Abstract
Purpose of Review
Recent studies have investigated the effect of treatments containing adipose-derived mesenchymal stem cells (ADMSCs) on human osteoarthritis. These have mostly used biologic adjuvants which may influence results. Thus, the purpose of this systematic review is to evaluate the current literature on these treatments when used in isolation.
Recent Findings
Five studies in this review used cultured ADMSCs, while four studies used stromal vascular fraction and three used micro-fragmented adipose tissue to deliver ADMSCs. No studies reported serious treatment-related adverse effects and all reported improvements in clinical measures for at least one dose. This was not necessarily reflected in imaging evaluations nor were improvements always maintained.
Summary
Current low-level evidence is limited due to variability in study methodology but indicates that treatments containing ADMSCs, when used in isolation, are safe and have the potential to reduce pain and improve function. Randomized controlled trials are now needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis and Current Treatments
Osteoarthritis (OA) is one of the world’s most disabling conditions [1]. Research has revealed that there are primary and secondary changes that occur in an osteoarthritic joint. The primary change is an alteration in the articular cartilage whereas secondary changes may include osteophyte formation [2, 3]. Traditional approaches in dealing with this damaged tissue have included bone marrow stimulation techniques such as microfracture, abrasion, or subchondral drilling. However, MRI evidence has shown that such treatments only partially rectify cartilage defects and results are not maintained after the 2–3-year time point [4]. Yet the biggest obstacle for researchers has been the elusiveness of techniques that regenerate hyaline cartilage instead of the less robust fibrocartilage [5, 6]. Other techniques include autologous chondrocyte implantation (ACI) and matrix-induced autologous chondrocyte implantation (MACI). These approaches offered renewed promise with many studies concluding that MACI significantly improved a number of different outcome measures compared to microfracture: it was found to be safer and needed a less complicated and invasive surgery [7]. Despite this, others have been more skeptical, citing concerns over arthrofibrosis frequency, delamination [8], and periosteal hypertrophy [9].
Mesenchymal Stem Cells
Stem cell therapies offer an alternative technique to regenerate or repair cartilage. Particularly, interest has focused on mesenchymal stem cells (MSCs), which not only are multipotent with the capability for self-renewal, but can also circumvent restrictions of cell availability in ACI [10].
MSCs may also have a number of other beneficial properties that have been reviewed elsewhere [11]. In brief, they have been shown to secrete growth factors and chemokines to increase angiogenesis and cell proliferation. MSCs also have anti-inflammatory and immunomodulatory properties, for example, by releasing cytokines such as TGF-β1 and IL-4 and by regulating the function and proliferation of T and B cells.
MSCs can now be obtained from a variety of tissues including adipose tissue, bone marrow, the infrapatellar fat pad, and umbilical cord blood. However, stem cells obtained from these different sources may express different surface markers. As a result of this, the International Society for Cellular Therapy has defined a minimum set of criteria to help characterize the MSC [12]. It must first, when cultured, adhere to plastic and then must express CD73, CD90, and CD105. It must not express CD11b, CD14, CD19, CD34, CD45, CD79 alpha, or any HLA-DR molecules. Lastly, MSCs should be able to differentiate into osteoblasts, adipocytes, and chondroblasts in in vitro experiments. However, many studies fail to comply with these criteria which presents an obstacle when analyzing the literature on the use of mesenchymal stem cells. Moreover, as Murphy et al. comment, these criteria are for cultured cells in vitro and in vivo cells have a different phenotype [11]. Therefore, they propose that these cells should be positive for CD44, CD73, CD90, CD105, CD146, and Stro-1 and negative for CD14, CD45, and CD11b. They also suggested that, based on the current literature, MSCs should not express or express very little CD34. However, more research should be carried out in order to clarify this characterization of in vivo stem cells.
Adipose-Derived Mesenchymal Stem Cells
Although bone marrow-derived stem cells (BMSCs) have been the subject of more study, there has been an upturn in work looking into the potential of adipose-derived mesenchymal stem cells (ADMSCs). This is owing to their ability to be cultured without passage for long periods of time, while retaining their phenotypic features [13].
