–2012.
Rollefstad S, et al. Treatment to lipid targets in patients with inflammatory joint diseases in a preventive cardio-rheuma clinic. Ann Rheum Dis. 2013;72:1968–74.
Solomon A, et al. Obesity and carotid atherosclerosis in African black and Caucasian women with established rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2012;14:R67.
Kremers HM, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450–7.
Tournadre A, et al. Metabolic profile during interleukin 6 inhibition in rheumatoid arthritisChanges in bodycomposition. J Cachexia Sarcopenia Muscle. 2017;8(4):639–46.
Metsios GS, et al. Association of physical inactivity with increased cardiovascular risk in patients with rheumatoid arthritis. Eur J Cardiovasc Prev Rehabil. 2009;16:188–94.
Sandberg ME, et al. Patients with regular physical activity before onset of rheumatoid arthritis present with milder disease. Ann Rheum Dis. 2014;73(8):1541–4.
Crepaldi G, et al. Cardiovascular Comorbidities Relate More than Others with Disease Activity in Rheumatoid Arthritis. PLoS One. 2016;11:e0146991.
Gonzalez A, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in nonrheumatoid arthritis patients? Ann Rheum Dis. 2008;67:64–9.
Yusuf S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52.
Choi HK, et al. Selection bias in rheumatic disease research. Nat Rev Rheumatol. 2014;10:403–12.
Hollan I, et al. Cardiovascular disease in autoimmune rheumatic diseases. Autoimmun Rev. 2013;12:1004–15.
Libby P, et al. Clinical implications of inflammation for cardiovascular primary prevention. Eur Heart J. 2010;31(7):777–83.
• Ruscitti P, et al. Subclinical atherosclerosis and history of cardiovascular events in Italian patients with rheumatoid arthritis.Results from a cross-sectional, multicenter GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Medicine. 2017;96(42):e8180 This cross sectional study points out the need of a good control of RA activity to prevent the occurrence of cardiovascular comorbidities.
Arts EE, et al. The effect of disease duration and disease activity on the risk of cardiovascular disease in rheumatoid arthritis patients. Ann Rheum Dis. 2015;74:998–1003.
Soubrier M, et al. Cardiovascular risk in rheumatoid arthritis. Joint Bone Spine. 2014;81:298–302.
Giacomelli R, et al. IL-1β at the crossroad between rheumatoid arthritis and type 2 diabetes: may we kill two birds with one stone? Expert Rev Clin Immunol. 2016;12:849–55.
Pasceri V, et al. 1999 A tale of two diseases: atherosclerosis and rheumatoid arthritis. [editorial comment]. Circulation, Vol. 100(21), pp. 2124–6.
Hollan I, et al. Vascular inflammation in systemic rheumatic diseases. Curr Med Lit Rheumatol. 2011;30(2):33–45.
Maes M, et al. Increased autoimmune activity against 5-HT: a key component of depression that is associated with inflammation and activation of cell-mediated immunity, and with severity and staging of depression. J Affect Disord. 2012;136(3):386–92.
Clark IA, et al. The roles of TNF in brain dysfunction and disease. Pharmacol Ther. 2010;128(3):519–48.
•• Arts EE, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74:668–74 This study has highlighted the inadequacy of commonly used cardiovascular risk stratification models in estimating CV risk in RA patients.
Crowson CS, et al. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110(3):420–4.
Gómez-Vaquero C, et al. SCORE and REGICOR function charts underestimate the cardiovascular risk in Spanish patients with rheumatoid arthritis. Arthritis Res Ther. 2013;15:R91.
Goodson NJ, et al. Mortality in early inflammatory polyarthritis:cardiovascular mortality is increased in seropositive patients. Arthritis Rheum. 2002;46:2010–9.
Hannawi S, et al. Atherosclerotic disease is increased in recent-onset rheumatoid arthritis: a critical role for inflammation. Arthritis Res Ther. 2007;9:R116.
Corrales A, et al. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: results of a population-based study. Ann Rheum Dis. 2014;73:722–7.
Peters MJL, et al. The interplay between infammation, lipids and cardiovascular risk in rheumatoid arthritis: why ratios may be better. Int J Clin Pract. 2010;64(10):1440–3.
Pingiottii E, et al. Surface expression of fractalkine receptor (CX3CR1) on CD4+/CD28- T cells in RA patients and correlation with atherosclerotic damage. Ann N Y Acad Sci. 2007;1107:32–41.
Evans MR, et al. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63(5):1211–20.
Ajeganova S, et al. Atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis—an inception cohort study. J Rheumatol. 2012;39(6):1146–54.
Piepoli MF, et al. European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2016;37:2315–81.
Ray KK, et al. The ACC/AHA 2013 guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: the good the bad and the uncertain: a comparison with ESC/EAS guidelines for the management of dyslipidaemias 2011. Eur Heart J. 2014;35:960–8.
Metsios GS, et al. Individualised exercise improves endothelial function in patients with rheumatoid arthritis. Ann Rheum Dis. 2014;73:748–51.
Lemmey AB, et al. Effects of high-intensity resistance training in patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum. 2009;61:1726–34.
Stavropoulos-Kalinoglou A, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819–25.
Estruch R, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90.
Sköldstam L, et al. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:208–14.
Catapano AL, et al. 2016 ESC/EAS guidelines for the management of dyslipidemias. Eur Heart J. 2016;37:2999–3058.
Roubille C, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(3):480–9.
del Rincón I, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Ann Rheum Dis. 2014;66:264–72.
van Sijl AM, et al. Confounding by indication probably distorts the relationship between steroid use and cardiovascular disease in rheumatoid arthritis: results from a prospective cohort study. PLoS One. 2014;9(1):e87965.
Howes LG, et al. Selective COX-2 inhibitors, NSAIDs and cardiovascular events – is celecoxib the safest choice? Ther Clin Risk Manag. 2007;3(5):831–45.
Lindhardsen J, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: a nationwide cohort study. Ann Rheum Dis. 2014;73(8):1515–21.
Bulgarelli A, et al. Treatment with methotrexate inhibits atherogenesis in cholesterol-fed rabbits. J Cardiovasc Pharmacol. 2012;59(4):308–14.
Souto A, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: a systematic review and meta-analysis. Arthritis Rheum. 2015;67:117–27.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Financial Disclosure
The authors received no specific funding for this work.
Additional information
Topical Collection on Rheumatoid Arthritis
Rights and permissions
About this article
Cite this article
Romano, S., Salustri, E., Ruscitti, P. et al. Cardiovascular and Metabolic Comorbidities in Rheumatoid Arthritis. Curr Rheumatol Rep 20, 81 (2018). https://doi.org/10.1007/s11926-018-0790-9
Published:
DOI: https://doi.org/10.1007/s11926-018-0790-9