Broadly, there are three ways of delivering ADMSCs: cultured ADMSCs, in stromal vascular fraction (SVF), and in micro-fragmented adipose tissue. SVF refers to a heterogeneous cell population that contains a small proportion of MSCs, whereas ADMSCs describe a very specific homogenous population of stem cells contained within the SVF and obtained through isolation then expansion. Micro-fragmented adipose tissue is the product of fragmentation of the adipose tissue without the use of enzymatic digestion [14, 15••]. Blood derivatives are washed off to obtain tissue that is enriched in pericytes and has a lower amount of hematopoietic cells than the enzymatically digested lipoaspirates such as the SVF.
As Park et al. have commented previously, the sudden popularity that these treatments have recently received has led to confusion—studies have described the treatments that they administer as ADMSCs when, in fact, cells they actually administered were from the SVF [16]. The Current Good Tissue Practice requirements state that any human-cell product that needs processing over and above “minimal manipulation” (in this case, expansion ex vivo) requires a license from the U.S. Food and Drug Administration [17]. Therefore, licenses must be obtained for administration of ADMSCs, but neither SVF nor micro-fragmented adipose tissue requires such expansion. This makes SVF and micro-fragmented adipose tissue more attractive propositions for surgeons administering these stem-cell based treatments to patients.
A number of reviews have provided an overview of treatments containing ADMSCs, but have always included studies which also use either bone marrow stimulation techniques or use MSC biological adjuvants (such as platelet-rich plasma, fibrin glue, hyaluronic acid). To our knowledge, no previous review has solely focused on adipose-derivative therapies when used alone without including studies that used a combination of additional techniques. In this review, we will evaluate only those studies that did not use combination techniques. This more focused approach will be beneficial for assessing the true efficacies of treatments containing ADMSCs without other techniques potentially clouding the data. Our aim is to provide the most reliable accounting of the likely clinical outcomes of such treatments.
Methods
A literature search was conducted in July 2018 for articles published between 2013 to the present using databases PubMed, EMBASE, CINHal, Scopus, and clinicaltrials.gov. Key terms in the search were “(mesenchymal stem cell OR mesenchymal stromal cell OR MSC OR stem cell OR stem AND cells) AND (cartilage OR chondral) AND (wound healing OR wound AND healing OR repair) AND (defect OR lesion OR osteoarthriti*).”
Articles were included that (1) assessed the effect of treatments containing ADMSCs on human patients with OA; (2) provided at least 6 months follow-up; (3) reported outcomes using pain or function scores; (4) were published in a peer-reviewed journal; (5) were published in the English language; and (6) for which the full text was freely available. Studies were excluded if they (1) used MSC biologic adjuvants such as scaffolds, platelet-rich plasma (PRP), fibrin glue, or hyaluronic acid (HA); (2) supplemented treatments containing ADMSCs with bone marrow stimulation techniques such as subchondral drilling, spongialization, abrasion, and microfracture; or were reports on (3) single-patient cases, reviews, animal data, in vitro data, conference proceedings, or ongoing clinical trials or results were otherwise not available (authors were contacted if this was the case).
The titles of the articles retrieved from each search were reviewed for relevance. Abstracts were then screened independently by two authors (CDSR and CKIR) to check if they met inclusion criteria. Full-text manuscripts were then retrieved for all potentially relevant articles and for those that authors remained uncertain about. Each was analyzed for criterion matching. A hand search was also performed on associated review articles to identify any articles that could have been missed by the search.
Results
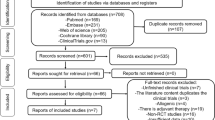
The search revealed 39 potentially relevant studies found on PubMed, EMBASE, CINHal, Scopus, and clinicaltrials.gov. Twelve studies were then included in this review (Fig. 1). One of the 12 articles was a 2-year follow-up of another included study, which was a prospective cohort study assessing ADMSC intra-articular injections at three doses [18•, 19••]. Four of the 12 studies were retrospective [20,21,22,23] and the remaining 8 were prospectively conducted [15••, 18•, 19••, 24, 25, 26••, 27••, 28•]. There was no randomized controlled trial found in the literature. Most studies were prospective cohort studies; others were retrospective reviews. The data extracted for each included study is summarized in Tables 1 and 2. Table 1 shows study details. Table 2 shows study results.
Details of Defects
In all of the 12 included studies, the cartilage defects were located in the knee. A few studies (4 out of 12: [18•, 19••, 20, 21]) specified the mean size of the defect at baseline, ranging from 407 to 540 mm2. The grade of the osteoarthritis varied across studies and each used different scales to report this: K-L scale [18•, 19••, 20, 21, 23,24,25, 27••, 28•], ICRS [21], and IKDC [26••]. Of those that used the K-L scale, lesions varied from grade I up to grade IV.
Treatment Administered
Of the 12 included studies, 4 studies used SVF assessing a total of 91 patients [20, 21, 24, 27••], 5 used cultured adipose-derived mesenchymal stem cells (ADMSCs) involving 63 patients [18•, 19••, 25, 26••, 28•], and 3 used micro-fragmented adipose tissue involving 82 patients [15••, 22, 23]. Of those that administered ADMSCs, which require cell culture before administration, the length of culture differed across studies: 2 studies cultured for 3 weeks [18•, 19••], 1 for 2 weeks [25], another for 2–3 weeks [26••], and 1 that administered multiple injections cultured for 3 and then 6 weeks [28•]. The resulting number of cells administered thus varied across studies, ranging from 2 million cells [25] to 100 million cells [18•, 19••]. The delivery method for cells also differed between studies. All studies with ADMSCs used an intra-articular injection technique [18•, 19••, 25, 26••, 28•], while 2 studies used this method for SVF [24, 27]. The remaining two studies which used SVF implanted the treatment preparation under arthroscopy [20, 21]. All 3 studies using micro-fragmented adipose tissue also injected intra-articularly [15••, 22, 23].
Seven studies involving the knees of 127 patients harvested adipose tissue from the abdomen [15••, 18•, 19••, 22, 23, 25, 26••], while 2 studies with 72 patients [20, 21]) used tissue from the buttock. Two studies harvested from multiple sites [24, 27••]; however, they did not specify numbers of patients for each site. One study of 18 patients did not report where they obtained the adipose tissue from [28•].
One study included patients that were treated with a MSC biologic adjuvant [21], a fibrin glue scaffold, in combination with the SVF. This was compared to SVF alone. Therefore, only the patient group that was treated with SVF alone (37 patients) was included in this analysis. Another retrospective cohort study [23] assessed a micro-fragmented adipose tissue approach combined with chondral shaving (SH) and compared this procedure to micro-fragmented adipose tissue added to shaving and meniscectomy (SM). As adipose-derived tissue was used in each of these cohorts, 21 patients and 14 patients respectively, both were included in this analysis. Six studies [20,21,22,23,24,25] included additional procedures such as aspiration of joint fluid, debridement, ACL/LCL reconstruction, and the aforementioned chondral shaving and meniscectomy. The remaining 6 studies did not apply any additional procedures [15••, 18•, 19••, 26••, 27••, 28•].
Follow-up
Total follow-up periods differed from 6 [25] to 29.2 months (mean) [21]. Only 3 studies, involving 49 patients, were followed for less than 12 months [18•, 25, 27••].
Outcome Measures
Differences between studies were also seen in the reported outcome measures and imaging evaluations used. The majority of studies reported validated objective and subjective pain or function scores such as Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Visual Analog Scale (VAS), Knee Society Score (KSS), Knee injury, and the Osteoarthritis Outcome Score (KOOS).
Crucially, a validated score to assess the appearance of actual articular cartilage repair was only used in four of the studies: Spasovski et al. used the Magnetic Resonance Observation of Cartilage Repair Tissue Score (MOCART) [26••], while Koh et al., Kim et al., and Jo et al. used the International Cartilage Repair Society (ICRS) score [18•, 20, 21]. This represents a significant deficiency in the literature.
A total of 9 studies out of 12 used some type of imaging evaluation to assess cartilage repair: second-look arthroscopy (2 studies of 72 patients) [20, 21], MRI (6 studies of 86 patients) [15••, 19••, 24, 25, 26••, 28•]; one study used both of these techniques (of 18 patients) [18•]. Clinical outcome scores were statistically improved for at least one dose group in all studies, as detailed in Table 2.
Adverse Events
Six of the 12 studies reported adverse events [18•, 22, 23, 25, 27••, 28•] with only one study showing a serious adverse event [25], namely unstable angina pectoris. Of those studies that documented treatment-related adverse effects, all were minor [25, 27••, 28•]. The most common was pain and swelling at the harvest site or the injection site [25, 27••]. Thus, according to these studies, treatment containing ADMSCs can be considered safe.
Does Outcome Change with Time?
Of the studies that recorded outcome measures at more than one time point, there were mixed results. At both 3 and 12 months after treatment, Fodor et al. showed that clinical outcomes were improved and results were relatively sustained [24]. Jo et al. found that in both the low- and medium-dose groups, WOMAC, VAS, KSS knee score, KSS function score, KOOS pain, and KOOS symptom outcomes tended to deteriorate from a peak response after 1 year. In the high-dose group, outcome scores such as the KSS function and KOOS sports were maintained after this time point [19••]. Spakowski et al. observed that, while all clinical scores statistically improved at 6 months, they tended to peak at 6 or 12 months with some then falling at 18 months [26••]. In the study by Cattaneo et al., the micro-fragmented adipose tissue approach plus chondral shaving (SH) group’s outcomes improved over all time points whereas in the micro-fragmented adipose tissue added to shaving and meniscectomy (SM) group, they improved to a peak of 6 months [23]. On the other hand, Hudetz et al. showed an improvement in VAS which persisted across all time points up to 12 months [15••]. However, this study only used VAS to measure clinical response. Song et al.’s was the only study to administer multiple injections [28•]. Interestingly, it was the third injection, at 48 weeks, that was associated with an increase in the WOMAC and SF-36 outcome improvement rates in the low- and high-dose groups and in the low- and mid-dose groups was associated with an increase in the NRS-11 outcome improvement rate. The authors suggested that this could represent a possible advantage of multiple injections.
Did Imaging Evaluations Correlate With Clinical Outcome Measurements?
The studies were varied in their answer to this question as well. Fodor et al. showed there were no significant differences on MRI 3 months after treatment, whereas clinical outcomes improved in this period [24]. The earlier of Jo et al.’s studies revealed that although clinical outcome measures improved only for the high-dose group, radiographic analysis showed no significant differences in measures such as K-L grade and joint space width between 0 and 6 months [18•]. The MRI, however, did show that the size of the cartilage defect decreased in a number of condyles at 6 months but this was only observed in the high-dose group. There also seemed to be a regeneration in cartilage in the medial femoral and tibial condyles, measured by differences in signal intensity on MRIs after 6 months. Similarly, the latter of Jo et al.’s studies showed that MRI findings were correlated with clinical outcomes observed after 24 months [19••]. The most significant changes in the sizes of the defects on MRI were seen in the highest dose group as were the most significant changes in clinical outcomes. In both Kim et al. and Koh et al., improvements in clinical outcomes (Tegner activity and IKDC) were associated with improvements in ICRS grade [20, 21]. Hudetz et al.’s VAS scores were related to their MRI outcomes [15••]. In contrast, Pers et al. found no correlation between MRI changes and clinical outcome measures [25]. Spasovski et al. found that their MRI results, specifically MOCART scores, were in line with their observed clinical results [26••]. However, this concordance was not seen with plain radiographs.
How Did Dose Affect Clinical Outcome Measurements?
Results from the four studies that used multiple doses reported variation in the dose dependence of clinical outcome measures. The most recent of the two studies conducted by Jo et al. concluded that the high-dose group achieved greater statistical significance in clinical outcome measure improvements over the 24 months (100 million ADMSCs) [19••]. They suggested that this was evidence for a possible dose-dependent response. Song et al. reported a similar finding for their high-dose group, who received 50 million ADMSCs [28•]. In the study by Pers et al., however, the low-dose group of 2 million ADMSCs achieved most statistical significance as measured by WOMAC, VAS, KOOS, SAS, and SF-36 scales after 6 months [25]. The authors suggested that this might have been due to the limited sample size used in the study although both Jo et al. and Song et al. used the same number of patients [18•, 19••, 28•]. The large variability in baseline WOMAC, KOOS, and SAS scores between patients in different dose groups was cited as another reason why this could have occurred.
Discussion
The Need for a Higher Level of Evidence to Assess Efficacy of Treatments
On the whole, treatments containing ADMSCs produced positive clinical outcomes. However, this could also reflect a publication bias. As outlined above and in Table 2, all studies showed a statistical improvement, in the very least, at one time point, one dose, and one outcome measure from WOMAC, VAS, KOOS, KSS, ROM, TUG, IKDC, Tegner activity scale, SAS, SF-36, HSS-KS, JKOM, and NRS-11 compared with baseline.
The heterogeneity of the results to date may be explained by a number of factors. There is considerable variability in the methods used by each study: harvest site, delivery method, follow-up period, and dosages vary, even in studies that used the same cell entity. As detailed by Kim et al., there are a number of potential patient-specific prognostic factors that may affect clinical outcomes, including patient age and lesion size [29]. In addition, most of the studies used other procedures such as debridement. Therefore, it is possible that the relative successes of approaches detailed in this review could be at least partially due to these additional procedures. The heterogeneity of results underscores the fact that the optimal type of ADMSC treatment (ADMSCs, SVF, micro-fragmented adipose tissue), harvest site, delivery method, and dosage has not yet been identified. Moreover, current studies generally have limited follow-up periods ranging from 6 to around 24 months. When treating a disease such as osteoarthritis where signs and symptoms evolve over years, longer term studies with follow-ups of 4–5 years must now be undertaken to observe if this initial efficacy is maintained. This highlights the importance of randomized, double-blinded controlled trials with longer follow-up periods as the gold standard to assess the efficacy of treatment containing ADMSCs.
Studies Need to Utilize More Sensitive Imaging Techniques to Support Clinical Data
Many of the studies reviewed showed signs of cartilage regeneration and/or the size of the initial cartilage defect decreasing [15••, 18•, 19••, 20, 21, 26••, 28•]. There were, however, some articles that did not show a correlation between clinical outcome measures and cartilage regeneration [24, 25]. This may suggest that other variables are contributing to the observed improvement in the clinical outcomes shown. Spasovski et al. commented that although MOCART scores showed improvement, this was not reflected in their radiographic findings [26••]. This was also seen in the results of Jo et al. [18•]. As discussed in the “Results” section of this article, most studies failed to use a validated outcome measure such as the MOCART score and ICRS grade to display real articular cartilage repair.
Radiographs have been shown to demonstrate tissue changes in OA that occur late in the development of disease and be less sensitive to earlier biochemical changes [15••, 30]. In contrast, techniques such as dGEMRIC are more sensitive to these early biochemical changes by measuring glycosaminoglycan (GAG) content of cartilage which is reduced in diseased cartilage. dGEMRIC, as used by Hudetz et al., may therefore provide a better option compared to radiography in displaying biochemical changes after treatment. Indeed, studies have shown that dGEMRIC can identify cartilage defects, even when OA changes have not been detected on radiographs [31]. In the current literature, it is clear that more studies should also utilize more sensitive imaging techniques such as dGEMRIC to detect diseased and regenerated cartilage.
Are These Treatments Safe?
None of the studies included in this review reported any serious treatment-related adverse effects. Pain and swelling at the injection or harvest site was the most common side effect but, in all cases, this resolved within a few days. In general, this low-level evidence suggests that the discussed treatments containing ADMSCs seem to be safe.
Optimizing Rehabilitation Procedures
Five of the studies in this review did not specify their rehabilitation procedures at all [15••, 25,26,27,28]. A long-term goal for this field must be to determine an optimum period of immobilization, the time at which partial and full weight bearing should be permitted, the range of motion allowed, and time points for full return which all varied across studies.
Future
In the past year, some research groups have attempted to evaluate the method of using micro-fragmented adipose tissue to deliver ADMSCs, which does not require the cell expansion or enzymatic treatment needed in ADSCs and SVF [15••, 22, 23]. This approach allows a more cost-effective, less labor-intensive technique, in comparison to others [22]. Owing to the novelty of this approach, only three of the studies in this review looked at such a technique; these studies collectively showed clinical improvements in pain and function as well as radiological improvement. This should act as a basis for future work that looks at gathering more high-level evidence for its use in a clinical setting. Further comparative studies looking at the use of ADSCs vs SVF vs micro-fragmented adipose tissue would be a clear step to achieve this goal.
Between studies that use the same treatment, we also call for a degree of standardization with regard to the outcome measures used, imaging evaluations used, harvest sites, mode of delivery, and dosage. Only then can we truly evaluate the relative safety and efficacies of different subtypes of treatment on osteoarthritis. We also encourage researchers to define background information about their samples beyond the simplicities of age and gender. For example, defect size was not specified in 8 of 12 studies reviewed [15••, 22,23,24,25, 26••, 27••, 28•]. Detailing defect sizes could be beneficial in explaining any difference in effectiveness between studies [29]. Furthermore, as discussed above, studies should use validated outcome measures such as the MOCART score and ICRS grade to measure actual cartilage repair after treatment.
Another significant challenge for this field is to find methods to encourage the generation of cartilage which is both stable and remains resistant to fibrous dedifferentiation. Indeed, in a study investigating the in vivo phenotypic stability of cartilage generated from stem cells, it was found that adipose-derived stem cells from the fat pad appeared to undergo fibrous dedifferentiation unlike bone marrow-derived stem cells [32]. In the future, more research must be undertaken to clarify the mechanisms driving this process and whether we can prevent this from occurring.
Limitations of Our Study
There are a number of inherent limitations with this review. Firstly, our search was limited to Scopus, MEDLINE, EMBASE, CINHal, and clinicaltrials.gov. Secondly, only articles published in English were screened. Further, a side effect of the fact that our aim was to focus specifically on studies that used treatments containing ADMSCs in isolation was that our search represents only a small subset of the literature involving these treatments for OA.
Conclusion
This review highlights the paucity of studies assessing treatments containing ADMSCs when used alone without biologic adjuvants or bone marrow stimulation techniques. Broadly, the current literature suggests that these treatments are associated with a low risk of serious side effects and can produce a decrease in pain and an improvement in function. Before clinicians can use these treatments on a wider scale, higher level studies with longer follow-up periods are imperative to display such improvements in pain and function compared to control groups. We have made a number of recommendations for the development of the field, including standardizing methodology within studies that use the same treatment subtype.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–30.
Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192(1):230–7.
Dequeker J, Luyten FP. The history of osteoarthritis-osteoarthrosis. Ann Rheum Dis. 2008;67(1):5–10.
Mithoefer K, WILLIAMS III RJ, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee: a prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911–20.
Kaul G, Cucchiarini M, Remberger K, Kohn D, Madry H. Failed cartilage repair for early osteoarthritis defects: a biochemical, histological and immunohistochemical analysis of the repair tissue after treatment with marrow-stimulation techniques. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2315–24.
Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. JBJS. 1993;75(4):532–53.
Basad E, Ishaque B, Bachmann G, Stürz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):519–27.
Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthr Cartil. 2011;19(7):779–91.
Marlovits S, Zeller P, Singer P, Resinger C, Vécsei V. Cartilage repair: generations of autologous chondrocyte transplantation. Eur J Radiol. 2006;57(1):24–31.
Roberts S, Genever P, McCaskie A, Bari CD. Prospects of stem cell therapy in osteoarthritis. Regen Med. 2011;6(3):351–66.
Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45(11):e54.
Dominici ML, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26(6):664–75.
Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013;22(11):2063–77.
•• Hudetz D, Borić I, Rod E, Jeleč Ž, Radić A, Vrdoljak T, et al. The effect of intra-articular injection of autologous microfragmented fat tissue on proteoglycan synthesis in patients with knee osteoarthritis. Genes. 2017;8(10):270. This was one of the first studies to use micro-fragmented fat tissue as a way of delivering ADMSCs.
Ha CW, Park YB. Mesenchymal stem cell injection for osteochondral lesions of the talus: letter to the editor. Am J Sports Med. 2014;42(6):NP34–5.
U.S. Department of Health and Human Services. Regulatory considerations for human cells, tissues, and cellular and tissuebased products: minimal manipulation and homologous use. Available from: https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM585403.pdf. Accessed 5th Aug 2018.
• Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–66. One of the pioneering clinical trials to use ADMSC treatment in isolation. This proof-of-concept clinical trial was followed up at a later time point (see [19••]).
•• Jo CH, Chai JW, Jeong EC, Oh S, Shin JS, Shim H, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med. 2017;45(12):2774–83. A recent clinical trial which was one of the few to include a longer term follow-up time point (2 years).
Koh YG, Choi YJ, Kwon OR, Kim YS. Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med. 2014;42(7):1628–37.
Kim YS, Choi YJ, Suh DS, Heo DB, Kim YI, Ryu JS, et al. Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold? Am J Sports Med. 2015;43(1):176–85.
Russo A, Condello V, Madonna V, Guerriero M, Zorzi C. Autologous and micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis. Journal of experimental orthopaedics. 2017;4(1):33.
Cattaneo G, De Caro A, Napoli F, Chiapale D, Trada P, Camera A. Micro-fragmented adipose tissue injection associated with arthroscopic procedures in patients with symptomatic knee osteoarthritis. BMC Musculoskelet Disord. 2018;19(1):176.
Fodor PB, Paulseth SG. Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthet Surg J. 2015;36(2):229–36.
Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med. 2016;5(7):847–56.
•• Spasovski D, Spasovski V, Baščarević Z, Stojiljković M, Vreća M, Anđelković M, et al. Intra-articular injection of autologous adipose-derived mesenchymal stem cells in the treatment of knee osteoarthritis. J Gene Med. 2018;20(1):e3002. A comprehensive study that assessed a range of outcome measures at several follow-ups, after injection with ADMSCs.
•• Yokota N, Yamakawa M, Shirata T, Kimura T, Kaneshima H. Clinical results following intra-articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee. Regen Ther. 2017;6:108–12. A recent study that highlighted the potential of treatments that deliver ADMSCs via SVF for osteoarthritis.
• Song Y, Du H, Dai C, Zhang L, Li S, Hunter DJ, et al. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. 2018;13(3):295–307. A pilot study which was one of the first in the field to explore the effects of multiple injections on clinical outcomes after ADMSC treatment.
Kim YS, Choi YJ, Koh YG. Mesenchymal stem cell implantation in knee osteoarthritis: an assessment of the factors influencing clinical outcomes. Am J Sports Med. 2015;43(9):2293–301.
Gray ML, Burstein D, Kim YJ, Maroudas A. Magnetic resonance imaging of cartilage glycosaminoglycan: basic principles, imaging technique, and clinical applications. J Orthop Res. 2008;26(3):281–91.
Williams A, Gillis A, McKenzie C, Po B, Sharma L, Micheli L, et al. Glycosaminoglycan distribution in cartilage as determined by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC): potential clinical applications. Am J Roentgenol. 2004;182(1):167–72.
Vinardell T, Sheehy EJ, Buckley CT, Kelly DJ. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng A. 2012;18(11–12):1161–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Complementary and Alternative Medicine.
Rights and permissions
About this article
Cite this article
Ranmuthu, C.D.S., Ranmuthu, C.K.I. & Khan, W.S. Evaluating the Current Literature on Treatments Containing Adipose-Derived Stem Cells for Osteoarthritis: a Progress Update. Curr Rheumatol Rep 20, 67 (2018). https://doi.org/10.1007/s11926-018-0776-7
Published:
DOI: https://doi.org/10.1007/s11926-018-0776-7